Abstract
Background
According to current guidelines, pancreatic cancer patients should be strictly selected for surgery, either palliative or resective.
Methods
Population-based study, including all patients undergoing surgery for pancreatic cancer in Italy between 2010 and 2012. Hospitals were divided into five volume groups (quintiles), to search for differences among volume categories.
Results
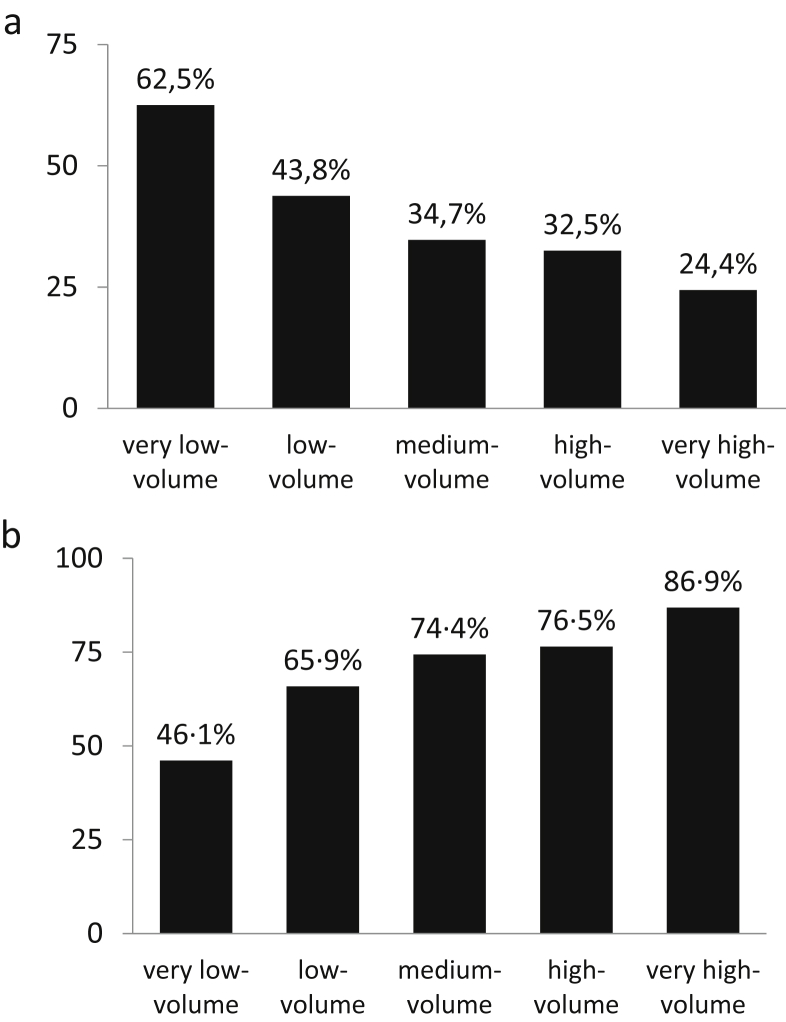
There were 544 hospitals performing 10 936 pancreatic cancer operations. The probability of undergoing palliative/explorative surgery was inversely related to volume, being 24.4% in very high-volume hospitals and 62.5% in very low-volume centres (adjusted OR 5.175). Contrarily, the resection rate in patients without metastases decreased from 86.9% to 46.1% (adjusted OR 7.429). As for resections, the mortality of non-resective surgery was inversely related to volume (p < 0.001). Surprisingly, mortality of non-resective surgery was higher than that for resections (8.2% vs. 6.7%; p < 0.01). Approximately 9% of all resections were performed on patients with distant metastases, irrespective of hospital volume group. The excess cost for the National Health System from surgery overuse was estimated at 12.5 million euro.
Discussion.
Discrepancies between guidelines on pancreatic cancer treatment and surgical practice were observed. An overuse of surgery was detected, with serious clinical and economic consequences.
Introduction
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States,1 as well as in Italy where it caused 10 722 deaths in 2012.2 It is the type of cancer with the worst prognosis, a 5-year survival rate of approximately 7%.3 Only a minority of patients affected by pancreatic cancer require surgery, either in terms of resective, palliative or exploratory operations.
Resection is indicated in the absence of distant metastases, and in the case of absent or limited invasion of peripancreatic vessels.4, 5, 6 Unfortunately, at the time of diagnosis, metastases are already manifest in 50% of cases, and the tumour invades adjacent major vessels in another 30% of cases.7 The role of palliative surgery (biliary or gastric by-passes) is also limited; guidelines from the USA,4, 5, 6 Europe4, 5, 6 and Italy6 are in agreement that an endoscopic biliary stent is the preferred method for treating malignant biliary obstruction which occurs in up to 70% of pancreatic cancer patients.7 Gastric outlet obstruction affects a minority of patients8 and is often a later event. Palliative gastrojejunostomy can be more effective than an enteral stent to relieve obstruction, but surgery should be reserved for individuals with a good prognosis and performance status.4, 6 Finally, exploratory surgery (laparoscopy or laparotomy) is sometimes performed on pancreatic cancer patients. In a few centres, diagnostic laparoscopy is routinely used prior to surgery, whereas it is selectively applied to patients with a higher risk of metastases in the majority of centres.4, 6 An exploratory laparotomy is carried out as a consequence of inadequate preoperative evaluation, namely a tumour deemed resectable, but intraoperatively unanticipated metastases or vascular invasion are found.
To date, nationwide data regarding the overall surgical treatment (both resective and non-resective operations) offered to pancreatic cancer patients are lacking. The aim of this study was to evaluate the operations performed for pancreatic cancer nationwide in Italy during the period 2010–2012. A possible variability of treatment depending on the hospital volume was investigated, since the volume–outcome relationship in pancreatic surgery is well established.9, 10, 11, 12, 13, 14, 15 An estimate of the clinical and economic consequences of potentially incorrect surgery was performed.
Methods
Data regarding pancreatic cancer surgery were obtained by the Italian Association for the Study of the Pancreas (AISP) from the Directorate of Health Care Planning of the Italian Ministry of Health whose database includes information on every inpatient discharged from all public and private hospitals in Italy.
Data were obtained for all acute, admitted patient episodes in Italy in the period January 2010–December 2012, having a principal diagnosis of malignant neoplasm of pancreas (codes 157.0-9 of the International Classification of Diseases, 9th revision, Clinical Modification). For reasons of homogeneity, the code 157.4 (malignant neoplasm of the islet of Langerhans) was excluded. The cancer was considered metastatic when codes 197 or 198 (secondary malignant neoplasm of respiratory and digestive systems or of other specified sites) were associated. To respect the legislation on data privacy and security, the data were made available anonymously by the Ministry of Health.
The operations considered for the data analysis are listed in Supplementary Table 1. The operations were categorised into resective and non-resective surgery (palliative/exploratory). Episodes coded as 99.85, hyperthermia for treatment of cancer (i.e. radiofrequency ablation of pancreatic cancer) were excluded because the indication for laparotomy could differ from the usual indications (165 episodes).
To evaluate a possible variability of surgical treatment depending on the volume of pancreatic surgery, hospitals were divided into quintiles to create cut-off points for sorting patients into five similarly sized groups (quintiles), listing hospitals according to the ascending number of resections performed by each centre over the three-year period.
The following items were investigated: non-resective surgery rates (both palliative and explorative surgery), resection rates in patients with or without distant metastases, operative mortality (defined as death occurring during hospital stay) of each operation as well as of each category of operation (explorative, palliative, resective).
Estimates on overuse of surgery and corresponding excess cost were carried out. Present guidelines on pancreatic cancer suggest to reduce the rate of surgical palliation in favour of endoscopic palliation, and to avoid resection in presence of metastases. The excess of palliative/exploratory surgery was estimated as the difference between the rate of each non-resective operation performed in the very high-volume group (used as a benchmark), and the rate recorded in the other volume categories. Furthermore, all resections performed in presence of distant metastases were considered incorrect. Avoidable costs were estimated according to the payer's perspective by means of a multi-step procedure. The first step was to compute the cost of hospitalisations using the national tariffs set by Ministerial Decree 18/10/2012. This value represents a cost estimate since Italy has a decentralised healthcare system, and tariffs are set at a regional level. The use of national tariffs was motivated by the desire to compute a national cost not influenced by the region where the patients were treated. The second step, average cost per hospitalisation, was computed separately for resective and non-resective surgery. This step was necessary since surgical treatment may fall into numerous diagnosis-related groups (DRGs), each with a different tariff, according to the combination of interventions coded in the case history. For the third step, different methods were used to estimate the average avoidable costs for resective and non-resective surgery. Regarding non-resective surgery, the estimate was based on the assumption that avoidable interventions would have been replaced by medical treatment. In that case, hospitalisation would have been classified as DRG 203 – malignancy of the hepatobiliary system or the pancreas. Therefore, the average avoidable cost was estimated as the difference between the average tariff computed in step 2 for non-resective interventions and the DRG 203 rate. Regarding resections in metastatic patients, the authors assumed that the appropriate treatment would have been non-resective treatment. Therefore, the average avoidable cost was estimated as the difference between the average tariff for the resective and the non-resective treatment computed in step 2. The last step was common to both operation categories and represented the total avoidable cost estimate by applying the average avoidable cost to the estimated number of avoidable cases.
The χ2 test was used for ordinal and nominal variables, and ANOVA for continuous variables. Logistic regression analysis was carried out to assess the relationship between hospital volume, other patient characteristics and the variables considered as outcomes of interest. A multivariate analysis was also carried out, taking all the significant variables affecting the different outcomes in the univariate analysis into consideration. Odds ratios (ORs) were adjusted according to sex, source of payment (National Health Service (NHS) or private payer), age and co-morbidity. Co-morbidities were classified according to the Charlson score.16 Age was not considered in the multivariate analysis, having already been age-adjusted in the Charlson score. A p value < 0.05 was considered statistically significant. Data analysis was carried out using SPSS version 13.0 for Windows (SPSS, Chicago, Illinois, USA).
Results
The final analysis included 10 936 operations, consisting of 6570 resections (60.1%), and 4366 non-resective operations (39.9%). Five hundred and forty-four hospitals in Italy carried out at least one pancreatic cancer operation. According to the number of operations performed, four hundred and eight hospitals (75%) performing nine or fewer resections in three years were classified as very low-volume, 76 hospitals (14%) performing 10 – 26 resections as low-volume, 37 hospitals (6.8%) performing 27 – 57 resections as medium-volume, 17 hospitals (3.1%) performing 58 – 141 resections as high-volume, and 6 hospitals (1.1%) performing >141 resections as very high-volume. Patient characteristics are summarised in Table 1; patients operated on in lower-volume hospitals tended to be older (p < 0.001) than those treated in higher-volume hospitals. The rate of non-resective surgery decreased progressively with increasing hospital volume, from 62.5% in very low-volume hospitals to 24.4% in very high-volume hospitals (Table 2, Fig. 1a). Of the resective operations, pancreaticoduodenectomy (PD) was the most frequent operation (37.2% of all operations) whereas exploratory laparotomy was the most frequent procedure among non-resective operations (16.3%). Multivariate analysis confirmed the independent effect of hospital volume on the type of surgery carried out (resective or non-resective). The odds ratio (OR) for undergoing a non-resective operation in the lowest-volume category compared with the highest-volume category was 5.172 (p < 0.001); adjustment for patient characteristics had no effect (adjusted OR 5.175, p < 0.001) (Table 3). Contrarily, in patients without distant metastases, the resection rate was 86.9% in very high-volume hospitals, progressively declining to 46.1% in very low-volume hospitals (Supplementary Table 2, Fig. 1b). The independent effect of hospital volume in influencing the type of surgery (resective or non-resective) on patients without metastases was confirmed by multivariate analysis (Supplementary Table 3). Of note, 604 resections (9.2% of overall resections) were performed on patients with distant metastases, with small variations among the different volume groups (very low-volume 8.5%, low-volume 8.9%, medium-volume 7.4%, high-volume 8.9%, very high-volume 11.8%).
Table 1.
Descriptive data of 10 936 operations for pancreatic cancer performed in Italy from 2010 to 2012, stratified in quintiles according to hospital volume of pancreatic cancer resections
| Volume category | No. of resections per year (3-yrs range) | No. of operations | No. of hospitals (%) | Mean age (SD) | Males (%) | Tumour site*: Head/body-tail (%) | Cases with metastases (%) | Mean Charlson comorbidity Index (SD) | Operations performed in public hospitals (%) | Operations funded by national health service (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Very low | 1.5 (0–9) | 2286 | 408 (75.0) | 71 [14] | 1185 (51.8) | 1330/516 (58.1%/22.6%) | 586 (25.6) | 5 [3] | 1697 (74.2) | 2240 (98) |
| Low | 5.5 (10–26) | 2120 | 76 (14.0) | 70 [14] | 1106 (52.2) | 1317/412 (62.1%/19.4%) | 482 (22.7) | 5 [2] | 1983 (93.5) | 2106 (99.3) |
| Medium | 13.5 (27–57) | 2165 | 37 (6.8) | 70 [14] | 1175 (54.3) | 1389/529 (64.1%/24.4%) | 406 (18.8) | 5 [2] | 1891 (87.3) | 2142 (98.9) |
| High | 33.5 (58–141) | 2388 | 17 (3.1) | 67 [15] | 1254 (52.5) | 1487/572 (62.3%/24.0%) | 484 (20.3) | 5 [2] | 1481 (62.0) | 2286 (95.7) |
| Very high | 91 (>141) | 1977 | 6 (1.1) | 66 [15] | 1035/942 (52.4) | 1191/471 (60.2%/23.8%) | 461 (23.3) | 5 [2] | 1401 (70.9) | 1890 (95.6) |
| Overall | 13.5 (0–432) | 10 936 | 544 | 69 [14] | 5755 (52.6%) | 6714/2500 (61.4%/22.9%) | 2419 (22.1) | 5 [2] | 8453 (77.3) | 10 664 (97.5) |
*in 1722 cases (15.7%) the tumour site could not be assessed (ICD-9codes: 157.9-8-3) SD = standard deviation.
Table 2.
Distribution of 10 936 operations for pancreatic cancer, according to the type of operation (resective or non-resective surgery) and to the hospital volume category. Percentages are calculated on the total number of operations in each volume category
| Overall hospitals % (n) | Very low-volume % (n) | Low-volume % (n) | Medium-volume % (n) | High-volume % (n) | Very high-volume % (n) | |
|---|---|---|---|---|---|---|
| Resective surgery | 60.1% (6570) | 37.5% (857) | 56.2% (1192) | 65.3% (1414) | 67.5% (1612) | 75.6% (1495) |
| Pancreatico-duodenectomy | 37.2% (4072) | 24.5% (561) | 38.2% (809) | 37.4% (809) | 42.7% (1020) | 44.2% (873) |
| Distal pancreatectomy | 13.7% (1501) | 8.0% (184) | 10.5% (222) | 15.9% (343) | 17.1% (408) | 17.4% (344) |
| Total pancreatectomy | 6.0% (657) | 1.7% (38) | 4.3% (91) | 9.3% (201) | 4.4% (104) | 11.3% (223) |
| Other resections | 3.1% (340) | 3.2% (74) | 3.3% (70) | 2.8% (61) | 3.3% (80) | 2.8% (55) |
| Non-resective surgery | 39.9% (4366) | 62.5% (1429) | 43.8% (928) | 34.7% (751) | 32.5% (776) | 24.4% (482) |
| Exploratory laparotomy | 16.3% (1784) | 22.0% (502) | 16.9% (358) | 16.0% (347) | 15.2% (362) | 10.9% (215) |
| Gastric by-pass | 8.8% (967) | 15.2% (347) | 8.7% (185) | 7.6% (164) | 6.6% (157) | 5.8% (114) |
| Double by-pass | 6.5% (713) | 10.1% (230) | 7.9% (167) | 5.2% (112) | 4.1% (99) | 5.3% (105) |
| Biliary by-pass | 6.1% (669) | 11.6% (266) | 7.4% (156) | 4.3% (93) | 4.7% (113) | 2.1% (41) |
| Exploratory laparoscopy | 2.1% (233) | 3.7% (84) | 2.9% (62) | 1.6% (35) | 1.9% (45) | 0.4% (7) |
Figure 1.
a: rates of non-resective operations in patients with pancreatic cancer, according to different hospital volumes. b: rates of resective operations in patients without distant metastases, according to different hospital volumes
Table 3.
Odds ratio (OR) for the risk of undergoing non-resective surgery in patients with pancreatic cancer. OR were adjusted for patients characteristics. Adjusted OR are reported for significant co-variates
| Variables | OR | 95% Confidence intervals | P | Adjusted OR | 95% Confidence intervals | P |
|---|---|---|---|---|---|---|
| Very high-volume | 1 | 1 | 1 | 1 | ||
| High-volume | 1.493 | 1.306–1.707 | <0.001 | 1.604 | 1.388-1.855 | <0.001 |
| Medium-volume | 1.647 | 1.439–1.886 | <0.001 | 1.721 | 1.485–1.995 | <0.001 |
| Low-volume | 2.415 | 2.112–2.760 | <0.001 | 2.301 | 1.987–2.664 | <0.001 |
| Very low-volume | 5.172 | 4.527–5.908 | <0.001 | 5.175 | 4.480–5.976 | <0.001 |
| Female | 1.018 | 0.943–1.099 | 0.651 | |||
| Charlson score | 1.505 | 1.472–1.539 | <0.001 | 1.516 | 1.481–1.552 | <0.001 |
| Body-tail tumour | 0.826 | 0.751–0.909 | <0.001 | 0.706 | 0.634–0.787 | <0.001 |
| Private hospital | 0.846 | 0.771–0.928 | <0.001 | 0.963 | 0.864–1.074 | 0.500 |
| Private funding | 0.631 | 0.485–0.821 | 0.001 | 0.845 | 0.630–1.133 | 0.260 |
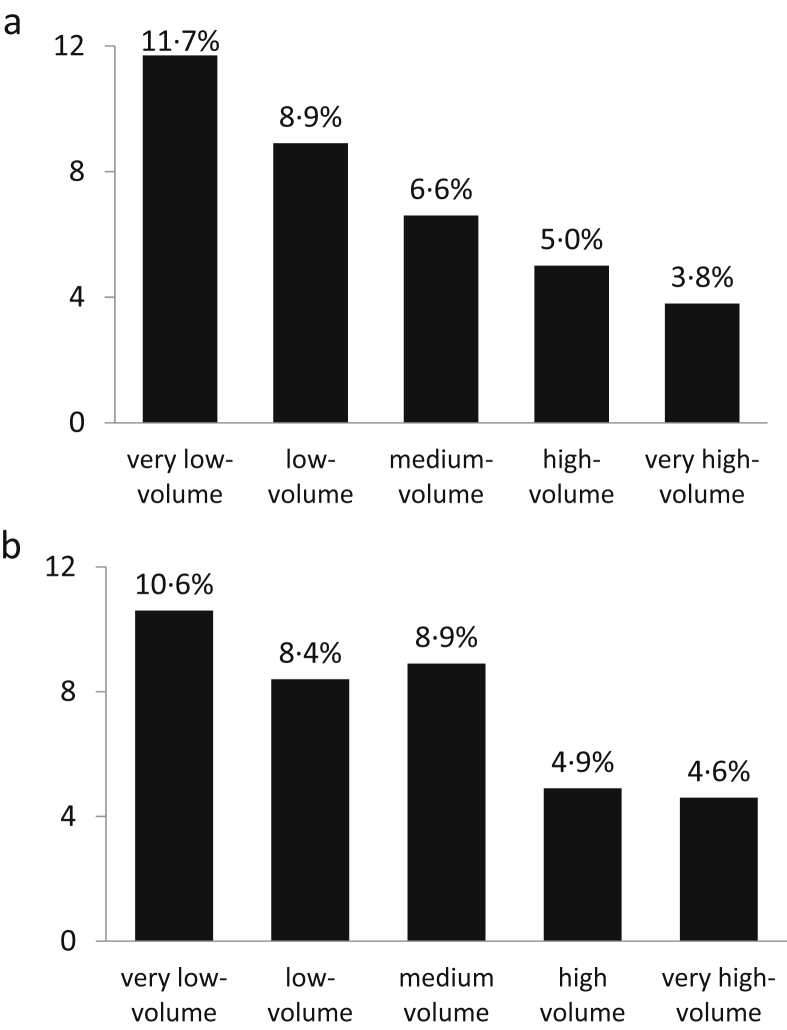
The operative mortality rates of resective and non-resective surgery are reported in Table 4. Mortality was higher for non-resective surgery than for resective surgery (8.2% vs. 6.7%, p < 0.01). All palliative operations had a mortality rate similar to or higher than that of PD: 7.0% for PD, 7.8% for biliary by-pass (p = 0.553), 9.3% for double by-pass (p < 0.05) and 14.4% for gastric by-pass (p < 0.001). The overall mortality rate for exploratory laparotomy was 5.2% and that of exploratory laparoscopy was 2.6%. The operative mortality rate was inversely related to hospital volume for both resective and non-resective surgery, declining from very low- to very high-volume hospitals (from 11.7% to 3.8%, p < 0.001, and from 10.6% to 4.6%, p < 0.001, respectively) (Fig. 2a,b, respectively). Multivariate analysis confirmed the independent effect of hospital volume on operative mortality, for both resective and non-resective surgery (Table 5).
Table 4.
Operative mortality of 10 936 operations for pancreatic cancer, stratified according to hospital volume and to type of operation (resective or non-resective). The p-values of the comparison between the very high-volume category and each other volume category are reported (chi-square, two tailed)
| Overall hospitals % (n) | Very low-volume % (n) | Low-volume % (n) | Medium-volume % (n) | High-volume % (n) | Very high-volume % (n) | |
|---|---|---|---|---|---|---|
| Resective surgery | 6.7% (437) |
11.7% (100) p < 0.0001 |
8.9% (106) p < 0.0001 |
6.6% (93) p = 0.001 |
5.0% (81) p = 0.121 |
3.8% (57) |
| Pancreatico-duodenectomy | 7.0% (287) | 14.1% (79) p < 0.0001 |
9.0% (73) p < 0.0001 |
6.8% (55) p < 0.01 |
5.4% (55) p < 0.01 |
2.9% (25) |
| Distal pancreatectomy | 2.4% (361) | 4.3% (8) p < 0.05 |
2.3% (5) p = 0.504 |
3.5% (12) P = 0.075 |
1.7% (7) p = 0.745 |
1.2% (4) |
| Total pancreatectomy | 13.5% (89) | 15.8% (6) p = 0.652 |
25.3% (23) p < 0.01 |
10.0% (20) p = 0.682 |
13.5% (14) p = 0.777 |
11.7% (223) |
| Other resections | 7.4% (25/340) | 9.5% (7) p = 0.350 |
7.1% (5) p = 0.649 |
9.8% (6) p = 0.343 |
6.3% (5) p = 0.781 |
3.6% (2) |
| Non-resective surgery | 8.2% (356) |
10.6% (151) p = 0.0001 |
8.4% (78) p = 0.01 |
8.9% (67) p < 0.01 |
4.9% (38) p = 0.894 |
4.6% (22) |
| Exploratory laparotomy | 5.2% (93) | 7.8% (39) p < 0.01 |
5.0% (18) p = 0.091 |
4.9% (17) p = 0.105 |
4.1% (15) p = 0.221 |
1.9% (4) |
| Gastric by- pass | 14.4% (139) | 16.4% (57) p = 0.360 |
15.7% (29) p = 0.520 |
16.5% (27) p = 0.426 |
7.6% (12) p = 0.284 |
12.3% (14) |
| Double by-pass | 9.3% (66) | 12.6% (29) p < 0.01 |
11.4% (19) p < 0.01 |
11.6% (13) p = 0.01 |
3.0% (3) p = 0.947 |
1.9% (2) |
| Biliary by-pass | 7.8% (52) | 9.4% (25) p = 0.512 |
5.8% (9) p = 0.825 |
8.6% (8) p = 0.688 |
7.1% (8) p = 0.904 |
4.9% (2) |
| Exploratory laparoscopy | 2.6% (6) | 1.2% (1) p = 0.772 |
4.8% (3) p = 0.552 |
5.7% (2) p = 0.517 |
0% (0) p = 1.00 |
0% (0) |
Figure 2.
a: mortality rates of 6570 resections for pancreatic cancer, according to different hospital volumes. b: mortality rates of 4366 non-resective operations (both palliative and exploratory), according to different hospital volumes
Table 5.
Odds ratio (OR) for the risk of operative mortality in 6570 pancreatic cancer resections and 4366 non-resective operations. OR were adjusted for patients characteristics. Adjusted odds ratios are reported for significant co-variates
| Variables | OR | CI 95% | P | Adjusted OR | CI 95% | P |
|---|---|---|---|---|---|---|
| Resective surgery | ||||||
| Very high-volume | 1 | 1 | 1 | 1 | ||
| High-volume | 1.335 | 0.944–1.887 | 0.102 | |||
| Medium-volume | 1.776 | 1.267–2.490 | 0.001 | 1.785 | 1.266–2.517 | 0.001 |
| Low-volume | 2.462 | 1.768–3.430 | <0.001 | 2.268 | 1.618–3.179 | <0.001 |
| Very low-volume | 3.333 | 2.379–4.668 | <0.001 | 3.429 | 2.438–4.824 | <0.001 |
| Female | 0.851 | 0.700–1.035 | 0.107 | |||
| Charlson score | 1.205 | 1.140–1.273 | <0.001 | 1.233 | 1.412–2.300 | <0.001 |
| Body-tail tumour | 0.390 | 0.285–0.533 | <0.001 | 0.395 | 0.287–0.543 | <0.001 |
| Private hospital | 0.711 | 0.555–0.911 | 0.007 | 0.801 | 0.617–1.038 | 0.094 |
| Private founding | 0.448 | 0.197–1.015 | 0.054 | |||
| Non-resective surgery | ||||||
| Very high-volume | 1 | 1 | 1 | 1 | ||
| High-volume | 1.077 | 0.629–1.843 | 0.788 | |||
| Medium-volume | 2.048 | 1.247–3.363 | 0.005 | 2.073 | 1.258–3.471 | 0.004 |
| Low-volume | 1.919 | 1.180–3.121 | 0.009 | 1.824 | 1.118–2.975 | 0.016 |
| Very low-volume | 2.470 | 1.560–3.913 | <0.001 | 2.630 | 1.656–4.178 | <0.001 |
| Female | 0.867 | 0.697–1.078 | 0.199 | |||
| Charlson score | 1.102 | 1.050–1.157 | <0.001 | 1.115 | 1.061–1.171 | <0.001 |
| Localization Body-Tail | 0.508 | 0.361–0.714 | <0.001 | 0.483 | 0.342–0.681 | <0.001 |
| Private hospital | 0.565 | 0.413–0.778 | <0.001 | 0.609 | 0.439–0.844 | 0.003 |
| Private founding | 0.281 | 0.069–1.149 | 0.077 | |||
Estimates regarding the overuse of surgery showed that, if all patients had received the standard of treatment offered by very high-volume hospitals, 1704 of the 4366 non-resective operations (39.7%) could have been avoided (Supplementary Table 4). In addition to these volume-related findings, another 604 resections performed on patients with metastases were considered incorrect (according to current guidelines), accounting for a total of 2308 operations (21.1%).
The estimation of avoidable costs due to the overuse of non-resective surgery in low-volume hospitals showed that more than 9.5 million euros could have been saved by the Italian NHS over a three-year period (Table 6). Furthermore, the excess expenses of resection instead of non-resective surgery in patients with distant metastases was estimated at 3 million euros. In all, the total cost for incorrect pancreatic cancer surgery during the study period amounted to approximately 12.5 million euros out of a total expenditure of approximately 128 million euros.
Table 6.
Excess cost (in euros) for pancreatic cancer surgery in Italy from 2010 to 2012, stratified according to hospital volume and type of operation (resective or non-resective). See text for the methods of estimation of avoidable costs
| Overall hospitals | Very low-volume | Low-volume | Medium-volume | High-volume | Very high-volume | |
|---|---|---|---|---|---|---|
| Total costs for pancreatic cancer surgery | 128 649 831 | 25 271 511 | 24 768 010 | 25 466 082 | 28 353 830 | 24 790 398 |
| Total avoidable costs | 12 574 169 | 5 433 230 | 2692 450 | 1 635 769 | 1923 310 | 889 411 |
| Avoidable costs for resective surgery (in patients with distant metastases) | 3 014 801 | 363 811 | 493 312 | 486 307 | 781 960 | 889 411 |
| Avoidable costs for non-resective surgery | 9 559 368 | 5069 419 | 2 199 138 | 1149 462 | 1 141 350 | – |
| Exploratory laparotomy | 2 650 066 | 1171 754 | 562 176 | 464 271 | 451 864 | – |
| Gastric by-pass | 1 735 076 | 1108 173 | 329 586 | 199 730 | 97 588 | – |
| Double by-pass | 1 129 492 | 891 838 | 237 654 | – | – | – |
| Biliary by-pass | 3 407 190 | 1653 764 | 872 075 | 394 324 | 487 028 | – |
| Exploratory laparoscopy | 637 544 | 243 889 | 197 647 | 91 137 | 104 870 | – |
Discussion
The volume–outcome relationship in pancreatic surgery is well-established,10, 11, 12, 13, 14, 15 and a recent meta-analysis confirmed a reduced risk of operative mortality in high-volume hospitals (OR 0.32).10, 11, 12, 13, 14, 15 This relationship has been documented in Italy as well: a fivefold increase in mortality risk in patients undergoing a PD in low-volume centres with respect to very high-volume centres was found (12.4% vs. 2.6%).15 Other studies focused on the effect of hospital volume on long-term survival, suggesting a possible detrimental effect of low-volume hospitals regarding survival after resection.12, 13, 144 For the first time, the present study points out that low-volume hospitals have also a different surgical approach to pancreatic cancer patients, characterized by an overuse of non-resective surgery: the probability of undergoing non-resective surgery is increased fivefold (adjusted OR 5.175) when patients are operated on in very low-volume hospitals with respect to the hospitals having the greatest experience. The overuse of health care services is an underinvestigated problem17: it is the provision of services where the harm outweighs the benefits, representing poor quality and contributing to high costs.18 When considering pancreatic cancer surgery, current guidelines clearly indicate that non-resective surgery for pancreatic cancer should be rarely performed,4, 5, 6 as shown in a study from Johns Hopkins: in such study a progressive reduction of palliative surgery (from >40 to <10%) in the period 1996–2010 was recorded, due to both a greater use of non-operative palliation and the ability of preoperatively recognising locally advanced or metastatic disease.19
The present study also showed that a patient without metastases has a lower probability to undergo resection, when he is operated in a low-volume hospital: the resection rate was 46.1% in very low-volume hospitals and 86.9% in very high-volume hospitals, (adjusted OR 7.429). This finding can partially be the consequence of the overuse of non-resective surgery in low-volume hospitals, but it also suggests that a patient with a potentially resectable cancer has a lower probability to receive resection in such hospitals.
Both clinical and non-clinical factors may be reasons for these variations between low-volume and high-volume hospitals. One factor could be related to the inadequate preoperative evaluation of resectability since correct staging of pancreatic cancer can be difficult. In a study aimed at evaluating the adequacy of staging using CT scans in peripheral centres, up to 68% of the scans were judged to be of unacceptable quality.20 Another factor might be the inadequate experience of the surgeon in performing resections. Pancreatic surgery is demanding and a surgeon lacking experience may overestimate vessel invasion or be unable to perform vascular resections. Restaging carried out in a very high-volume centre, after previously unsuccessful resection attempts at other centres, showed that the majority of patients actually had anatomically resectable tumours.21, 22 Overindication of surgical palliation for treating malignant obstructive jaundice may be a third factor. There is evidence that surgical clinical practice and evidence-based medicine often differ,21, 22 even in academic medical centres.21, 22 Furthermore, a recent survey suggested that specialisation may influence surgical decision making, and that surgeons recommend interventions more frequently when they are not specialised in the treatment of a specific disease.25 Another possible explanation is the unavailability of endoscopic palliation in low-volume centres, with travel requirements to receive the optimal treatment. However, this should not be a barrier in Italy; our Country has a high population density (about sevenfold the USA) and in vast majority of cases referral hospitals are in a short distance from low-volume centres. Finally, the personal interest of surgeons should be considered.26, 27 In Italy, the reason of incorrect surgical attitude cannot be the economic self-interest of physicians, since the Italian NHS guarantees public universal coverage and physician payments are based on a fixed salary instead of fee-for-service. However, other non-financial conflicts of interest may play a role: pancreatic surgery is appealing to a general surgeon, who may feel challenged by the difficulty of such an operation, and in some low-volume hospitals, even by-pass surgery may be a gratifying procedure.
Another unexpected finding of the present study was the high mortality rate in palliative and exploratory surgery, even higher than after resections. Previous reports from single Institutions have suggested very low operative mortality after surgical palliation,28, 29 but a recent analysis from a database of the American College of Surgeons showed non-negligible mortality rates after palliative surgery (6.5%) and laparotomy alone (5%).30 The present study found higher mortality rates, probably due to the unselected nature of the survey; the analysis was carried out on the entire population of Italian hospitals and hospitalisations, thus different from the previous study which included only a selection of American hospitals. This mortality risk suggests extreme caution in selecting patients for surgical palliation. The present guidelines indicate a gastric by-pass as an alternative to an enteral stent when patients have good performance status but, in the light of the 12% mortality of gastric or double by-passes, patients should be carefully selected. On the other hand, since the mortality risk of by-pass surgery is similar to or higher than that of a PD, a possible role for palliative PD instead of by-pass surgery in highly selected patients should be evaluated in future studies.
At present, all guidelines clearly contraindicate a resection in the case of distant metastases,4, 5, 6 but approximately 9% of all resections were performed on metastatic patients, with only small variations among the different volume groups (range 7.4%–11.8%). To note, the higher rate of resection in metastatic cancer was recorded in the centres with the highest experience. This attitude is censurable and should be considered an additional example of surgery overuse in this context. We can speculate that some surgeons decide to perform a palliative resection in case of intraoperative unexpected finding of limited metastatic disease and locally resectable tumour. As already discussed, surgical clinical practice and evidence-based medicine often differ.23, 24 One study from the Heidelberg group reported the results of resection in M1 patiens, concluding that in case of liver or peritoneal metastasis resection cannot be recommended.31 Further hypotheses for this misconduct can be considered, though unlikely: a miscoding between pancreatic adenocarcinoma and endocrine malignancy, for which resection can be indicated even in metastatic cases; finally, the possibility of resection after complete radiologic regression of metastases obtained by primary chemotherapy with Folfirinox or similar polichemotherapy schemes. However, the number of metastatic patients who actually have such a response is very limited (only a couple of case reports have been published), and the benefit of resection in term of survival is far to be demonstrated.
The present study highlights the need of a multidisciplinary tumour board as a criteria to treat pancreatic cancer patients: the presence of such a team would have been enough to prevent the overuse of palliative surgery instead of endoscopic palliation and the abuse of resection in metastatic patients.
The overuse of medical services contributes to less efficient care and causes avoidable medical care cost burdens. The appropriateness in service provision, besides ensuring that the right treatment is provided to the right patient, can become a major source of savings. The possible increase of costs and resource utilization after pancreaticoduodenectomy in low-volume hospitals has been previously reported.32, 33, 34 The present study estimated that approximately 10% of public resources for pancreatic cancer surgery were spent on incorrect resective and non-resective surgery. As an overall result, 12.5 million euros could have been saved during the study period.
A possible bias of this study is its reliance on the use of administrative data to obtain clinical results. The limitations of using administrative data are well known and are mainly related to possible heterogeneity in coding practices. However, high-cost procedures, such as operations, are usually coded accurately,35 thus providing a reliable source for research. Administrative databases are an invaluable source of information in real world clinical practice, and the Italian Ministry of Health database collects detailed information on every inpatient discharged from any public and private hospital in Italy.
In conclusion, the present nationwide study points out a number of new findings regarding pancreatic cancer surgery: (i) the overuse of palliative/explorative surgery in low-volume hospitals; (ii) the lower probability of patients without metastases to undergo resection in low-volume-hospitals; (iii) the high mortality rate of non-resective surgery; (iv) the non-negligible rate of pancreatic resections in patients with distant metastases and (v) the excess cost for the NHS deriving from the overuse of pancreatic cancer surgery. Efforts to centralise pancreatic surgery should be intensified; 89% of Italian centres were in very low- or low-volume hospitals, with serious clinical consequences for patients and relevant excess cost. Intervention strategies, such as continuing medical education for surgeons and public information for patients, are needed. Most importantly, interventions by public health authorities are suggested in order to provide implementation of the surgical guidelines and to define the minimal requirements for hospitals in providing pancreatic surgery, possibly including them into the Italian NHS accreditation criteria.
Funding
No funding source.
Conflicts of interest
None to declare.
Footnotes
The paper is based on a previous communication at the Combined EPC & IAP Meeting 2014, Southampton, June 24–28: Overuse of Surgery for Pancreatic Cancer Treatment. An Analysis of the Italian Association for the Study of Pancreas (AISP) on a Nationwide Database.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2015.11.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cancer Facts and Figures 2014 – American Cancer Society. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf [accessed 05.09.15].
- 2.Istat – Istituto Nazionale di Statistica, Principali cause di morte in Italia– Anno 2012. http://www.istat.it/it/files/2014/12/Principali_cause_morte_2012.pdf?title=Principali+cause+di+morte+in+Italia+-+03%2Fdic%2F2014+-+Testo+integrale.pdf [accessed 05.09.15].
- 3.De Angelis R., Sant M., Coleman M.P., Francisci S., Baili P., Pierannunzio D. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Version 2. 2015 http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [accessed 05.09.15].
- 5.Seufferlein T., Bachet J.B., Van Cutsem E., Rougier P., ESMO Guidelines Working Group Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl. 7):vii33–vii40. doi: 10.1093/annonc/mds224. [DOI] [PubMed] [Google Scholar]
- 6.AIOM. Linee guida. Carcinoma del Pancreas Esocrino. Edizione 2014. http://www.aiom.it/C_Common/Download.asp?file=/$Site$/Attivita_Scientifica/Linee_Guida/2014/2014_LG_AIOM_Carcinoma_pancreas.pdf [accessed 02.06.15].
- 7.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad C., Lillemoe K.D. Surgical palliation of pancreatic cancer. Cancer J. 2012;18:577–583. doi: 10.1097/PPO.0b013e3182797dfe. [DOI] [PubMed] [Google Scholar]
- 9.Gooiker G.A., van Gijn W., Wouters M.W., Post P.N., van de Velde C.J., Tollenaar R.A., Signalling Committee Cancer of the Dutch Cancer Society Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485–494. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer J.D., Siewers A.E., Finlayson E.V., Stukel T.A., Lucas F.L., Batista I. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 11.van Heek N.T., Kuhlmann K.F., Scholten R.J., de Castro S.M., Busch O.R., van Gulik T.M. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkmeyer J.D., Sun Y., Wong S.L., Stukel T.A. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilimoria K.Y., Bentrem D.J., Feinglass J.M., Stewart A.K., Winchester D.P., Talamonti M.S. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626–4633. doi: 10.1200/JCO.2007.15.6356. [DOI] [PubMed] [Google Scholar]
- 14.Gooiker G.A., Lemmens V.E., Besselink M.G., Busch O.R., Bonsing B.A., Molenaar I.Q. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101:1000–1005. doi: 10.1002/bjs.9468. [DOI] [PubMed] [Google Scholar]
- 15.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Balzano G., Zerbi A., Capretti G., Rocchetti S., Capitanio V., Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 17.Korenstein D., Falk R., Howell E.A., Bishop T., Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172:171–178. doi: 10.1001/archinternmed.2011.772. [DOI] [PubMed] [Google Scholar]
- 18.Keyhani S., Falk R., Howell E.A., Bishop T., Korenstein D. Overuse and systems of care: a systematic review. Med Care. 2013;51:503–508. doi: 10.1097/MLR.0b013e31828dbafe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kneuertz P.J., Cunningham S.C., Cameron J.L., Torrez S., Tapazoglou N., Herman J.M. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: trends and lessons learned from a large, single institution experience. J Gastrointest Surg. 2011;15:1917–1927. doi: 10.1007/s11605-011-1665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morak M.J., Hermans J.J., Smeenk H.G., Renders W.M., Nuyttens J.J., Kazemier G. Staging for locally advanced pancreatic cancer. Eur J Surg Oncol. 2009;35:963–968. doi: 10.1016/j.ejso.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Truty M.J., Thomas R.M., Katz M.H., Vauthey J.N., Crane C., Varadhachary G.R. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg. 2012;215:41–51. doi: 10.1016/j.jamcollsurg.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalski C.W., Kleeff J., Bachmann J., Alkhatib J., Erkan M., Esposito I. Second-look operation for unresectable pancreatic ductal adenocarcinoma at a high-volume center. Ann Surg Oncol. 2008;15:186–192. doi: 10.1245/s10434-007-9535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slim K., Panis Y., Chipponi J., Société Française de Chirurgie Digestive Half of the current practice of gastrointestinal surgery is against the evidence: a survey of the French Society of Digestive Surgery. J Gastrointest Surg. 2004;8:1079–1082. doi: 10.1016/j.gassur.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Melis M., Karl R.C., Wong S.L., Brennan M.F., Matthews J.B., Roggin K.K. Evidence-based surgical practice in academic medical centers: consistently anecdotal? J Gastrointest Surg. 2010;14:904–909. doi: 10.1007/s11605-010-1175-1. [DOI] [PubMed] [Google Scholar]
- 25.Wilson N.P., Wilson F.P., Neuman M., Epstein A., Bell R., Armstrong K. Determinants of surgical decision making: a national survey. Am J Surg. 2013;206:970–977. doi: 10.1016/j.amjsurg.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J., Andersen R., Brook R., Kominski G., Albert P.S., Wenger N. The effects of payment method on clinical decision-making: physician responses to clinical scenarios. Med Care. 2004;42:297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 27.Armour B.S., Pitts M.M., Maclean R., Cangialose C., Kishel M., Imai H. The effect of explicit financial incentives on physician behavior. Arch Intern Med. 2001;161:1261–1266. doi: 10.1001/archinte.161.10.1261. [DOI] [PubMed] [Google Scholar]
- 28.Lyons J.M., Karkar A., Correa-Gallego C.C., D'Angelica M.I., DeMatteo R.P., Fong Y. Operative procedures for unresectable pancreatic cancer: does operative bypass decrease requirements for postoperative procedures and in-hospital days? HPB. 2012;14:469–475. doi: 10.1111/j.1477-2574.2012.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillemoe K.D., Cameron J.L., Hardacre J.M., Sohn T.A., Sauter P.K., Coleman J. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322–328. doi: 10.1097/00000658-199909000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartlett E.K., Wachtel H., Fraker D.L., Vollmer C.M., Drebin J.A., Kelz R.R. Surgical palliation for pancreatic malignancy: practice patterns and predictors of morbidity and mortality. J Gastrointest Surg. 2014;18:1292–1298. doi: 10.1007/s11605-014-2502-8. [DOI] [PubMed] [Google Scholar]
- 31.Shrikhande S.V., Kleeff J., Reiser C., Weitz J., Hinz U., Esposito I. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14:118–127. doi: 10.1245/s10434-006-9131-8. [DOI] [PubMed] [Google Scholar]
- 32.Sutton J.M., Wilson G.C., Paquette I.M., Wima K., Hanseman D.J., Quillin R.C., 3rd Cost effectiveness after a pancreaticoduodenectomy: bolstering the volume argument. HPB. 2014;16:1056–1061. doi: 10.1111/hpb.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka R., Yasunaga H., Hasegawa K., Horiguchi H., Fushimi K., Aoki T. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg. 2014;101:523–529. doi: 10.1002/bjs.9420. [DOI] [PubMed] [Google Scholar]
- 34.Enomoto L.M., Gusani N.J., Dillon P.W., Hollenbeak C.S. Impact of surgeon and hospital volume on mortality, length of stay, and cost of pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:690–700. doi: 10.1007/s11605-013-2422-z. [DOI] [PubMed] [Google Scholar]
- 35.Quan H., Parsons G.A., Ghali W.A. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.