Abstract
Background
Posthepatectomy liver failure is one of the most feared complications in extended hepatic resections. In 2012, a novel two-stage liver resection was developed, able to induce rapid and extensive hypertrophy by portal vein ligation and in situ liver splitting – Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS). The technique became more widely employed but its use remained controversial due to reporting of high complication and mortality rates.
Method
A national audit was performed to gather information about the safety of the procedure and to better understand the complications. The audit was offered to all high-volume hepatobiliary centers in Italy.
Results
Of all Italian centers approached in January 2012, 12 centers with experience in ALPPS enrolled and participated in collection of data. Fifty patients underwent ALPPS between 2012 and 2014. In 48/50 patients completion of hepatectomy was performed successfully. Major morbidity occurred in 54% with a 20% 90-day mortality. Uni- and multivariate analysis showed that ALPPS for cholangiocarcinoma and a peak of bilirubin over 5 mg/dl between stages was associated with increase of 90-day mortality and worse survival.
Discussion
It is proposed that a moratorium be introduced for classic ALPPS in cholangiocarcinoma and to abort ALPPS in patients who develop an interstage increase in bilirubin, due to the high risk of liver failure and mortality.
Introduction
When the liver remnant is too small to sustain post-resection liver function, portal vein occlusion techniques such as portal vein embolization (PVE) or portal vein ligation (PVL) are used routinely to increase the future residual liver volume.1, 2 In 2012 a new surgical technique was introduced that combines PVL with in situ splitting of the liver parenchyma, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS).3, 4 Despite rapid and impressive FLR hypertrophy and several studies demonstrating the potential of ALPPS to extend limitations for resectability, concerns about complications and mortality were brought forward, which led to ongoing controversy about its safety.
Given that ALPPS has exceedingly rare indications and is associated with a mortality that is clearly above 5%, single center series are insufficient to gain knowledge about the safety of ALPPS. An international registry initiated by the University of Zurich, reported a 90-day mortality of 9%, but was based on voluntary reporting and likely underestimates true mortality due to incomplete reporting in about 20% of patients and a potential selective reporting bias due to the voluntary character of the registry.5 Concerns about the safety of ALPPS led us to initiate a collaborative national registry in Italy to gather data prospectively from monitored surgical centers to assess true mortality and its risks.
The primary aim was to assess the safety of ALPPS in the experience of Italian surgeons. The secondary objective was to identify risk factors to provide guidance on how to use this surgical innovation.
Methods
Study design
All high-volume hepatobiliary centers in Italy were approached in January 2012 and offered the opportunity to participate in a national audit of patients treated with an ALPPS procedure. Participating centers committed to include all consecutive patients in their hospital undergoing ALPPS. Others, not reported herein, either refused to send their data or did not perform any ALPPS at the time.
The Italian ALPPS registry was approved by the respective ethical committees of each center. All patients entered into the registry approved inclusion of their data into an anonymized database. Data entry was performed into electronic spreadsheets by the surgeons performing the procedures. Data entered were monitored by the study center in Maggiore Hospital and complete data entry of all ALPPS patients performed in each center was confirmed. In March 2012, data entry commenced at the Department of Surgery at Maggiore Hospital. Following the enrollment of the 50th patient on February 28th 2014, the analysis data set for this study was created and analyzed.
Variables
The main outcomes of this study were 90-day mortality and complications as well as independent predictors of survival after ALPPS by uni- and multivariate analysis. Liver remnant volumes were assessed using cross-sectional imaging by computed tomography (CT) or magnetic resonance imaging (MRI) and standardized future liver remnant (sFLR) was assessed in each patient using the Vauthey formula: −794.41 + 1267.28 × body surface area (m2).6, 7 Volumetry was performed using dedicated volume rendering software. In patients with bilobar involvement, FLR was calculated by subtracting the tumor volume (clean FLR).
Liver function tests were documented preoperatively and every other day postoperatively. Preoperative cholestasis was defined, similarly to previous studies, as preoperative bilirubin >2.9 mg/dl.8 Complications were classified according to the Clavien classification of surgical complications9 and grade ≥ IIIA was considered a “major” complication. Posthepatectomy liver failure (PHLF) was classified according to the ISGLS definition.10 Data on oncologic staging were entered according to the pathology assessments at each center.
Surgical technique
The ALPPS surgical technique was performed according to its inaugural description.11 The nomenclature defined in the first report from the International ALPPS registry was used to describe ALPPS resection types.12 All cases were performed by the same surgeon of each single center.
Study size and bias
Based on literature reports, a mortality of 10% (5 patients) was expected and it was planned that the first analysis of the registry using safety endpoints should be performed after enrollment of 50 patients. Since ALPPS was used for three very different oncological indications and due to the small number of cases, patients were classified into 3 groups for analysis: liver metastases, biliary malignancies and hepatocellular carcinoma.
The biases intrinsic to registry reports with voluntary data entry were prospectively addressed. Since many participating centers were at the beginning of their learning curve for ALPPS, the audit was only offered to high-volume hepatobiliary centers to limit its potential impact when analyzing outcomes.
All consecutive patients at each center were enrolled into the registry to reduce reporting bias (principle of consecutivity). Centers were advised to also enter patients who did not proceed to complete resection in either stage to reduce reporting bias (principle of intent to treat). Entries were confirmed at the time of analysis by a retrospective audit through the study center in Maggiore Hospital (principle of audit). These principles were introduced to address concerns voiced about voluntary registry reports and to allow us to give a valid assessment of the safety of the procedure.
Statistical analysis
Data were expressed in median and range and the Kruskal–Wallis or median test were used for comparisons. Fisher's Exact test was used for comparisons of categorical variables. Univariate logistic regression analysis was applied in order to investigate risk factors for mortality and major complications. Survival analysis was performed with using a Cox Proportional Hazard Model. Variables found different in the univariate analysis with an α-value of 0.10 or which were clinically significant according to our judgement were included in a multivariate model and then excluded through a backward elimination procedure with α ≤ 0.05. Receiver operating characteristic (ROC) curve analysis was performed to identify the cut-off value of peak bilirubin between stages in predicting 90-day mortality. The cut-off value was determined by seeking the largest sum of the sensitivity and specificity values, while maintaining the lowest likelihood ratio of a negative test and the highest likelihood ratio of a positive test. All analyses were performed using the statistical software R (Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
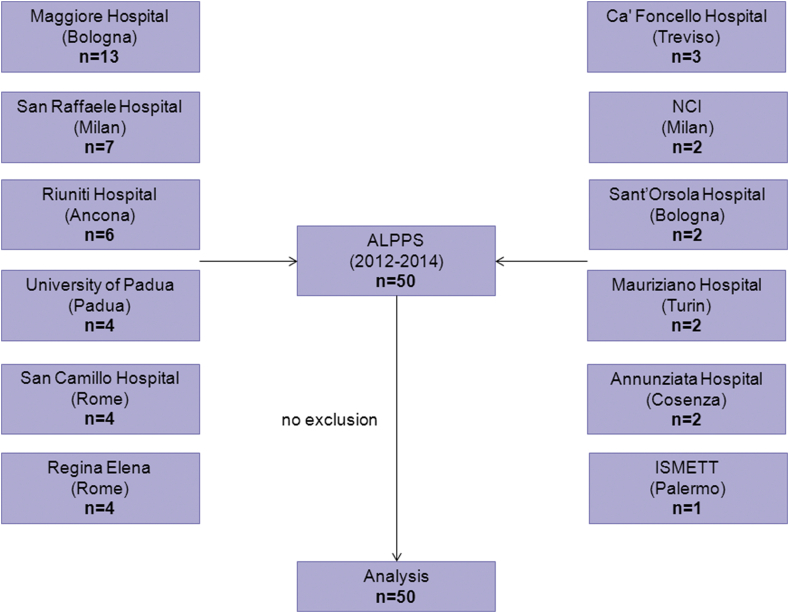
Twelve centers enrolled and participated in collection of data: Maggiore Hospital (Bologna), San Raffaele Hospital (Milan), Riuniti Hospital (Ancona), Regina Elena Cancer Institute (Rome), San Camillo Hospital (Rome), University Hospital of Padua (Padua), Cà Foncello Hospital (Treviso), National Cancer Institute (Milan), Annunziata Hospital (Cosenza), Sant'Orsola-Malpighi Hospital (Bologna), Mauriziano Umberto I Hospital (Turin) and Mediterranean Institute for Transplantation and Advanced Specialized Therapies (Palermo). Fifty patients were registered between March 2012 and February 2014 and all were included into the study with complete documentation of the procedures, laboratory data and information on complications and outcome (Fig. 1).
Figure 1.
Flow chart of patients enrolled in this study. Twelve major HPB centers participated and all provided complete and monitored datasets
Descriptive data of the study population
Descriptive variables of the entire patient cohort are shown in Table 1.
Table 1.
Descriptives of all ALPPS patients in Italy 2012–2014 (n = 50)
| Variable | n = 50 (%)a |
|---|---|
| Age, median (range), years | 62 (36–79) |
| Sex, female/male | 23/27 |
| BMI, median (range), kg/m2 | 26 (19–32) |
| Indication, | |
| Primary indication | 46 (94) |
| Salvage ALPPS | 4 (6) |
| Tumor type, | |
| CRLM | 21 (44) |
| Non-CRLM | 1 (2) |
| HCC | 8 (15) |
| IHCC | 8 (15) |
| PHCC | 11 (22) |
| GBCA | 1 (2) |
| Parenchyma, | |
| Normal parenchyma | 19 (38) |
| Damaged parenchyma | 31 (62) |
| Cirrhosis, | 6 (12) |
| Cholestasis, | 8 (16) |
| Chemotherapy, | 17 (34) |
| Oxaliplatin-based regimen | 9 (53) |
| Irinotecan-based regimen | 8 (47) |
| sFLR prior to stage 1, median (range), % | 20 (10–35) |
| FLR/BW prior to stage 1, median (range), % | 0.42 (0.21–0.73) |
| Time intervals, median (range), days | |
| stage 1 to imaging prior to stage 2 | 7 (3–15) |
| imaging prior to stage 2 to stage 2 | 2 (0–23) |
| stage 1 to stage 2 | 10 (4–37) |
| sFLR prior to stage 2, median (range), % | 35 (19–59) |
| FLR/BW prior to stage 2, median (range), % | 0.73 (0.39–1.22) |
| Liver Hypertrophy, median (range), % | |
| Normal parenchyma | 74 (33–153) |
| Chemotherapy | 56 (15–227) |
| Cirrhosis | 50 (14–178) |
| Cholestasis | 96 (37–190) |
BMI, body mass index; CRLM, colorectal liver metastases; HCC, hepatocellular carcinoma; IHCC, intrahepatic cholangiocarcinoma; PHCC, perihilar cholangiocarcinoma; GBCA, gallbladder cancer; sFLR, standardized future liver remnant; BW, body weight.
% – unless otherwise stated.
Of the 22 patients who underwent ALPPS for liver metastases (Table 2), two had previously undergone minor hepatic resection. Preoperative chemotherapy was performed in 16 of 22 patients (73%) receiving a median of 9 cycles (range 2–12) but with different regimens. The median time window between drug administration and surgery was 84 days (range 10–432).
Table 2.
Data stratified by indication for ALPPS for metastatic tumors, biliary tumors and HCC
| Metastases (n = 22) | Biliary (n = 20) | HCC (n = 8) | P-value | |
|---|---|---|---|---|
| Patients characteristics | ||||
| Age, median (range), years | 59.5 (45–79) | 66.0 (54–77) | 56.0 (36–74) | 0.026 |
| Sex, female/male, n | 13/9 | 12/8 | 2/6 | 0.261 |
| BMI, median (range), kg/m2 | 26.4 (19.6–30.4) | 24.9 (19.1–29) | 27.1 (20.8–32.3) | 0.098 |
| Chemotherapy, n (%) | 16 (73) | 1 (5) | 0 | <0.0001 |
| Salvage ALPPS, n (%) | 3 (14) | 1 (5) | 0 | 0.504 |
| Biliary stenting, n (%) | 0 | 6 (30) | 0 | 0.006 |
| Baseline labor values | ||||
| Bilirubin, median (range), mg/dl | 0.50 (0.12–1.10) | 1.49 (0.36–10.7) | 0.70 (0.40–0.96) | 0.017 |
| INR, median (range) | 1.03 (0.85–1.15) | 1.01 (0.06–1.18) | 1.12 (0.99–1.37) | 0.151 |
| Creatinine, median (range), mg/dl | 0.84 (0.05–1.16) | 0.79 (0.51–1.07) | 0.70 (0.49–0.83) | 0.119 |
| Liver volumes (prior to stage 1) | ||||
| FLR, median (range), cc | 328.0 (135–410) | 293.5 (173–466) | 373.0 (203–593) | 0.030 |
| sFLR, median (range), % | 19.0 (10–27) | 20.5 (10–33) | 23.5 (15–35) | 0.057 |
| FLR/BW, median (range), % | 0.40 (0.21–0.58) | 0.44 (0.22–0.67) | 0.51 (0.31–0.73) | 0.059 |
| Liver volumes (prior to stage 2) | ||||
| Time intervala, median (range), days | 7 (4–11) | 7 (3–13) | 8.5 (6–15) | 0.427 |
| Increase, median (range), % | 60.5 (15–227) | 76.5 (37–190) | 56.5 (14–178) | 0.663 |
| sFLR, median (range), % | 33 (19–40) | 40 (23–59) | 39.5 (31–52) | 0.024 |
| FLR/BW, median (range), % | 0.66 (0.39–0.85) | 0.89 (0.5–1.22) | 0.84 (0.63–1.09) | 0.018 |
| Intraoperative data (stage 1) | ||||
| Type of liver resection, n (%) | 0.019 | |||
| Right hepatectomy | 12 (55) | 3 (15) | 4 (50) | |
| Right trisectionectomy | 10 (45) | 17 (85) | 4 (50) | |
| Operative time, median (range), min | 307.5 (138–510) | 350.0 (241–745) | 335.0 (258–480) | 0.274 |
| Blood transfusions, median (%), units | 2 (32) | 2 (35) | 2 (38) | 0.805 |
| Pringle maneuver, n (%) | 8 (36) | 7 (35) | 2 (25) | 0.925 |
| Intraoperative data (stage 2) | ||||
| Operative time, median (range), min | 183 (50–320) | 180 (52–726) | 188 (125–720) | 0.607 |
| Blood transfusions, median (%), units | 2 (41) | 2 (44) | 3 (50) | 0.929 |
| Clinical outcomes | ||||
| Clavien ≥ IIIA morbidity, stage 1, n (%) | 2 (9) | 10 (50) | 2 (25) | 0.012 |
| Clavien ≥ IIIA morbidity, stage 2, n (%) | 8 (36) | 8 (44) | 3 (37.5) | 0.923 |
| 90-day mortality, stage 1, n (%) | 0 | 2 (10) | 0 | 0.452 |
| 90-day mortality, stage 2, n (%) | 1 (4.5) | 6 (30) | 1 (12.5) | 0.017 |
| PHLF grade B/C, stage 1, n (%) | 0 | 4 (22) | 0 | 0.028 |
| PHLF grade B/C, stage 2, n (%) | 2 (9) | 9 (50) | 3 (37.5) | 0.015 |
| Peak bilirubin (interstage) > 5 mg/dl, n (%) | 0 | 7 (35) | 1 (13) | 0.008 |
Of 20 patients who underwent surgery for biliary tumors (Table 2), 11 had perihilar cholangiocarcinoma, 8 intrahepatic cholangiocarcinoma and one patient had a gallbladder tumor. Preoperative median serum bilirubin was 0.7 (range 0.36–10.7) mg/dl. One patient underwent surgery with a preoperative bilirubin level of 10.7 mg/dl because it was neither possible to perform biliary drainage percutaneously nor endoscopically. Biliary drainage was performed in six cases.
None of the eight patients with hepatocellular carcinoma (HCC) (Table 2) were candidates for liver transplantation according to the respective tumor boards because their tumors were beyond Milan criteria.13 Six of these patients had cirrhosis by histology, classified as Child-Pugh A with a median preoperative MELD score of 7 (range 6–10).
Four patients underwent ALPPS as a salvage procedure after lack of volume increase after conventional methods to increase remnant volume (Table 2), in 3 patients after portal vein embolization and in one patient after portal vein ligation.
The three types of tumor types differed in age, preoperative chemotherapy and biliary stenting (Table 2).
Overall, 31 right trisectionectomy ALPPS and 19 right hepatectomy ALPPS were performed (Table 2). The median surgical time for stage 1 was 321 min (range 138–745) and comparable among groups. Pringle maneuver was performed in 17/50 cases for a median clamping time of 30 min (range 5–110). Thirty-four percent of patients received blood transfusions, at a comparable rate per group. The diseased hemi-liver bile duct was ligated (n = 9), preserved (n = 27) or drained externally (n = 14) during stage 1. Associated extrahepatic procedures performed during stage 1 included three simultaneous right hemicolectomies, two rectal anterior resections, one diaphragmatic resection and one splenectomy.
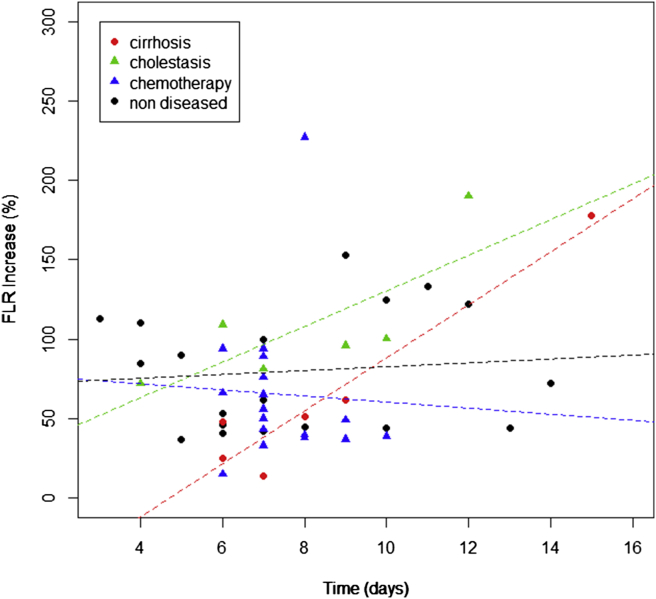
The sFLR prior to stage 1 was at a median of 20% (range 10–35) and FLR/BW at 0.42% (range 0.21–0.73) and increased to 35% (range 19–59) and 0.73% (range 0.39–1.22) prior to stage 2, respectively (Table 2). The median hypertrophy between the first and second stage was 63% (range 14–227). Livers from patients with preoperative cholestasis developed a higher percent of hypertrophy (96%, range 37–190) compared to normal livers. Livers of patients who received preoperative chemotherapy (56%, range 15–227) or had cirrhosis (50%, range 14–178) (Fig. 2) developed a lower degree of hypertrophy. Stage 2 was performed in 180 min (median, range 50–726). During the second stage, 4 extrahepatic procedures were performed: one ileostomy closure, one portal vein thrombectomy, one inferior vena cava replacement and one loop-ileostomy due to anastomotic leak of a colorectal anastomosis.
Figure 2.
Waiting time between stages and hypertrophy in different quality of liver parenchyma
Outcome data
Overall 90-day mortality was 20% (Table 3). Two patients died after stage 1 due to sudden cardiac death and septic shock on postoperative day 7 and 5, respectively, and did not proceed to stage 2.
Table 3.
Analysis of 90-day mortality
| n | Age (years) | Tumor Type | Bilirubin prior to stage 1 (mg/dl) | Type of hepatectomy | sFLR prior to stage 2 (%) | FLR/BW prior to stage 2 (%) | PHLFb | Direct cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 72 | GBCA | 2.9 | Trisectionectomy | 26% | 0.58% | Yes | Septic Shock |
| 2 | 76 | IHCC | 10.7 | Trisectionectomy | 40% | 0.93% | Yes | Septic Shock |
| 3a | 64 | PHCC | 3.7 | Trisectionectomy | – | – | – | Cardiogenic shock |
| 4a | 77 | PHCC | 1.01 | Trisectionectomy | – | – | – | Septic shock |
| 5 | 73 | PHCC | 0.62 | Trisectionectomy | 23% | 0.50% | No | Septic shock |
| 6 | 74 | METS | 0.69 | Trisectionectomy | 23% | 0.50% | Yes | Septic shock |
| 7 | 54 | IHCC | 1 | Trisectionectomy | 53% | 1.13% | Yes | Septic shock |
| 8 | 60 | IHCC | 0.4 | Trisectionectomy | 60% | 1.30% | Yes | Hemorrhagic Shock |
| 9 | 73 | IHCC | 5.9 | Trisectionectomy | 30% | 0.64% | Yes | Septic shock |
| 10 | 74 | IHCC | 0.36 | Trisectionectomy | 26% | 0.53% | Yes | Septic shock |
Mortality after stage 2 was 16%. The direct cause of death was septic shock in 7 patients and hemorrhagic shock in one patient. 90-day mortality after stage 2 for CRLM (4.5%) and HCC (12.5%) was significantly lower (p = 0.017) as compared to biliary malignancies (30%) (Table 2). Among these, two patients experienced liver failure between stages according to the “50-50 criteria”,14 whereas, according to the ISGLS definition,10 2 grade B and 1 grade A PHLF were observed. After stage 2, all deaths but one met any of both criterion (Table 3).
Overall, 160 complication events were recorded and 54% of patients experienced major complications. The biliary group experienced more complications after stage 1 (p = 0.012). Overall, five patients underwent a relaparotomy (10%). Patients were discharged after a median hospital stay of 27 days (range 15–127). Histology revealed negative resection margins in all patients but one with CRLM, who had a positive margins by histology.
The median follow-up after discharge was 364 days. One-year overall survival (OS) for CRLM, biliary tumors and HCC was 91%, 60% and 75%, respectively. One-year disease-free survival (DFS) was 55%, 45% and 62%, respectively.
Risk factors for complications and mortality
ROC curve analysis of peak bilirubin between stages to predict 90-day mortality was performed. The cut-off value was 5 mg/dl. The area under the curve was 0.69 (0.44–0.94) with a sensitivity value of 50% and a specificity value of 92.5%.
On univariate analysis for major complications, cholestasis (p = 0.004), preoperative biliary drainage (p = 0.014), biliary tumors versus metastatic tumors (p = 0.030), peak bilirubin >5 mg/dl (p = 0.004) between stages were significantly associated with major complications.
In the univariate analysis for 90-day mortality age >65 years (p = 0.006), biliary tumors (p = 0.019) and peak bilirubin >5 mg/dl between stages (p = 0.004) were significantly correlated (Table 4).
Table 4.
Univariate logistic regression for 90-day mortality after ALPPS
| Variable | 95% CI | OR | P-value |
|---|---|---|---|
| Peak bilirubin (interstage) > 5 mg/dl | 2.35–78.07 | 12.33 | 0.004 |
| Age >65 years | 2.23–77.61 | 10.54 | 0.006 |
| Biliary vs Metastases | 2.19–276.50 | 14.00 | 0.019 |
| HCC vs Metastases | 0.11–83.00 | 3.00 | 0.458 |
| Male gender | 0.33–6.00 | 1.36 | 0.671 |
| Preoperative biliary drainage | 0.28–13.83 | 2.25 | 0.393 |
| Preoperative cholestasis | 0.65–21.67 | 3.86 | 0.120 |
| FLR (prior to stage 1) | 0.98–1.00 | 0.99 | 0.059 |
| sFLR (prior to stage 1) | 0.74–1.01 | 0.87 | 0.070 |
| FLR/BW (prior to stage 1) | 4.967e–07–1.21 | 0.002 | 0.080 |
| Right trisectionectomy vs. right hepatectomy | 1.21–142.39 | 7.37 | 0.070 |
| FLR (prior to stage 2) | 0.99–1.00 | 1.00 | 0.356 |
| FLR/BW (prior to stage 2) | 0.0004–6.20 | 0.08 | 0.282 |
| sFLR (prior to stage 2) | 0.83–1.02 | 0.93 | 0.168 |
| Time interval (between stages) | 0.67–1.28 | 1.17 | 0.760 |
| FLR increase >60% | 0.61–22.20 | 3.00 | 0.210 |
HCC, hepatocellular carcinoma; FLR, future liver remnant; TLV, total liver volume; BW, body weight; 95% CI, 95% confidence interval; OR, odds ratio.
Multivariate Cox regression analysis for 90-day survival revealed that peak bilirubin >5 mg/dl between stages was the only independent prognostic factors for reduced survival (95% CI = 3.34–57.92; HR = 13.91; p = 0.0005).
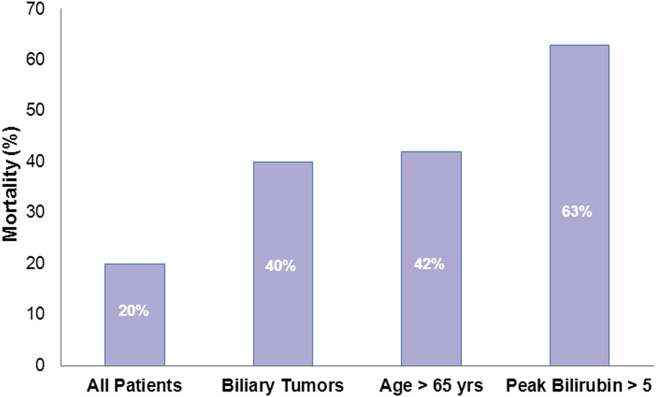
Short-term clinical outcome by peak bilirubin between stages >5 mg/dl is shown in Fig. 3. Eight of 50 patients (16%) with a peak bilirubin >5 mg/dl had a 90-day mortality rate of 63% compared with a mortality of 9.5% in patients with an interstage peak bilirubin <5 mg/dl.
Figure 3.
Ninety-day mortality in high risk subgroups in the Italian experience with ALPPS
Discussion
This closely monitored audit of 50 consecutive patients undergoing ALPPS in a single country in Europe shows an overall mortality of 20% for this controversial procedure, which is twice as high as the 9% previously reported from the International ALPPS Registry.12 This suggests significant underreporting of adverse outcomes in the voluntary International Registry.5 Future observational registries on high risk surgical innovations like ALPPS should follow the principles of consecutivity, intent-to-treat and a close audit to provide a more valid view of the safety of a surgical innovation. This Italian national audit, although certainly at the beginning of the learning curve for a complex surgical procedure like ALPPS, contributes to evaluate its safety.
Such an high rate of mortality has to be attributed to the large number of biliary tumors which have traditionally not been the target of two-stage hepatectomy. ALPPS should be discouraged in its current form in this patient population. Nadalin et al.15 reported that all their postoperative deaths occurred in patients affected by cholangiocarcinoma combining a stented biliary system and a cholestatic liver. In the global analysis performed by the International ALPPS registry,12 90-day mortality of biliary tumors, PHCC, IHCC and GBCA was 27%, 13% and 33% respectively. The only cohort studies that reported acceptable outcomes with ALPPS did not include biliary cancers (Alvarez et al.11 and Hernandez-Alejandro et al.16). Of course, other considerations like size of the remnant liver may also have an important impact on outcomes beyond tumor type. However, based on these data, for biliary tumors, time-honored methods to increase remnant volume like classic portal vein embolization,2 hepatic vein embolization17 should remain standard of care, as has been postulated by many experienced centers.15, 18 Variation on ALPPS like the novel hybrid ALPPS19 may cautiously be explored in this patient population in the context of registered research protocols.
Conversely, ALPPS may not represent an absolute contraindication for biliary tumors but rather it may be offered to well-selected patients. In patients with biliary tumors who did not develop cholestasis after stage 1, there was only one liver-related death and a major morbidity rate of 37.5%.
We would like to point out that paying attention to interstage bilirubin levels in ALPPS may be as important as the primary indication. Indeed, this study shows that increased bilirubin after stage 1 remains a prohibitive risk factor for mortality as has been shown preoperatively for major hepatectomies in general. In our multivariate model, a peak of bilirubin level >5 mg/dl after stage 1 was the strongest predictors of 90-day survival. We conclude that ALPPS stage 2 should not be performed when a significant increase in serum bilirubin or more generally a decrease of liver function occurs after stage 1. Aborting the second part of this two-stage hepatectomy may prevent liver failure and death after stage 2. Therefore, we suggest to abandon use of plastic bags or leaving of any other foreign bodies in abdominal cavity. Hence, a second surgical stage does not have to be performed with necessity and aborting without a complication incurred may even allow return to chemotherapy as further salvage treatment.
We would encourage centers to perform more sophisticated liver function testing, i.e. indocyanine green clearance,20 99mTc-mebrofenin or 99mTc-Galactosyl Serum Albumin scintigraphy,21 if available, to corroborate the observation that in ALPPS interstage liver function is the key to achieve good outcomes. The importance of elevated bilirubin as a risk factor, reflecting liver functional reserve prior to major hepatectomy, has been long recognized by cohort analyses22, 23 and has been also included into diagnostic scores for liver failure after resection either in combination with INR10, 14or in isolation as the Bilirubin >7 mg/dl score.24
Interestingly, patient with biliary tumors show the highest degree of hypertrophy (96%, p = 0.02), possibly induced by the regenerative stimulus of cholestasis.25 Nevertheless, despite the higher regeneration rate, five patients died of irreversible liver failure. We conclude that ALPPS hypertrophy may result in dysfunctional liver volume despite an appropriate volume for resection in some patients. While direct assessment of liver function prior to stage 2 such as indocyanine green or HIDA may help to differentiate ‘ineffective’ from ‘effective’ hypertrophy, both tests are influenced by elevated serum bilirubin levels.26 We ought to explore how nonfunctional hypertrophy can be reliably differentiated from functional hypertrophy and regional liver function test may be key tools for this in the future.
An additional research question comes from the observation that significant liver dysfunction may arise after ALPPS stage 1, induced by deportalization of one part of the liver and the rapid growth of the other, and that this dysfunction may be profound enough to cause rapid hyperbilirubinemia despite the “auxiliary liver” in place. As for now, our study demonstrates that high bilirubin levels are a reliable contraindication to delay or omit stage 2. In clinical practice surgeons performing ALPPS should become familiar to not perform the second stage in case of elevated bilirubin levels which are a surrogate clinical marker of liver dysfunction after the vascular redistribution in stage 1 ALPPS.
This study is also the largest series of ALPPS in cirrhosis to our knowledge. A median volume increase of 50% within a median of 7.5 days in 6 cirrhotic livers in this series is impressive. One of these six patients died within 90 days of ALPPS. Although, there is considerable risk in cirrhotic patients, ALPPS may be a salvage option in selected patients with HCC and fibrosis or cirrhosis and in whom PVE has been unsuccessful, as has been proposed by members of our group before.27
Long term survival data beyond 2 years after ALPPS are not yet available, but OS at 1 year for metastatic tumors in our series (91%) appears to be at least no higher to that reported by the International ALPPS Registry in 141 patients with CLRM (76%)12 and comparable to two-stage hepatectomy for CLRM (87%).28 DFS for CRLM (55%) was similar to DFS reported by the International Registry (59%) and following 2-stage hepatectomy (60%)28, 29 with an acceptable recurrence rate (10/21 patients, 48%), despite the controversial debate about it.30 Prospective and randomized cohorts with secondary endpoints OS and DFS are currently underway (NCT02215577).
The Italian registry was developed early in 2012 to audit national outcomes of a procedure that has been criticized for its high mortality rate starting with the inaugural publication.31 The emerging importance of the novel in-situ split procedure3 for liver resection with small remnants was recognized by Italian surgeons as reflected by a long list of single center reports.27, 32, 33, 34 The lasting importance of ALPPS has been underscored by a recent consensus conference (1st International Consensus Meeting On ALPPS, February 27th and 28th 2015, Hamburg, Germany).
This Italian audit of consecutive cases, has demonstrated that ALPPS has a 90-day overall mortality of 20%, higher than previously reported by single centers and the International Registry. While classic ALPPS is an important and reasonably safe (4.5% 90-day mortality) tool for bilateral liver metastases to induce increase in residual liver volume, it should not be currently used in patients with biliary tumors and in those who develop interstage elevation of bilirubin levels to avoid adverse outcomes.
Disclosure
The authors declare that they have nothing to disclose.
Acknowledgments
Members of the ALPPS Italian Registry Group are co-authors in this study: Roberto Montalti, Marco Vivarelli (Riuniti Hospital, Ancona); Gian Luca Grazi (Regina Elena National Cancer Institute, Rome); Giovanni Vennarecci, Giuseppe Maria Ettorre (San Camillo Hospital, Rome); Marco Massani, Nicolò Bassi (Regional Reference Center of Hepato-Biliary-Pancreatic Surgery, Treviso); Christian Cotsoglou, Vincenzo Mazzaferro (National Cancer Institute, Milan); Bruno Nardo (Annunziata Hospital, Cosenza), Fabio Forchino, Alessandro Ferrero (Mauriziano Umberto I Hospital, Turin); Salvatore Gruttadauria (Mediterranean Institute for Transplantation and Advanced Specialized Therapies, Palermo); Giorgio Ercolani, Antonio Daniele Pinna (S. Orsola-Malpighi Hospital, Bologna).
Footnotes
Best Poster Award at the 1st International ALPPS Consensus Meeting ALPPS, February 27th and 28th 2015, Hamburg, Germany.
Contributor Information
Elio Jovine, Email: elio.jovine@ausl.bologna.it.
ALPPS Italian Registry Group:
Roberto Montalti, Marco Vivarelli, Gian Luca Grazi, Giovanni Vennarecci, Giuseppe Maria Ettorre, Marco Massani, Nicolò Bassi, Christian Cotsoglou, Vincenzo Mazzaferro, Bruno Nardo, Fabio Forchino, Alessandro Ferrero, Salvatore Gruttadauria, Giorgio Ercolani, and Antonio Daniele Pinna
Conflicts of interest
None declared.
References
- 1.Broering D.C., Hillert C., Krupski G., Fischer L., Mueller L., Achilles E.G. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6:905–913. doi: 10.1016/s1091-255x(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 2.Abulkhir A., Limongelli P., Healey A.J., Damrah O., Tait P., Jackson J. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 3.Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 4.De Santibañes E., Clavien P.A. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- 5.Schadde E., Raptis D.A., Schnitzbauer A.A., Ardiles V., Tschuor C., Lesurtel M. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the International ALPPS Registry. Ann Surg. 2015;262:780–786. doi: 10.1097/SLA.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 6.Mosteller R.D. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 7.Vauthey J.N., Abdalla E.K., Doherty D.A., Gertsch P., Fenstermacher M.J., Loyer E.M. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 8.Farges O., Regimbeau J.M., Fuks D., Le Treut Y.P., Cherqui D., Bachellier P. Multicentre European study of preoperative biliary drainage for hilarcholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez F.A., Ardiles V., Sanchez Claria R., Pekolj J., de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy(ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814–821. doi: 10.1007/s11605-012-2092-2. [DOI] [PubMed] [Google Scholar]
- 12.Schadde E., Ardiles V., Robles-Campos R., Malago M., Machado M., Hernandez-Alejandro R. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829–838. doi: 10.1097/SLA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 14.Balzan S., Belghiti J., Farges O., Ogata S., Sauvanet A., Delefosse D. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadalin S., Capobianco I., Li J., Girotti P., Königsrainer I., Königsrainer A. Indications and limits for Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35–42. doi: 10.1055/s-0033-1356364. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Alejandro R., Bertens K.A., Pineda-Solis K., Croome K.P. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194–201. doi: 10.1016/j.surg.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Hwang S., Lee S.G., Ko G.Y., Kim B.S., Sung K.B., Kim M.H. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–616. doi: 10.1097/SLA.0b013e31819ecc5c. [DOI] [PubMed] [Google Scholar]
- 18.Kokudo N., Shindoh J. How can we safely climb the ALPPS? Updates Surg. 2013 Sep;65:175–177. doi: 10.1007/s13304-013-0215-2. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Kantas A., Ittrich H., Koops A., Achilles E.G., Fischer L. Avoid “All-Touch” by hybrid ALPPS to achieve oncological efficacy. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000845. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Lau L., Christophi C., Muralidharan V. Intraoperative functional liver remnant assessment with indocyanine green clearance: another toehold for climbing the “ALPPS”. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000608. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.de Santibañes E., Alvarez F.A., Ardiles V. How to avoid postoperative liver failure: a novel method. World J Surg. 2012;36:125–128. doi: 10.1007/s00268-011-1331-0. [DOI] [PubMed] [Google Scholar]
- 22.Melendez J., Ferri E., Zwillman M., Fischer M., DeMatteo R., Leung D. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001 Jan;192:47–53. doi: 10.1016/s1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 23.Llovet J.M., Brú C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 24.Mullen J.T., Ribero D., Reddy S.K., Donadon M., Zorzi D., Gautam S. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862–4. [DOI] [PubMed] [Google Scholar]
- 25.Otao R., Beppu T., Isiko T., Mima K., Okabe H., Hayashi H. External biliary drainage and liver regeneration after major hepatectomy. Br J Surg. 2012;99:1569–1574. doi: 10.1002/bjs.8906. [DOI] [PubMed] [Google Scholar]
- 26.Cieslak K.P., Runge J.H., Heger M., Stoker J., Bennink R.J., van Gulik T.M. New perspectives in the assessment of future remnant liver. Dig Surg. 2014;31:255–268. doi: 10.1159/000364836. [Epubahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Vennarecci G., Laurenzi A., Levi Sandri G.B., Busi Rizzi E., Cristofaro M., Montalbano M. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:982–988. doi: 10.1016/j.ejso.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Wicherts D.A., Miller R., de Haas R.J., Bitsakou G., Vibert E., Veilhan L.A. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 29.Adam R., Laurent A., Azoulay D., Castaing D., Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldhafer K.J., Donati M., Jenner R.M., Stang A., Stavrou G.A. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg. 2014;38:1504–1509. doi: 10.1007/s00268-013-2401-2. [DOI] [PubMed] [Google Scholar]
- 31.Aloia T.A., Vauthey J.N. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012 doi: 10.1097/SLA.0b013e318265fd3e. 256:e9; author reply e16–9. [DOI] [PubMed] [Google Scholar]
- 32.Ratti F., Cipriani F., Gagliano A., Catena M., Paganelli M., Aldrighetti L. Defining indications to ALPPS procedure: technical aspects and open issues. Updates Surg. 2014;66:41–49. doi: 10.1007/s13304-013-0243-y. [DOI] [PubMed] [Google Scholar]
- 33.Ratti F., Schadde E., Masetti M., Massani M., Zanello M., Serenari M. Strategies to increase the resectability of patients with colorectal liver metastases: a multi-center case-match analysis of ALPPS and conventional two-stage hepatectomy. Ann Surg Oncol. 2015 Jun;22:1933–1942. doi: 10.1245/s10434-014-4291-4. [DOI] [PubMed] [Google Scholar]
- 34.Cillo U., Gringeri E., Feltracco P., Bassi D., D'Amico F.E., Polacco M. Totally laparoscopic microwave ablation and portal vein ligation for staged hepatectomy: a new minimally invasive two-stage hepatectomy. Ann Surg Oncol. 2015 doi: 10.1245/s10434-014-4353-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]