Abstract
Background
This study seeks to examine the impact of orthotopic liver transplantation (OLT) on Health-Related Quality of Life (HRQoL) and mental health in patients with different MELD scores.
Methods
Patients who has undergone orthotopic liver transplant (OLT) or were on the waiting list for OLT were submitted to HRQoL and depression/anxiety assessment by questionnaire: Short-Form 36 (SF-36), Questions on Life Satisfaction (FLZ-M), Patient Health Questionnaire-4 (PHQ-4). Data were analysed following division of patients into three groups: pretransplant patients with a MELD score <10, ≥10, and OLT recipients.
Results
The surveys were sent to 940 consecutive patients within one week in June 2013. Of these 940 patients, 869 (92.4%) met the inclusion criteria. In total, 291 (33.5%) eligible questionnaires (OLT group: 235, MELD <10: 25; MELD _10: 31) were suitable for analysis. General health (GH), vitality (VIT), and mental health (MH) were lower in both pretransplant groups compared to the OLT group (all p < 0.05). Anxiety and depression were higher in the MELD <10 group than in the OLT group (anxiety: p < 0.05; depression: p < 0.01).
Discussion
Patients with low MELD scores seem to benefit from OLT with regards to HRQoL and mental health.
Introduction
Many patients with end stage liver failure suffer from serious physical impairment such as reduced kidney function,1 ascites2 or recurrent hemorrhage,3 peritonitis4 and encephalopathy.5 These complications are often life-threatening and after the first episode of decompensation of liver cirrhosis, the mortality rate approaches 85% within five years.2 The only curative treatment option is orthotopic liver transplantation (OLT).
Since 2002, in the US, the allocation of deceased donor liver transplants has been based on the model for end-stage liver disease (MELD) score although for several diseases, an exceptional MELD score exists. The score is an objective assessment based on a mathematical formula using logarithmic values of serum bilirubin, serum creatinine, and institutional normalized ratio (INR). The MELD score was designed to detect patients with a high risk of mortality related to end-stage liver disease with the intention of prioritizing these patients for liver transplantation.6 In general, patients with a MELD score of ≥15 should be considered for the liver transplant waiting list since they profit from significant survival benefit with OLT.7, 8, 9, 10 Nevertheless, physical impairment and mortality rates can be increased in patients with lower MELD scores as well.11 These impairments potentially decrease the patients' Health-Related Quality of Life (HRQoL), the person's well-being which indicates the ability to participate actively in daily life. There are several standardized measurement instruments to evaluate the HRQoL after solid organ transplantation12, 13 and the SF-36 survey is most frequently used.14
In addition to HRQoL, another important psychosocial outcome factor of OLT is the presence of anxiety and depression.15, 16 Mental disorders are known to have a negative impact on HRQoL.17 Apart from that, these conditions appear to increase mortality after OLT.15, 18 Besides the Hospital Anxiety and Depression Scale (HADS),19 the Patient Health Questionnaire (PHQ) is a frequently used instrument to measure anxiety and depression.20 The PHQ-4 is an ultra brief version of the survey.21
Apart from mere survival rates, HRQoL and mental health should be considered further important outcome factors in liver transplant recipients and patients on the liver transplant waiting list. Several studies have demonstrated that the overall HRQoL improves dramatically after OLT compared to pretransplantation.22, 23 However, there is some evidence that the patients' actual liver function represented by MELD score does not appear to be related to HRQoL.24, 25 Yet, there are contradictory findings on the question of whether patients with higher preoperative MELD scores benefit more from OLT with regards to HRQoL than patients with low scores.23, 26 Furthermore, it is unclear whether HRQoL improves in patients with low MELD scores. The present study seeks to evaluate the influence of OLT at a single centre on patients on the liver transplant waiting list with low MELD scores (<10) and high MELD scores (≥10).
Methods
Study design
All surviving patients who had received OLT at the University Hospital of Leipzig between 1993 and June 2013 were identified in June 2013. Moreover, recipients of double transplants (sequential or simultaneous liver and kidney transplants) and all patients who were on the waiting list for a liver transplantation in June 2013 were included.
The written SF-36 survey form was sent to all patients via mail. Return envelopes were included free of charge. A period of two weeks was envisaged for questionnaire return. All patients who had not returned the questionnaire after two weeks were contacted a second time via mail within another 14 days. Altogether, patients had four weeks for their responses. Prior to the study, informed consent of all patients and the consent of the local ethics committee (ID: 414-12-17122012) were obtained.
Patients
The following patients were included: age between 18 and 70 years, a returned questionnaire with at least 50% of the questions answered, records of medication and patient history available.
These patients groups were analysed based on presence on the liver transplant waiting list with a MELD score <10, on the liver transplant waiting list with a MELD score ≥10 and patients who has undergone OLT. The MELD score was calculated from the patients' records and blood test results retrospectively and the latest available MELD score was added to the database.
Short Form-36
The SF-36 survey27 includes 36 questions about current health status. It serves as an assessment tool of health-related QoL of probands over the age of 14. Eight dimensions of QoL are evaluated. These eight dimensions summarize two main sections, the physical component summary (PCS) and mental component summary (MCS).
These eight dimensions include; Physical Functioning (PF, 10 items), Role-Physical (RP, 4 items), Bodily Pain (BP, 2 items), General Health Perceptions (GH, 6 items), Vitality (VIT, 4 items), Social Functioning (SF, 2 items), Role-Emotional (RE, 3 items) and Mental Health (MH, 5 items). In each dimension, the range of possible scores is between 0 and 100 points. As a consequence, it is easy to compare between scales and patients.28 In the present study, the standard version of the SF-36 was used. At the end of the survey period, all SF-36 questionnaires were scanned and digitalized.
Questionnaire on Life Satisfaction (FLZ-M)
The Questions on Life Satisfaction (FLZ-M) survey was used in order to assess life satisfaction.29 It includes 8 aspects of daily life: friends/acquaintances, leisure time/hobbies, health, income/financial security, occupation/work, housing/living conditions, family life/children, partner relationship/sexuality. The possible scores for each dimension range from −12 to 20. Negative scores are an indicator of dissatisfaction whereas positive scores indicate satisfaction. If the weighted satisfaction value is 0, this means that the item is of no subjective importance to the individual. High scores indicate high subjective importance.29
Patient Health Questionnaire-4 (PHQ-4)
The test used in the present study is an ultra brief screening tool for anxiety and depression. It is a validated measurement instrument to detect mental disorders. It consists of 4 items. The GAD-2 detects anxiety (“Over the last 2 weeks, how often have you been bothered by (i) Feeling nervous, anxious, or on edge (ii) Not being able to stop or control worrying”). The PHQ-2 was designed to reveal depressive and anxious symptoms (Over the last 2 weeks, how often have you been bothered by (i) little interest or pleasure in doing things (ii) feeling down, depressed or hopeless). It is based on the full version of the PHQ test. For each item, there are 4 possible answers (0 = “not at all”, 1 = “on several days”, 2 = “more than half the time” and 3 = “nearly every day”). The sum of both scores can be created in order to obtain a summation score. The scores are rated as follows: normal (0–2), mild (3–5), moderate (6–8) and severe (9–12).21
Statistical analysis
Microsoft Excel (Microsoft, Redmond, USA) and SPSS 20.0 (IBM, Armonk, USA) were used for collection and analysis of the data. The data were digitized and computed with the Evasys© system (Electric Paper Evaluations Systeme GmbH, Lüneburg, Germany) and then imported into SPSS.
The data were checked for Gaussian distribution using the Kolmogrov–Smirnov test. Since there was no Gaussian distribution, non-parametric statistical tests (Kruskal–Wallis test and the Mann–Whitney-U-test) were used to check for statistical differences between the groups.
Categorial variables were analysed with the chi-square-test. All p-values < 0.05 were considered statistically significant.
Results
Basic characteristics
It was determined that 689 patients had undergone OLT at the University of Leipzig between 1993 and June 2013 and only known surviving patients were contacted. The liver transplant waiting list comprised 251 patients at that time (June 2013). Questionnaires were sent to these 940 patients (73.3% OLT recipients, 26.7% waiting list patients) and were asked to complete the SF-36 survey. In 27 cases, relatives of the patients informed us that the patient was deceased. Forty three patients were excluded from the analysis because they were 70 years of age or older. Of these 43 patients, there were 41 OLT recipients (4.6%) and 2 patients from the waiting list (0.2%). Another patient (0.1%) from the waiting list was excluded since there was no documented MELD-score available. In total, there were 869 (92.4%) patients who met the inclusion criteria. There were 291 (33.5%) eligible questionnaires (235 OLT recipients, 56 patients from the waiting list).
On the waiting list group, 25 (44.6%) had a MELD score <10 (mean = 7.6, SD = 1.1, range = 6–9), and 31 (55.4%) had a MELD score ≥10 (mean = 12.9, SD 3.5, range 10–25).
In the transplanted group, there were 127 (54%) patients who had received a liver transplant within the last 5 years, 38 (16%) within 5–10 years and 70 (30%) more than 10 years prior to the study time.
The three groups did not differ significantly with regard to demographic variables (Table 1). One patient did not provide information on family status. For 14 patients, no details were provided on their relationship status.
Table 1.
Basic characteristics
| MELD <10 |
MELD ≥10 |
Transplanted |
p | |
|---|---|---|---|---|
| n (%)/mean | n (%)/mean | n (%)/mean | ||
| Gender | 0.536 | |||
| Male | 14 (56.0%) | 17 (54.8%) | 149 (63.4%) | |
| Female | 11 (44.0%) | 14 (45.2%) | 86 (36.6%) | |
| Total | 25 (100%) | 31 (100%) | 235 (100%) | |
| Age (years) | 54.2 (SD = 6.8) | 56.1 (SD = 10.1) | 56.6 (SD = 9.2) | 0.096 |
| Underlying disease | 0.653 | |||
| Alcoholic liver cirrhosis | 17 (68.0%) | 16 (51.6%) | 131 (55.7%) | |
| Cryptoenic liver cirrhosis | 3 (12%) | 5 (16.1%) | 22 (9.4%) | |
| Cholestatic bile duct diseases | 3 (12%) | 2 (6.5%) | 14 (6.0%) | |
| Viral hepatitis | 0 (0%) | 2 (6.5%) | 12 (5.1%) | |
| Cystic liver disease | 0 (0%) | 1 (3.2%) | 14 (6.0%) | |
| Acute liver failure | 0 (0%) | 0 (0%) | 16 (6.8%) | |
| Autoimmune hepatitis | 1 (4.0%) | 2 (6.5%) | 8 (3.4%) | |
| Not specified | 1 (4.0%) | 3 (9.7%) | 18 (7.7%) | |
| Total | 25 (100%) | 31 (100%) | 235 (100%) | |
| Comorbidities | ||||
| Arterial hypertension | 8 (32.0%) | 14 (45.2%) | 166 (70.6%) | 0.000 |
| Diabetes mellitus | 10 (40.0%) | 17 (54.8%) | 108 (46.0%) | 0.517 |
| Chronic kidney failure | 5 (20.0%) | 4 (12.9%) | 135 (57.4%) | 0.000 |
| Committed relationship | 0.109 | |||
| Yes | 10 (40.0%) | 21 (67.7%) | 160 (68.1%) | |
| No | 11 (44.0%) | 7 (22.6%) | 67 (28.5%) | |
| Not specified | 4 (16%) | 3 (9.7%) | 8 (3.4%) | |
| Family status | 0.544 | |||
| Unmarried | 6 (24.0%) | 3 (9.7%) | 34 (14.5%) | |
| Married | 12 (48.0%) | 20 (64.5%) | 157 (66.8%) | |
| Divorced | 5 (20.0%) | 6 (19.4%) | 30 (12.8%) | |
| Widowed | 2 (8.0%) | 2 (6.5%) | 13 (5.5%) | |
| Not specified | 0 (0%) | 0 (0%) | 1 (0.4%) | |
| Religious | ||||
| Yes | 3 (12.0%) | 5 (16.1%) | 41 (17.4%) | 0.782 |
| No | 22 (88.0%) | 26 (83.9%) | 194 (82.6%) | |
ns, statistically not significant.
The distribution of the comorbidities arterial hypertension (AH), diabetes mellitus (DM) and chronic kidney failure (CKF) is shown in Table 1. No patient suffered from end-stage renal failure. AH and CKF were significantly more frequent in the OLT group (Table 1).
SF-36 results
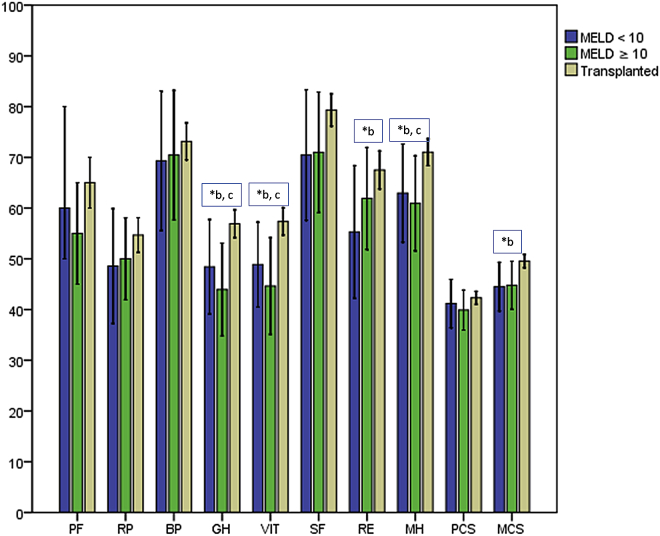
The highest scores in all dimensions and PCS and MCS were found in the OLT group, with statistical significance for GH (p = 0.002), VIT (p = 0.002), MH (p = 0.011) and MCS (p = 0.016). For PF (p = 0.427), RP (p = 0.271), BP (p = 0.696), SF (p = 0.092), RE (p = 0.059) and PCS (p = 0.464), no significant differences were found (Fig. 1) Post-hoc testing revealed no statistically significant differences between the MELD <10 and ≥10 groups (PF: p = 0.397, RP: p = 0.362, BP: p = 0.696, GH: p = 0.627, VIT: p = 0.811, SF: p = 0.826, RE: p = 0.262, MH: p = 0.780, PCS: p = 0.558, MCS: p = 0.639). A comparison between patients with a MELD score <10 and the transplanted patients showed significantly higher scores for GH (p = 0.030), VIT (p = 0.021), RE (p = 0.029), MH (p = 0.020) and MCS (p = 0.021) in the transplanted group. The differences in the other dimensions were not statistically significant (PF: p = 0.878, RP: p = 0.135, BP: p = 0.397, SF: p = 0.102, PCS: p = 0.668).
Figure 1.
SF-36 results from the different groups. PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health perceptions; VIT, vitality; SF, social functioning; RE, role-emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary. * indicates statistical significance between b: MELD <10 group and OLT group; c: MELD ≥10 and OLT group
Patients with a MELD score ≥10 had significantly reduced scores for GH (p = 0.003), VIT (p = 0.003) and MH (p = 0.033) when compared to the OLT group (PF: p = 0.195, RP: p = 0.476, BP: p = 0.811, SF: p = 0.105, RE: p = 0.265, PCS: p = 0.234, MCS = 0.054).
FLZ-M results
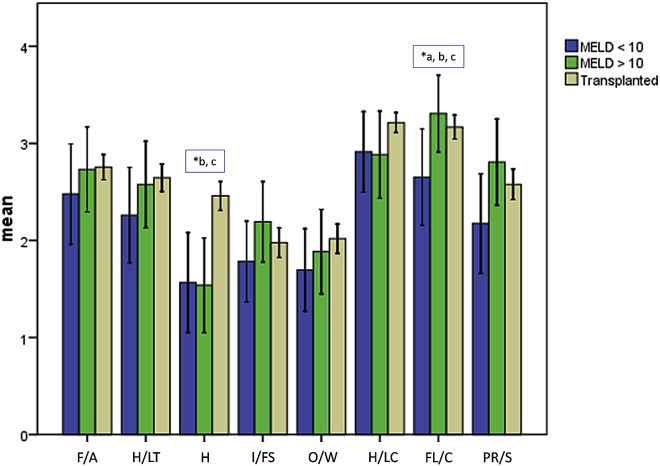
When comparing the three groups, significant differences were found on the scales health (H, p = 0.000) and family life/children (FL/C, p = 0.049), with the highest values in the OLT group (H) and the MELD >10 group (FL/C). The dimensions friends/acquaintances (F/A, p = 0.593), hobbies/leisure time (H/LT, p = 0.298), income/financial security (I/FS, p = 0.418), occupation/work (O/W, p = 0.353), housing/living conditions (H/LC, p = 0.170), partner relationship/sex (PR/S, p = 0.245). The results are shown in Fig. 3. Post hoc testing revealed a significantly higher FL/C score in patients with MELD >10 compared to MELD ≥10 (p = 0.044). The remaining scales did not differ significantly (F/A: p = 0.395, H/LT: p = 0.336, H: p = 0.939, I/FS: p = 0.207, O/W: p = 0.770, H/L: p = 1.0, PR/S: p = 0.130). When comparing the MELD <10 group with the OLT group, significantly lower scores for “FL/C” (p = 0.026) and “health” (p = 0.001) were shown (F/A: p = 0.316, H/LT: p = 0.116, I/FS: p = 0.232, O/W: p = 0.221, H/L: p = 0.180, PR/S: p = 0.120). The H-score was significantly higher in the OLT group than in the MELD ≥10 group (p = 0.000). The differences on the other scales were not significant (F/A: p = 0.921, H/LT: p = 0.840, I/FS: p = 0.680, O/W: p = 0.373, H/L: p = 0.143, FL/C: p = 0.345, PR/S: p = 0.620).
Figure 3.
FLZ-M results from the different groups. F/A, friends/acquaintances; LT/H, leisure time/hobbies; H, health; I/FS, income/financial security; O/W, occupation/work; H/LC, housing/living conditions; FL/C, family life/children; PR/S, partner relationship/sexuality. * indicates statistical significance between a: MELD <10 and MELD ≥10 group; b: MELD <10 group and OLT group; c: MELD ≥10 and OLT group
PHQ-4 results
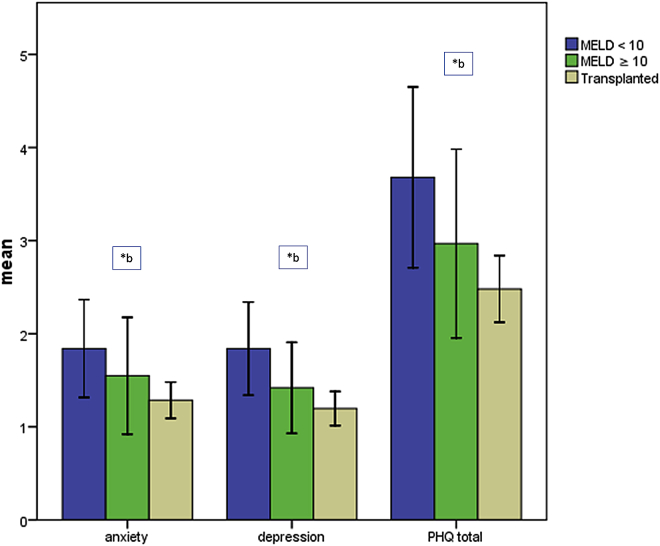
The lowest values on the scales anxiety, depression and summation score were found in the OLT group, the highest in the MELD <10 group. The MELD ≥10 group showed intermediate results (Fig. 2). Statistical significance was found for depression (p = 0.010) and summation score (p = 0.013). The differences in the anxiety scale were not significant (p = 0.051). The comparison between the MELD <10 and ≥10 group (anxiety: p = 0.210, depression: p = 0.126, summation score: p = 0.139) as well as the comparison between the MELD ≥10 and the OLT group (anxiety: p = 0.518, depression: p = 0.220, summation score: p = 0.289) did not show significant differences for all three scales. When comparing the MELD <10 and the OLT group, significantly lower scores were found in the OLT group for anxiety (p = 0.015), depression (p = 0.004) and summation score (p = 0.004).
Figure 2.
PHQ-4 results from the different groups. * indicates statistical significance between a: MELD <10 and MELD ≥10 group; b: MELD <10 group and OLT group; c: MELD ≥10 and OLT group
Discussion
The present study shows that OLT recipients have a higher HRQoL and lower levels of depression and anxiety compared to patients on the waiting list. It could be shown that both patients with MELD scores <10 and patients with MELD scores ≥10 have a reduced HRQoL compared to OLT recipients, especially in the dimensions of mental health (GH, VIT and MH). Patients with MELD <10 even showed significantly lower scores on the scales RE and MCS compared to the transplanted group. The FLZ-M revealed significantly reduced scores for “health” and “family life/children” for patients with a MELD score below 10 compared to the other groups. The depression and anxiety scores were significantly higher in the MELD <10 group than in the OLT group. There were no differences between patients with a higher MELD score and OLT recipients with regards to affective status.
Several studies have shown the positive effects of OLT on HRQoL in liver transplant candidates22, 23 with scores for PCS ranging between 37 and 62, and for MCS scores between 48 and 55.30, 31, 32, 33 Our examination showed intermediate results (PCS: 42.3, MCS: 49.6).
Contrary to the results in the present study, Estraviz et al found that patients with reduced liver function (Child B and C) benefited more from OLT than patients with Child A cirrhosis. Similarly, Togashi and colleagues found significantly higher HRQoL scores after living donor liver transplantation amongst patients with a MELD score >15 compared to patients whose MELD score was below 15. They found significantly reduced HRQoL scores preoperatively in the high MELD group compared to the low MELD group as well.26 Two further examinations found comparable results,34, 35 whereas Mabrouk and colleagues found a worse HRQoL in OLT recipients who had preoperative MELD scores >15.23 On the other hand, there are studies indicating that MELD score does not depict the dimensions of HRQoL. One examination that was carried out on patients listed for liver transplantation found no correlation between liver function measured by MELD score and dynamic liver function tests and HRQoL. Instead, ascites and butyryl cholinesterase serum concentration were found to be independent predictors of HRQoL.24 These findings were confirmed by two further studies that found no correlation between MELD score and HRQoL prior to OLT.25, 36
The present study is one of the few that uses the FLZ to assess life satisfaction in OLT recipients and transplant candidates. With the exception of the dimension “family/children” we supported the findings of the study of Goetzmann and colleagues,37 that current liver function did not influence psychosocial variables in patients on the liver transplant waiting list. Both pretransplant groups in our study showed significantly reduced “health” scores compared to the OLT group. A possible explanation for the positive effect of OLT on the dimension “health” is the fact that OLT cures the underlying disease and thus improves general health. These findings are consistent with the findings for GH on the SF-36.
The occurrence of depression and anxiety before and after liver transplantation has been examined in several studies. In most studies, mental health is reduced in patients on the waiting list38, 39 and improves significantly after OLT.40, 41, 42 Nonetheless, there is little evidence as to the influence of liver function on the mental health of these patients. Santos et al found a tendency for high scores of anxiety related to lower MELD scores.43 These findings tally with the results of the present study. In contrast to these results, Zahn et al showed that OLT recipients with MELD scores ≥13 had significantly more depressive symptoms than patients with lower MELD scores.44
The interpretation of these contrary findings and understanding them in the context of the present study is difficult. A possible explanation is the presence of response shift effects. Such effects indicate a change in an individual's values, internal standards and conceptualization of quality of life on HRQoL examinations.45 These effects could be a possible explanation for the contradictory findings when comparing the influence of MELD score before and after OLT. This means that patients with a more serious liver disease (high MELD score) realize after being cured by OLT that their health status before OLT was even worse than they realized. Since the present study was cross-sectional, with only one evaluation cycle, these effects are less likely to influence the results. We found, similar to the findings in the studies on pretransplant patients mentioned above, no differences in patients with different MELD scores. Both groups had lower scores, especially on the mental scales of the SF-36, compared to OLT recipients. The benefit of OLT appeared to be even greater in patients with MELD scores <10. This is remarkable since OLT recipients had significantly more comorbidities (AH and CKF). Although these findings have to be interpreted with caution due to the relatively small sample size of the pretransplant groups, these findings support the theory that even patients with low MELD scores have impaired mental health and a reduced HRQoL that significantly increase after OLT. The knowledge of suffering from a life-threatening disease but not receiving a life-saving liver transplantation in the near future might lead to higher levels of anxiety and depression and a reduced quality of life in these patients.
Evaluations of quality of life (QoL) are currently not implemented in the organ distribution systems of western countries. A routinely performed QoL screening in transplant candidates on the waiting list might help to identify mentally most impaired patients independent from the MELD status. Thus, not only should the survival benefit be taken into account regarding organ distribution, but also the potential to ameliorate the HrQoL and mental health.
Even though response shift effects are unlikely, the cross-sectional, retrospective design of the present study is one of its shortcomings. In a prospective examination, factors impacting the HRQoL could be identified more precisely. Moreover, there were unequal group sizes with more OLT recipients than patients on the waiting list. One of the strengths of our study is its relatively large patient sample size. Furthermore, this is one of the few examinations dealing with the question of whether OLT improves HRQoL in patients with low MELD scores.
In summary, OLT improves HRQoL, especially the mental HRQoL in patients on the liver transplant waiting list. Both patients with low and high MELD scores benefit from OLT. Apparently, liver function measured by MELD score does not depict HRQoL. Depression and anxiety appear to be even more present in pretransplant patients with low MELD (<10) scores than in patients with higher MELD scores. Thus, these patients especially would potentially benefit from OLT with regards to their mental health.
Financial support and industry affiliations
There was no financial support of any kind contributing to this publication.
No grants were accepted/used for this publication.
None of the authors has any personal or institutional financial interest in drugs, materials, or devices described in this submission.
Acknowledgements
We want to thank Ms Dorothea Weinert and Mr Jerome Denis Braun from the transplantation office for their support in creating the patient list.
Footnotes
The paper is not based on a previous communication to a society or meeting.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Sampaio M.S., Martin P., Bunnapradist S. Renal dysfunction in end-stage liver disease and post-liver transplant. Clin Liver Dis. 2014;18:543–560. doi: 10.1016/j.cld.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraldes J.G., Tandon P. Soft and hard endpoints in acute variceal bleeding. Hepatology. 2015;61:762–765. doi: 10.1002/hep.27583. [DOI] [PubMed] [Google Scholar]
- 4.Terg R., Casciato P., Garbe C., Cartier M., Stieben T., Mendizabal M. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62:1056–1060. doi: 10.1016/j.jhep.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Saab S. Evaluation of the impact of rehospitalization in the management of hepatic encephalopathy. Int J Gen Med. 2015;8:165–173. doi: 10.2147/IJGM.S81878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal A.K., Kamath P.S. Model for end-stage liver disease. J Clin Exp Hepatol. 2013;3:50–60. doi: 10.1016/j.jceh.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merion R.M., Schaubel D.E., Dykstra D.M., Freeman R.B., Port F.K., Wolfe R.A. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 8.Cuomo O., Perrella A., Arenga G. Model for End-Stage Liver Disease (MELD) score system to evaluate patients with viral hepatitis on the waiting list: better than the Child-Turcotte-Pugh (CTP) system? Transplant Proc. 2008;40:1906–1909. doi: 10.1016/j.transproceed.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 9.Freeman R.B. Model for end-stage liver disease (MELD) for liver allocation: a 5-year score card. Hepatology. 2008;47:1052–1057. doi: 10.1002/hep.22135. [DOI] [PubMed] [Google Scholar]
- 10.Huo T.I., Wu J.C., Lee S.D. MELD in liver transplantation: the da Vinci code for the Holy Grail? J Hepatol. 2005;42:477–478. doi: 10.1016/j.jhep.2005.02.003. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 11.Somsouk M., Kornfield R., Vittinghoff E., Inadomi J.M., Biggins S.W. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl. 2011;17:129–136. doi: 10.1002/lt.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwish Murad S., Heimbach J.K., Gores G.J., Rosen C.B., Benson J.T., Kim W.R. Excellent quality of life after liver transplantation for patients with perihilar cholangiocarcinoma who have undergone neoadjuvant chemoradiation. Liver Transpl. 2013;19:521–528. doi: 10.1002/lt.23630. [DOI] [PubMed] [Google Scholar]
- 13.Crossen J.R., Keeffe E.B., Benner K.G., Garvey-Schray A., Whalen J., Mesch J. Psychological assessment of quality of life following liver transplantation. J Clin Psychol Med Settings. 1994;1:71–81. doi: 10.1007/BF01991725. [DOI] [PubMed] [Google Scholar]
- 14.Baranyi A., Krauseneck T., Rothenhäusler H.B. Posttraumatic stress symptoms after solid-organ transplantation: preoperative risk factors and the impact on health-related quality of life and life satisfaction. Health Qual Life Outcomes. 2013;11:111. doi: 10.1186/1477-7525-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corruble E., Barry C., Varescon I., Durrbach A., Samuel D., Lang P. Report of depressive symptoms on waiting list and mortality after liver and kidney transplantation: a prospective cohort study. BMC Psychiatry. 2011;11:182. doi: 10.1186/1471-244X-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMartini A., Dew M.A., Chaiffetz D., Fitzgerald M.G., Devera M.E., Fontes P. Early trajectories of depressive symptoms after liver transplantation for alcoholic liver disease predicts long-term survival. Am J Transplant. 2011;11:1287–1295. doi: 10.1111/j.1600-6143.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller L.R., Paulson D., Eshelman A., Bugenski M., Brown K.A., Moonka D. Mental health affects the quality of life and recovery after liver transplantation. Liver Transpl. 2013;19:1272–1278. doi: 10.1002/lt.23728. [DOI] [PubMed] [Google Scholar]
- 18.Rogal S.S., Dew M.A., Fontes P., DiMartini A.F. Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant. 2013;13:928–935. doi: 10.1111/ajt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jay C.L., Butt Z., Ladner D.P., Skaro A.I., Abecassis M.M. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K., Spitzer R.L., Williams J.B., Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 22.Yang L.S., Shan L.L., Saxena A., Morris D.L. Liver transplantation: a systematic review of long-term quality of life. Liver Int. 2014;34:1298–1313. doi: 10.1111/liv.12553. [DOI] [PubMed] [Google Scholar]
- 23.Mabrouk M., Esmat G., Yosry A., El-Serafy M., Doss W., Zayed N. Health-related quality of life in Egyptian patients after liver transplantation. Ann Hepatol. 2012;11:882–890. [PubMed] [Google Scholar]
- 24.Jara M., Bednarsch J., Malinowski M., Lüttgert K., Orr J., Puhl G. Predictors of quality of life in patients evaluated for liver transplantation. Clin Transplant. 2014;28:1331–1338. doi: 10.1111/ctr.12426. [DOI] [PubMed] [Google Scholar]
- 25.Derck J.E., Thelen A.E., Cron D.C., Friedman J.F., Gerebics A.D., Englesbe M.J. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99:340–344. doi: 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 26.Togashi J., Sugawara Y., Akamatsu N., Tamura S., Yamashiki N., Kaneko J. Quality of life after adult living donor liver transplantation: a longitudinal prospective follow-up study. Hepatol Res. 2013;43:1052–1063. doi: 10.1111/hepr.12060. [DOI] [PubMed] [Google Scholar]
- 27.Morfeld M., KI, Bullinger M. Hogrefe; Göttingen: 2011. SF 36-Fragebogen zum Gesundheitszustand. [Google Scholar]
- 28.JE W. SF-36® Health Survey Update [cited 2015 2nd January 2015]. Available from: http://www.sf-36.org/tools/sf36.shtml.
- 29.G H, P H Questions on Life Satisfaction (FLZ M) – a short questionnaire for assessing subjective quality of life. Eur J Psychol Assess. 2000;16:150–159. [Google Scholar]
- 30.Chen P.X., Yan L.N., Wang W.T. Health-related quality of life of 256 recipients after liver transplantation. World J Gastroenterol. 2012;18:5114–5121. doi: 10.3748/wjg.v18.i36.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugler C., Gottlieb J., Warnecke G., Schwarz A., Weissenborn K., Barg-Hock H. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplantation. 2013;96:316–323. doi: 10.1097/TP.0b013e31829853eb. [DOI] [PubMed] [Google Scholar]
- 32.Duffy J.P., Kao K., Ko C.Y., Farmer D.G., McDiarmid S.V., Hong J.C. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. doi: 10.1097/SLA.0b013e3181f5f23a. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan K.M., Radosevich D.M., Lake J.R. Health-related quality of life: two decades after liver transplantation. Liver Transpl. 2014;20:649–654. doi: 10.1002/lt.23855. [DOI] [PubMed] [Google Scholar]
- 34.Castaldo E.T., Feurer I.D., Russell R.T., Pinson C.W. Correlation of health-related quality of life after liver transplant with the Model for End-Stage Liver Disease score. Arch Surg. 2009;144:167–172. doi: 10.1001/archsurg.2008.563. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigue J.R., Nelson D.R., Reed A.I., Hanto D.W., Curry M.P. Is Model for End-Stage Liver Disease score associated with quality of life after liver transplantation? Prog Transplant. 2011;21:207–214. doi: 10.1177/152692481102100305. [DOI] [PubMed] [Google Scholar]
- 36.Saab S., Ibrahim A.B., Shpaner A., Younossi Z.M., Lee C., Durazo F. MELD fails to measure quality of life in liver transplant candidates. Liver Transpl. 2005;11:218–223. doi: 10.1002/lt.20345. [DOI] [PubMed] [Google Scholar]
- 37.Goetzmann L., Wagner-Huber R., Klaghofer R., Muellhaupt B., Clavien P.A., Buddeberg C. Waiting for a liver transplant: psychosocial well-being, spirituality, and need for counselling. Transplant Proc. 2006;38:2931–2936. doi: 10.1016/j.transproceed.2006.08.171. [DOI] [PubMed] [Google Scholar]
- 38.Martins P.D., Sankarankutty A.K., OeC Silva, Gorayeb R. Psychological distress in patients listed for liver transplantation. Acta Cir Bras. 2006;21(Suppl. 1):40–43. doi: 10.1590/s0102-86502006000700010. [DOI] [PubMed] [Google Scholar]
- 39.Santos G.G., Gonçalves L.C., Buzzo N., Mendes T.A., Dias T.P., da Silva R.C. Quality of life, depression, and psychosocial characteristics of patients awaiting liver transplants. Transplant Proc. 2012;44:2413–2415. doi: 10.1016/j.transproceed.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Karam V., Castaing D., Danet C., Delvart V., Gasquet I., Adam R. Longitudinal prospective evaluation of quality of life in adult patients before and one year after liver transplantation. Liver Transpl. 2003;9:703–711. doi: 10.1053/jlts.2003.50148. [DOI] [PubMed] [Google Scholar]
- 41.Burra P., De Bona M., Canova D., Feltrin A., Ponton A., Ermani M. Longitudinal prospective study on quality of life and psychological distress before and one year after liver transplantation. Acta Gastroenterol Belg. 2005;68:19–25. [PubMed] [Google Scholar]
- 42.Martín-Rodríguez A., Fernández-Jiménez E., Pérez-San-Gregorio M.A., Pérez-Bernal J., Gómez-Bravo M.A. Longitudinal study of liver transplant recipients' quality of life as a function of their perception of general health: at waiting list and at 3, 6, and 12 months post-transplantation. Transplant Proc. 2013;45:3653–3655. doi: 10.1016/j.transproceed.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Santos G.R., Boin I.F., Pereira M.I., Bonato T.C., Silva R.C., Stucchi R.S. Anxiety levels observed in candidates for liver transplantation. Transplant Proc. 2010;42:513–516. doi: 10.1016/j.transproceed.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Zahn A., Seubert L., Jünger J., Schellberg D., Weiss K.H., Schemmer P. Factors influencing long-term quality of life and depression in German liver transplant recipients: a single-centre cross-sectional study. Ann Transplant. 2013;18:327–335. doi: 10.12659/AOT.883962. [DOI] [PubMed] [Google Scholar]
- 45.Sprangers M.A., Schwartz C.E. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]