Abstract
Hypertonic dextrose injections (prolotherapy) is an emerging treatment for symptomatic knee osteoarthritis (OA) but its efficacy is uncertain. We conducted a systematic review with meta-analysis to synthesize clinical evidence on the effect of prolotherapy for knee OA. Fifteen electronic databases were searched from their inception to September 2015. The primary outcome of interest was score change on the Western Ontario and McMaster Universities Arthritis Index (WOMAC). Three randomized controlled trials (RCTs) of moderate risk of bias and one quasi–randomized trial were included, with data from a total of 258 patients. In the meta-analysis of two eligible studies, prolotherapy is superior to exercise alone by a standardized mean difference (SMD) of 0.81 (95% CI: 0.18 to 1.45, p = 0.012), 0.78 (95% CI: 0.25 to 1.30, p = 0.001) and 0.62 (95% CI: 0.04 to 1.20, p = 0.035) on the WOMAC composite scale; and WOMAC function and pain subscale scores respectively. Moderate heterogeneity exists in all cases. Overall, prolotherapy conferred a positive and significant beneficial effect in the treatment of knee OA. Adequately powered, longer-term trials with uniform end points are needed to better elucidate the efficacy of prolotherapy.
Knee osteoarthritis (OA) is the most common form of chronic arthritis worldwide and a major cause of pain and disability1,2,3. It carries considerable economic burden with its high prevalence, loss of work time and utilization of healthcare service resources4,5. The effectiveness of current treatment for KOA is limited. Current guidelines suggest that the management of knee OA should take a multidisciplinary approach including non-pharmacological, pharmacological, surgical and complementary therapies6,7. Despite efforts by the Osteoarthritis Research Society International (OARSI) Treatment Guidelines Committee, there are limited generally accepted core sets of treatments for patients with knee OA8. A safe and effective treatment option that complements the current therapy remains a top priority in clinical practice and research. In the United States, assessment of knee OA treatment has been identified as a “top 100” research priority by the National Academy of Medicine9, and the Agency for Healthcare Research and has called for new knee OA therapy10.
Hypertonic dextrose injection, “prolotherapy”, is an injection-based treatment used for a variety of painful chronic musculoskeletal pain conditions, including knee OA11. The core practice principle of prolotherapy is injection of relatively small volumes (0.5–6 ml) of an irritant solution, usually hypertonic dextrose, at painful ligament and tendon attachments, as well as in adjacent joint spaces11. The basic science of the mechanism of action on connective tissue and cells of various type (e.g. fibroblast/chondrocytes etc.) is not well understood. However, animal studies have reported that peritendinous dextrose injection consistently resulted in fibroblast and vascular proliferation, dense collagen deposition and increase in ligament thickness, energy absorption and ultimate load bearing ability12,13,14,15. Animal model data has also suggested cartilage-specific anabolic growth as a result of intra-articular dextrose injection16. The hypothesized mechanisms for pain relief include: (i) stimulation of local healing among chronically injured extra- and intra-articular tissue; (ii) reduction of joint instability through the strengthening of stretched or torn ligaments, and (iii) stimulation of cellular proliferation17.
Prolotherapy is practiced throughout the world; the strongest interest appears to be among physicians and patients in primary care18,19. Human studies have assessed the role of prolotherapy for various musculoskeletal conditions20,21,22,23,24. Systematic reviews of the clinical effectiveness of prolotherapy in chronic low back pain and lateral epicondylitis had been conducted in the past which yielded mixed and positive results respectively, though the strength of evidence was limited by clinical heterogeneity amongst studies and the presence of co-interventions25,26. In recent years, prolotherapy has been used to treat patients with knee OA refractory to other conservative care27. Results from several published clinical trials have shown positive effects of prolotherapy in knee OA but the findings have not been synthesized28,29,30,31. Therefore, we conducted a systematic review with the aim of more comprehensively assessing the efficacy of prolotherapy for knee OA in order to clarify its potential role as a non-surgical treatment modality.
Methods
We follow the PRISMA Reporting Guidelines for Systematic Review and Meta-analysis32:
Search methods for identification of studies
Potential studies were identified by searching the electronic databases listed below, with search period starting from their inception till September 2015 (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL)
OVID MEDLINE
EMBASE
Global Health,
NHS Health Technology Assessment Database,
Digital Dissertation Consortium,
International Pharmaceutical Abstract
BIOSIS Preview.
AMED
Inspec
Ovid Nursing Database
CinicalTrial.gov (http://clinicaltrials.gov/),
Drugs@FDA (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm),
European Medicines Agency public assessment reports (EPAR, http://www.ema.europa.eu/ema),
Pharmaceuticals and medical devices agency of Japan (http://www.pmda.go.jp/english/service/approved.html).
Types of studies
This systematic review included randomized controlled trials (RCTs) and quasi-randomized controlled trials that compared prolotherapy injections to saline injections, water injections or exercise therapy. Co-interventions were allowed, as long as they were uniform across all groups. There were no limitations on publication dates. The protocol was registered at PROSPERO (CRD42015015901).
Types of participants
We selected studies that included participants aged 18 years or over with (i) a diagnosis of knee OA according to the criteria from the American College of Rheumatology33, (ii) knee pain for at least 3 months and (iii) had reported the Western Ontario and McMaster Universities Arthritis Index (WOMAC)34 or the Visual Analogue Scales (VAS) for pain as one of the outcomes35. We excluded studies that included participants who had undergone total knee replacement.
Types of intervention
For inclusion, dextrose prolotherapy injections had to be administered to at least one group within the trial. For comparison groups we included studies that provided injections with 0.9% normal saline or water; or exercise therapy. Consistent with the clinical practice of prolotherapy, at least part of the injection protocol had to include an intra-articular injection, with or without additional injections to the peri-articular ligament or tendon attachments.
Outcome measures
In accordance with international consensus on the core set of outcome measures for phase III clinical trials in OA, eligible trials needed to include assessment of either self- reported pain or self-reported physical function36. Following this recommendation, the primary outcome of interest is the Western Ontario and McMaster Universities Arthritis Index (WOMAC)37. Secondary outcomes of pain include the Visual Analogue Scales (VAS) for pain35, Knee Pain Scale (KPS)38 and Wong-Baker Scale39.
Eligibility Assessment and Data Extraction
Two reviewers (RS and VC) independently screened electronically retrieved titles and abstracts, evaluated potentially relevant full texts, and determined study eligibility. For eligible studies, data were extracted independently by the two authors (RS and DC) using a piloted data extraction form. For each eligible study, the following data were extracted: study design, clinical settings, participant characteristics, features of interventions, outcomes, duration of follow up and adverse events. An attempt was made to contact study authors regarding these methodological elements if not reported.
Risk of bias assessment
The risk of bias among included studies was assessed by the Cochrane’s risk of bias tool40 by two reviewers independently (XYW and RH). The following risk of bias domains were evaluated: sequence generation, allocation concealment, blinding of participants and research personnel, blinding of outcome assessors, incomplete outcome data, and selective outcome reporting. Discrepancies in study selection, data extraction and risk of bias assessment results were resolved by group consensus. In the group consensus process, the two reviewers discussed reasons for discrepancy with a goal to achieve consensus after clarification. If a consensus could not be reached, a third reviewer Vincent Chung (VC) was included as an arbitrator. A decision was made after reevaluation of the included studies and further discussion between the three reviewers. This has been clarified in the revised manuscript.
Statistical Analysis
All meta-analyses were conducted using the STATA software41. A random effect model was used to pool study results, taking into account possible variations in effect sizes across trials42. Changes in continuous outcomes were pooled as standardized mean differences (SMD), as different scaling of outcome measurements across trials were expected. 95% confidence intervals (CI) were calculated for all estimates. I square (I2) statistic was calculated to estimate heterogeneity across studies. An I2 level of less than <25%, 25–50% and greater than 50% were regarded as indicators of low, moderate and high levels of heterogeneity respectively43.
Minimal Clinically Important Difference (MCID) for WOMAC and VAS
The effect estimates were interpreted according to the values of the established Minimal Clinically Important Difference (MCID). It has been reported that a 12-point increment on WOMAC composite scale indicates ‘good to very good’ improvement when the scale is transformed onto a 0–100 point scale44,45.
The MCID for VAS global pain assessment score and VAS pain on motion were based on a study in 2005; of which the MCID for improvement in the global VAS is 18.3 mm whereas that for VAS in motion is 19.9 mm44.
Results
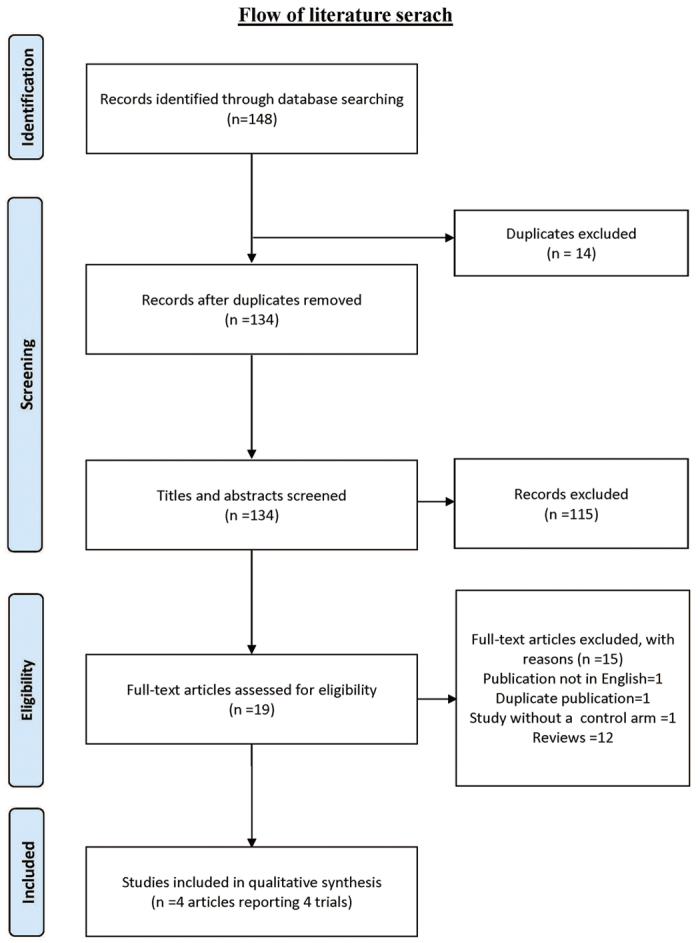
We identified 134 citations from all searches and excluded 14 duplicates. After screening the titles and abstracts, we retrieved 19 full texts for further assessment. Of these, 15 were excluded for the following reasons: duplicate publication as conference abstract (n = 1), publication not in English (n = 1), trial without a control arm (n = 1) and narrative reviews (n = 12). Four full texts which reported results from four clinical trials were eligible for inclusion28,29,30,31.(Fig. 1)
Figure 1. Flow of literature search.
Characteristics of included trials
Characteristics of included trials are summarized (Table 1). The four included clinical trials28,29,30,31 recruited a total of 258 patients with a diagnosis of knee OA based on American College of Rheumatology (ACR) guidelines including baseline radiological severity as graded by a Kellgren-Lawrence (K-L) score (Table 2)46. The follow up period ranged from five weeks to one year post-enrollment. The injection protocols varied; two studies referred to an existing published injection protocol47.
Table 1. Characteristics of included studies.
| Source & no. of arms | Intervention | Injection route and solution concentration (%) | Total no of participants | Number of case analyzed | Age +/− standard deviation | Sex Female (%) | Weight +/− standard deviation | Body mass index (BMI) | No of injections received +/−standard deviation | Outcomes assessed | Trial duration (Time for end points assessment) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rabago30 (2 arms) | Dextrose OR Dextrose plus Morrhuate | Intra-articular dextrose 25%, Extra-articular dextrose 15%, Sodium Morrhuate 5% | 27 | 24 | 54.5+/−6.8 | 54.2 | Not reported | 25% of the subjects with BMI ≦25 37.5% of the subjects with BMI 26–30 37.5% of the subjects with BMI ≧31 | 4.5+/−0.9 | 52 weeks | |
| WOMAC composite -WOMAC-pain -WOMAC-stiff -WOMAC-function | (week 5,9,12,24,52) | ||||||||||
| Saline OR Exercise | Intra-articular and extra-articular saline 0.9% | 18 | 13 | 57.4+/−7.6 | 61.5 | Not reported | 30.8% of the subjects with BMI ≦25, 30.8% of the subjects with BMI 26–30, 38.5% of the subjects with BMI ≧31 | 4.3+/−0.8 | Cartilage volume | (at week 52 only) | |
| Rabago22 (3 arms) | Dextrose | Intra-articular dextrose 25%, Extra-articular dextrose 15%, | 33 | 30 | 56.8+/−7.9 | 63 | Not reported | 33% of the subjects with BMI ≦25, 20% of the subjects with BMI 25–30, 47% of the subjects with BMI, ≧30 | 3.95+/−1 | WOMAC composite WOMAC-pain WOMAC-stiff WOMAC-function KPS (each knee) | 52 weeks (week 5,9,12,24,52) |
| Saline | Intra-articular and extra-articular saline 0.9% | 31 | 29 | 56.8+/−6.7 | 69 | Not reported | 28% of the subjects with BMI ≦25, 38% of the subjects with BMI 25–30, 34% with BMI ≧30 | 3.71+/−1.1 | |||
| Exercise | N/A | 33 | 31 | 56.4+/−7.0 | 68 | Not reported | 19% of the subjects with BMI ≦25, 39% of the subjects with BMI 25–30, 42% of the subjects with BMI ≧30 | N/A | |||
| Dumais et al.28 (2 arms with cross over) | Dextrose | Intra-articular dextrose 20%, Extra-articular dextrose 15% | 21 | 18 | 57.3+/−12.6 | 38.9 | 90.1 (22) | 32.2+/−7.2 | 3.7+/−0.7 | WOMAC composite -WOMAC-pain -WOMAC-stiff -WOMAC-function Pain intensity | 32 weeks (data collected at week 16 before cross- over; then reassessed at week 32) |
| Exercise | N/A | 24 | 18 | 56.2+/−10.9 | 55.6 | 92.4 (17.2) | 34.3+/−5.7 | N/A | Functional impairment Wong-Baker Descriptive Numerical VAS Combined pain score Timed UGT | ||
| Reeves et al.21 (2 arms) | Dextrose | Intra-articular dextrose10% | 36 | 35 | 60.0+/−12.5 | 51.4 | 86.6 (19.2) | Not reported | 3 | VAS- rest VAS- walking VAS-stair use Swelling Buckling/2 mon Flexion range | 24 weeks (assessed at week 24 only) |
| Water | Intra-articular bacteriostatic water | 35 | 32 | 65.3+/−11.0 | 34.3 | 90.5(19.1) | Not reported | 3 |
KEY:
BMI = Body Mass Index.
VAS = Visual Analogue Scale.
UGT = Up and Go Test.
WOMAC = Western Ontario and McMaster Universities Arthritis Index.
KPS = Knee Pain Scale.
Table 2. Distribution of OA knee severity grading among participants of included trials.
| Study | Interventions and controls | Kellgren Lawerence Grade 2 or above | |
|---|---|---|---|
| Reeves 2010 | Dextrose | 36 | |
| Normal saline | 25 | ||
| Kellgren-Lawerenc Grade 1 or 2(mild) | Kellgren-Lawerence grade 3 or 4 (moderate to severe) | ||
| Dumais28 | Dextrose | 3 | 15 |
| Exercise | 2 | 16 | |
| Rabago22 | Dextrose | 11 | 14 |
| Normal Saline | 12 | 9 | |
| Exercise | 9 | 14 | |
| Rabago30 | Dextrose (Dextrose alone or dextrose + morrhuate) & Control (normal saline or exercise) | 157 | 96 |
Risk of bias
Risk of bias amongst included studies was moderate overall (Table 3). None provided a detailed publicly accessible protocol of study methods including planned a priori statistical analysis methods (e.g. study protocols on trial registration websites such as ClinicalTrials.gov) that allowed assessment of selective outcome reporting, but three of them have provided a detailed analysis plan via personal communication29,30,31. Sequence generation was considered to have a low risk of bias in only two studies, and two had a low risk of bias in allocation concealment. Two studies blinded both patients and investigators, but two did not. Two studies reported blinding of assessors but not the remaining two. Risk of bias incurred from incomplete outcome data varied, with drop-out rates ranged from 0% to 27.8%. Since three out of four trials did not provide trial protocol, this can be regarded as a major source of bias.
Table 3. Risk of bias assessment.
| Source | Sequence generation | Allocation concealment | Blinding of participants and researchers | Blinding of outcome assessment | Incomplete outcome data addressed | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Reeves21 | Low. Random sequence was generated by random number table. | Unclear. Relevant information was not reported. | Low. Identical control solution was used | Low. Films were separated in different packets so reading 1 film would not influence reading of the next. X ray films were read by the chief investigator. A database coordinator loaded results onto the database. | High. Lost to follow up was 9/77 = 11.7% > 10% | Unclear. No protocol was provided. |
| Dumais28 | Unclear. Authors did not provide information on how random sequence was generated. | Low. Opaque sealed envelopes were used. | High. It was an open-labeled trial. | High. It was an open-labeled trial. | High. Lost to follow up was 3/21 = 14.3% in group A. 6/24 = 25% in group B. Both groups >10% | Unclear. No protocol was provided. |
| Rabago22 | Low. Random sequence was generated by computer. | Low. Allocation concealment of injectant was achieved through the use of sealed opaque envelopes (personal communication, Rabago 2015) | Low. Both active and control solutions looked similar. | Low. The injector, outcome assessor, principal investigator, and participants were blinded to injection group by preparation of syringes off site; blinding was formally assessed among injection participants and injector using questionnaire. | Low. No lost to follow-up cases. | Low. No protocol was provided,but analysis plan was clearly described. |
| Rabago30 | High. Non-randomized study design. | High. Non-randomized study design. | High. Single-arm uncontrolled study/at-home exercise “control” was used. | High. Primary outcome was self-reported QOL. | High. Lost to follow up was 3/27 = 11.1% in intervention group. 5/18 = 27.8% in control group. Both >10% | Unclear. No protocol was provided. |
Effect of interventions
The effect of interventions with different comparison groups and follow up duration is summarized in Table 4. Dumais et al.28 used WOMAC version constructed on a five point (0–4) Likert scale: composite (0–96), pain (0–20), stiffness (0–8) and function (0–68). Lower score indicated better knee related outcomes. Rabago et al.30,31 used a WOMAC version constructed on the same five point (0–4 point scale) Likert scale. Each of the individual subscales was then converted to a 0–100 score scale, with higher score reflecting improvement.
Table 4. Effectiveness of prolotherapy injection by comparison type for WOMAC composite, WOMAC pain, WOMAC Function scores and pain intensity.
|
Outcome: WOMAC Composite at 12–16 week |
||||||||
|---|---|---|---|---|---|---|---|---|
| Comparison | n | Scale Range ^ | Pre-treatment mean score | Standard deviation | Post-treatment adjusted mean change | Standard error | Duration of follow up | |
| Dumais28 | Dextrose | 18 | 0–96 | 44.4 | 13.7 | −21.8 € | 2.95 | 16 week |
| Exercise | 18 | 0–96 | 36.2 | 16.8 | −6.1 € | 3.28 | ||
| Rabago22 | Dextrose | 30 | 0–100 | 63.1 | 15.0 | 13.31 | 3.32 | 12 week |
| Normal saline | 29 | 0–100 | 62.7 | 14.3 | 8.19 | 3.37 | ||
| Exercise | 31 | 0–100 | 60.5 | 11.3 | 4.26 | 3.36 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 59.9 | 12.2 | 14.2 | 2.7 | 12 week |
| Control (normal saline or exercise) | 13 | 0–100 | 67.0 | 10.8 | 7.0 | 3.4 | ||
| Outcome: WOMAC Pain at 12–16 week | ||||||||
| Dumais28 | Dextrose | 18 | 0–20 | 9.5 | 2.9 | −5.0 € | 0.78 | 16 week |
| Exercise | 18 | 0–20 | 8.7 | 4.0 | −1.9 € | 0.73 | ||
| Rabago22 | Dextrose | 30 | 0–100 | 66.8 | 14.9 | 11.78 | 3.62 | 12 week |
| Normal saline | 29 | 0–100 | 66.7 | 16.1 | 5.79 | 3.67 | ||
| Exercise | 31 | 0–100 | 63.2 | 13.1 | 4.89 | 3.66 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 62.3 | 12.9 | 15.4 | 3.0 | 12 week |
| Control (normal saline or exercise) | 13 | 0–100 | 71.9 | 13.5 | 3.5 | 3.9 | ||
| Outcome: WOMAC Function at 12–16 week | ||||||||
| Dumais28 | Dextrose | 18 | 0–68 | 33.6 | 10.7 | −14.6 € | 2.14 | 16 week |
| Exercise | 18 | 0–68 | 26.8 | 12.8 | −3.6 € | 2.52 | ||
| Rabago22 | Dextrose | 30 | 0–100 | 65.2 | 15.8 | 14.61 | 3.40 | 12 week |
| Normal saline | 29 | 0–100 | 67.6 | 17.5 | 6.63 | 3.44 | ||
| Exercise | 31 | 0–100 | 61.9 | 12.7 | 4.89 | 3.43 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 62.6 | 12.9 | 14.3 | 2.8 | 12 week |
| Control (normal saline or exercise) | 13 | 0–100 | 68.5 | 11.3 | 7.8 | 2.8 | ||
| Outcome: pain intensity at 16–24 week (measured on a Visual Analogue scale VAS of 0–100 mm) | ||||||||
| Dumais28 # | Dextrose | 18 | 0–100 | 48.6 | 21.8 | −29.70 € | 4.57 | 16 week |
| Exercise | 18 | 0–100 | 38.3 | 24.8 | −9.92 € | 4.58 | ||
| Reeves 2002$ | Dextrose | 36 | 0–100 | 21.5 | 22.4 | −5.4 | 2.4 | 24 week |
| Water | 35 | 0–100 | 27.3 | 20.2 | −10.4 | 2.5 | ||
| Reeves 2002£ | Dextrose | 36 | 0–100 | 39.4 | 28.2 | −13.9 | 3.1 | |
| Water | 35 | 0–100 | 38.3 | 22.0 | −9.80 | 3.2 | ||
| Reeve 2002& | Dextrose | 36 | 0–100 | 53.3 | 28.0 | −13.7 | 3.2 | |
| Water | 35 | 0–100 | 58.3 | 26.0 | −12.3 | 3.2 | ||
| Outcome: pain intensity at 16–24 week (Wong Baker/Knee pain scale) | ||||||||
| Dumais28 * | Dextrose | 18 | WBS 0–5 | 2.7 | 1.2 | −1.27 € | 0.33 | 16 week |
| Exercise | 18 | WBS 0–5 | 2.3 | 1.1 | −0.19 € | 0.26 | ||
| Rabago22 ¥ | Dextrose | 43 | KPS 0–5 | 1.8 | 0.8 | −0.92 | 0.25 | 24 week |
| Normal saline | 41 | KPS 0–5 | 1.7 | 0.7 | −0.26 | 0.25 | ||
| Exercise | 47 | KPS 0–5 | 1.7 | 0.8 | −0.33 | 0.24 | ||
| Outcome: WOMAC Composite at 52 week | ||||||||
| Rabago22 | Dextrose | 30 | 0–100 | 63.1 | 15.0 | 15.32 | 3.32 | |
| Normal saline | 29 | 0–100 | 62.7 | 14.3 | 7.59 | 3.36 | ||
| Exercise | 31 | 0–100 | 60.5 | 11.3 | 8.24 | 3.33 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 59.9 | 12.2 | 17.6 | 3.2 | |
| Control (normal saline or exercise) | 13 | 0–100 | 67.0 | 10.8 | 8.6 | 5.0 | ||
| Outcome WOMAC Pain at 52 week | ||||||||
| Rabago22 | Dextrose | 30 | 0–100 | 66.8 | 14.9 | 14.18 | 3.62 | |
| Normal saline | 29 | 0–100 | 66.7 | 16.1 | 7.38 | 3.67 | ||
| Exercise | 31 | 0–100 | 63.2 | 13.1 | 9.24 | 3.63 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 62.3 | 12.9 | 18.1 | 3.8 | |
| Control (normal saline or exercise) | 13 | 0–100 | 71.9 | 13.5 | 4.6 | 5.0 | ||
| Outcome WOMAC function at 52 week | ||||||||
| Rabago22 | Dextrose | 30 | 0–100 | 65.2 | 15.8 | 16.25 | 3.39 | |
| Normal saline | 29 | 0–100 | 67.6 | 17.5 | 5.46 | 3.44 | ||
| Exercise | 31 | 0–100 | 61.9 | 12.7 | 7.31 | 3.40 | ||
| Rabago30 | Dextrose (dextrose alone or dextrose + morrhuate) | 24 | 0–100 | 62.6 | 12.9 | 18.6 | 2.9 | |
| Control (normal saline or exercise) | 13 | 0–100 | 68.5 | 11.3 | 9.8 | 4.8 | ||
Keys:
#The VAS score in Dumais 2012 represents a global assessment of disease activity, with minus sign (−) indicating improvement.
$The VAS score in Reeves 2002 represents measurement of pain intensity at rest, with minus sign (−) indicating improvement.
£The VAS score in Reeves 2002 represents measurement of pain intensity with walking, with minus sign (−) indicating improvement.
&The VAS score in Reeves 2002 represents measurement of pain intensity with stair use, with minus sign (−) indicating improvement.
*Wong Baker = Wong Baker Faces Pain Rating scale from 0–5, with minus sign (−) indicating improvement.
¥The Knee pain scale assessed severity on a 0–5 ordinal scale on each individual knee, with minus sign (−) indicating improvement.
^Dumais adopted WOMAC version constructed on a five point Likert scale: composite (0–96), pain (0–20), stiffness (0–8) and function (0–68). Lower score indicates better knee related metrics, minus sign (−) indicates pain reduction.
^Rabago adopted WOMAC version constructed on a five point Likert scale: composite (0–96), pain (0–20), stiffness (0–8) and function (0–68); values were then converted to a 0–100 scale for each of the four domains. Composite WOMAC score reflect a weighted average. Higher score indicates better knee related metrics, of which a positive sign (+) indicates improvement.
€The values in standard deviation were converted to standard error.
Dextrose Injection versus Exercise on WOMAC index
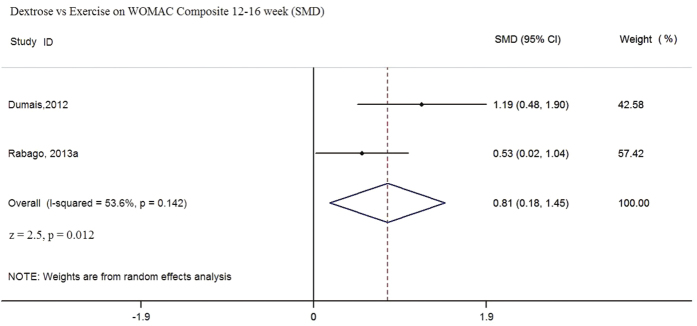
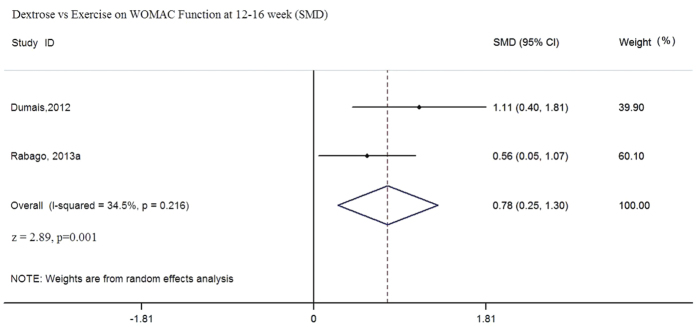
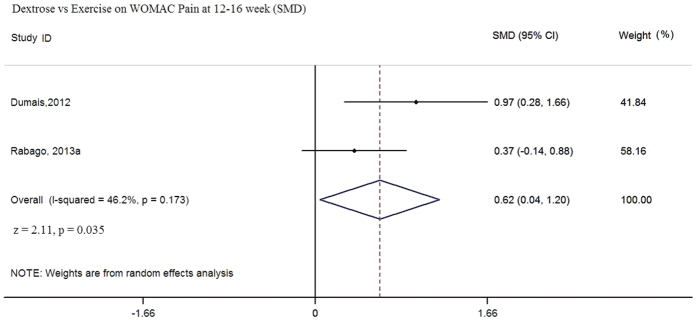
In this comparison, two RCTs (n = 97) were eligible for pooling using follow-up data from 12 to 16 weeks28,30. Although the same WOMAC tool was used, different measurement scales were adopted in these two trials; therefore, SMDs were calculated in the random effect meta-analyses. Pooled results favored prolotherapy in terms of WOMAC composite and WOMAC function scores (Figs 2 and 3). The pooled SMD of WOMAC composite score is 0.81 (95% CI: 0.18 to 1.45, p = 0.012, I2 = 53.6%); whereas the SMD of WOMAC-function score is 0.78 (95% CI: 0.25 to 1.30, p = 0.001, I2 = 34.5%). For WOMAC pain (Fig. 4), pooled results indicated that the SMD is 0.62 (95% CI: 0.04 to 1.20, p = 0.035, I2 = 46.2%). While the p value indicates statistically significant result as defined by p < 0.05, the lower end of the 95% CI is very close to zero, suggesting the imprecision of possible treatment effect. Overall, sizes of the SMD indicated that dextrose injection provides substantially stronger improvement when compared to exercise, but the lower end of 95% CI has covered SMD values of small effect sizes (i.e. SMD ≤ 0.2)48. Such uncertainty is also reflected in the low to moderate degree of heterogeneity among all three meta-analyses, given all with I2 values ranging from 34.5% to 53.6%49. Although it is not included in meta-analysis due to differences in data collection time, Rabago et al.22 also reported WOMAC outcomes at 26 and 52 week follow-up. Dextrose injections outperformed exercise on the composite and function-subscale score (26 and 52 weeks) and the pain-subscale score (26 weeks) respectively30.
Figure 2. Dextose vs Exercise on WOMAC Composite 12–16 week (SMD).
Figure 3. Dextrose vs Exercise on WOMAC Function at 12–16 week (SMD).
Figure 4. Dextrose vs Exercise on WOMAC Pain at 12–16 week (SMD).
Dextrose versus Saline Injection on WOMAC index at 12 weeks and 52 weeks
In two trials, dextrose injections, either on their own30 or mixed with sodium morrhuate31, were found to be superior to normal saline in improving WOMAC composite and subscale scores to levels above the minimal clinical important difference (MCID) at 12 and 52 weeks35. (Table 4)
Pain intensity on VAS at 16 and 24 weeks after prolotherapy
At 16 weeks, Dumais et al. reported an improvement in the VAS global pain score of 29.7 points (SE: 4.57), above the MCID at 24 weeks, Reeves et al. also reported improvement in VAS pain during walking of 13.9 (SE: 3.10) and during stair climb of 13.7 (SE 3.2)29, which were slightly lower than the relevant MCID44. Pooling of the two trials was not possible as the VAS measured slightly different outcomes; the former represented a global assessment of pain whereas the latter measured pain level during movement.
Change in pain severity at 16 weeks and 24 weeks after prolotherapy
At 16 week, Dumais et al. reported statistically significant improvement in Wong Baker Scale scores after prolotherapy (adjusted mean change: 1.27, SE: 0.33, p = 0.003)28. At 24 weeks, Rabago et al.22 also reported a statistically significant improvement in WOMAC pain scores (adjusted mean change 15.50 ± SE 3.56, p < 0.05) and on the KPS (adjusted mean change: 0.92, SE: 0, 25, p < 0.05). The pain reduction magnitude of active participants was significantly greater when compared with their control groups (p < 0.05)30.
Side effects
All four trials monitored side effects and adverse effects. Only one trial reported self-limited bruises after both dextrose (n = 3) and saline injections (n = 5)30. This was an expected side effect and deemed to be of minimal clinical relevance due to its transient nature
Discussion
Pooling data from two RCTs, we report that peri- and intra-articular hypertonic dextrose knee injections in three to five sessions have a statistically significant and clinically relevant effect in the improvement of WOMAC composite score, functional and pain subscale at 12 to 16 weeks compared to formal at-home exercise. Self-reported outcomes favoring prolotherapy are also observed in unpooled data when dextrose prolotherapy groups are compared with other control groups. The majority of the effect sizes are higher than the MCID, and the benefits were sustained up to 1 year. The risk of bias level in the included studies was moderate. Overall, prolotherapy injections appear to be safe, but no study was powered to detect rare adverse events.
While the overall direction of the effect is positive, some uncertainty of the effect size still exists due to the low to moderate heterogeneity and wide confidence intervals. In addition, this systematic review identified several limitations of existing trials. None of the included studies provided a publicly accessible protocol, therefore the potential for selective outcome reporting exists; however, the analyses are generally straightforward and well-reported. The sample size was small with only four trials eligible for review and only portions of two trials could be pooled in meta-analyses. Small sample size led to a wide confidence interval; thus the effect sizes reported may be imprecise. However, pooled results provide guidance for the sample size needed in future clinical studies50. Due to a lack of uniform longer-term follow up data across both studies, pooling of results could only be done with data collected between 12 to 16 week follow-up. Given that prolotherapy is hypothesized to work by healing and regeneration over several months51,52, the effects reported here may underestimate longer term benefits. A recent open label study reported progressive improvement in WOMAC scores for most study participants through 2.5 ± 0.6 years (range 1.6–3.5 years) of follow up after prolotherapy27. Finally, low to moderate quantified heterogeneity was reported in pooled analyses; this is likely attributable to multiple factors, including differences in patient characteristics, control treatment, study design, injection protocol methods, dextrose concentration, follow-up duration and outcomes assessment methods.
While the positive findings in the evaluated studies suggest efficacy, their limitations restrict a rigorous determination of efficacy and clinical utility. The current analysis suggests studies with the following characteristics can be considered in the future: 1) study duration of at least one year. Prolotherapy is hypothesized to be a regenerative injection therapy; serial monthly injections are typically performed to stimulate hypothesized tissue effects; 2) uniform self-reported outcomes and time points using the WOMAC outcome measures37; 3) objectively determined physical function and health status outcomes such as hospitalization, nursing home placement and decline in health53,54. Inclusion of objective assessment including magnetic resonance imaging31 and intra-articular and serum biomarkers55. 4) a priori subgroup analysis to identify the phenotype of patients who respond most favorably to prolotherapy;5) because the performance of prolotherapy appears to be superior to saline and exercise, future studies may consider formal effectiveness designs using exercise or other non-injection matched control comparators. This avoids injection-related discomfort and risk among controls, saves study conduct time and cost, and offers a comparison relevant to clinical practice; 6) overall quality of life and cost effectiveness evaluation using the EuroQol-5D, a commonly used measure that allows comparison to other interventions56; 7) determination of the MCID of WOMAC score specific to prolotherapy.
Investigation of prolotherapy is in an early stage and several treatment protocol issues warrant further study to determine optimal strategy, including dextrose concentration and volume, treatment frequency and duration, and specific utility of intra compared with extra-articular injections. Studies in this review used the approach of dextrose injections into the intra-articular joint space and the extra-articular soft tissue attachment. Recently, more superficial application of hypertonic dextrose has been reported to provide an analgesic effect in the treatment of a variety of peripheral neuropathic pain conditions57,58. Sensorineural effects of dextrose have been hypothesized31. Given that the etiology of knee OA pain is multifactorial, future trials may consider adding superficial dextrose injection as part of the protocol. Concomitant basic science studies using in vitro and animal model methodologies are warranted to better elucidate the mechanism of action of dextrose in the context of musculoskeletal pathology. Finally, studies in this review used palpation guidance for injections; future investigators should also consider the use of ultrasound guidance to improve injection accuracy and precision, limiting procedural variability59.
Strengths and weaknesses of this systematic review
The main limitation of this systematic review is the limited number of studies and their relatively small sample size. We considered whether or not to perform a meta-analysis in addition to systematic narrative review, given that the small sample sizes of included trials might generate an unstable pooled effect size60. A meta-analyses is included and resulted in increased statistical rigor by increasing the overall sample size, providing a more precise estimate61. In a narrative review, evaluation of efficacy in RCTs is based primarily on the p-values of individual studies, a so-called ‘vote counting’ approach, and the risk of arriving at a biased conclusion may even be higher62. Because most of the results in the included trials were positive, readers may in the case of narrative review be tempted to draw an over-confident conclusion63. A meta-analysis, with calculation of I2 value, is able to express current best evidence and heterogeneity among studies quantitatively64. Specifically, we determined that the I2 value of WOMAC composite score is 53.6%, indicating considerable heterogeneity on the effect estimate, a finding that can be communicated only through meta-analysis65.
Strengths include the conduct of a comprehensive literature search, duplicate study selection and data extraction by two independent reviewers, and appraising risk of bias of all included studies using a validated tool independently by two reviewers.
Conclusion
The results of this systematic review indicate that hypertonic dextrose prolotherapy conferred a positive, significant beneficial effect meeting criteria for clinical relevance in the treatment of knee OA, compared with saline injection and exercise. However, moderate heterogeneity existed among these trial results. Larger, long-term trials with uniform outcomes and high methodological standards are needed for more a more comprehensive assessment of the overall treatment effect of prolotherapy.
Additional Information
How to cite this article: Sit, R. W. S. et al. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: A systematic review and meta-analysis. Sci. Rep. 6, 25247; doi: 10.1038/srep25247 (2016).
Supplementary Material
Footnotes
Author Contributions R.W.S.S. is the first author of this article, responsible for the study design, review of literatures and completion of manuscript. V.C.H.C. is the corresponding author in this study; he provides methodology support and acts as another reviewer in literatures search K.D.R. and D.R. are the authors of the three papers involved in this systematic review; both of them provide valuable advice on the study design, data interpretation and discussion. K.K.W.C. provides expert opinions on the practical aspect of prolotherapy and S.Y.S.W. as a senior author to overlook the whole study. D.C.C.C., R.S.T.H. and X.W. provide full statistical support in this article.
References
- Brooks P. M. The burden of musculoskeletal disease–a global perspective. Clin. Rheumatol. 25(6), 778–781(2006). [DOI] [PubMed] [Google Scholar]
- Fransen M. et al. The epidemiology of osteoarthritis in asia. Int. J. Rheum. Dis. 14(2), 113–121 (2011). [DOI] [PubMed] [Google Scholar]
- Litwic A., Edwards M. H., Dennison E. M. & Cooper C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105, 185–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Hawker G. A., Laporte A., Croxford R. & Coyte P. C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 44(12), 1531–1537 (2005). [DOI] [PubMed] [Google Scholar]
- Cross M. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73(7), 1323–1330 (2014). [DOI] [PubMed] [Google Scholar]
- Hochberg M. C. et al. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit. Care Res. (Hoboken). 64(4), 465–474 (2012). [DOI] [PubMed] [Google Scholar]
- Bruyere O. et al. An algorithm recommendation for the management of knee osteoarthritis in europe and internationally: a report from a task force of the european society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Semin. Arthritis. Rheu. 44(3), 253–263 (2014). [DOI] [PubMed] [Google Scholar]
- McAlindon T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 22(3), 363–388 (2014). [DOI] [PubMed] [Google Scholar]
- The national academies of sciences, engineering, medicine. Initial national priorities for comparative effectiveness research. (2009) Available at: http://Www.iom.edu/reports/2009/ComparativeEffectivenessResearchPriorities.aspx. (Date of access: April 2014).
- Samson D. J. et al. Treatment of primary and secondary osteoarthritis of the knee. Evid. Rep. Technol. Assess (Full Rep.). 157, 1–157 (2007). [PMC free article] [PubMed] [Google Scholar]
- Rabago D., Best T. M., Beamsley M. & Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin. J. Sport Med. 15(5), 376–380 (2005). [DOI] [PubMed] [Google Scholar]
- Oh S. et al. Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: a potential model to study carpal tunnel syndrome? Hand (N. Y.). 3(1), 34–40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii Y. et al. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch. Phys. Med. Rehab. 90(2), 333–339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii Y. et al. Effects of hypertonic dextrose injections in the rabbit carpal tunnel. J. Orthop. Res. 29(7), 1022–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii Y. et al. Effects of multiple injections of hypertonic dextrose in the rabbit carpal tunnel: a potential model of carpal tunnel syndrome development. Hand (N.Y.). 9(1), 52–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. S. et al. Intra-articular injection of a nutritive mixture solution protects articular cartilage from osteoarthritic progression induced by anterior cruciate ligament transection in mature rabbits: a randomized controlled trial. Arthritis Res. Ther. 9(1), R8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A., Borg-Stein J. & Nguyen R. T. Regenerative injection therapy for osteoarthritis: fundamental concepts and evidence-based review. P.M. & R. 4(5, Supplement), S104–S109 (2012). [DOI] [PubMed] [Google Scholar]
- Rabago D., Slattengren A. & Zgierska A. Prolotherapy in primary care practice. Prim. Care. 37(1), 65–80 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel L. M. & Best T. M. Prolotherapy: A clinical review of its role in treating chronic musculoskeletal pain. P.M. & R. 3(6, Supplement), S78–S81 (2011). [DOI] [PubMed] [Google Scholar]
- Jahangiri A., Moghaddam F. R. & Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J. Orthop. Sci. 19(5), 737–743 (2014). [DOI] [PubMed] [Google Scholar]
- Reeves K. D. & Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J. Altern. Complement. Med. 6(4), 311–320 (2000). [DOI] [PubMed] [Google Scholar]
- Rabago D. et al. Hypertonic dextrose and morrhuate sodium injections (prolotherapy) for lateral epicondylosis (tennis elbow): results of a single-blind, pilot-level, randomized controlled trial. Am. J. Phys. Med. Rehabil. 92(7), 587–596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol G. A. & Reeves K. D. Regenerative injection of elite athletes with career-altering chronic groin pain who fail conservative treatment: a consecutive case series. Am. J. Phys. Med. Rehabil. 87(11), 890–902 (2008). [DOI] [PubMed] [Google Scholar]
- Kim W. M., Lee H. G., Jeong C. W., Kim C. M. & Yoon M. H. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J. Altern. Complement Med. 16(12), 1285–1290 (2010). [DOI] [PubMed] [Google Scholar]
- Dagenais S., Yelland M. J., Del Mar C. & Schoene M. L. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst. Rev. 2(2), CD004059 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D. et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br. J. Sports Med. 43(7), 471–481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D., Mundt M., Zgierska A. & Grettie J. Hypertonic dextrose injection (prolotherapy) for knee osteoarthritis: long term outcomes. Complement Ther. Med. 23(3), 388–395 (2015). [DOI] [PubMed] [Google Scholar]
- Dumais R. et al. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: a randomized crossover study. Pain Med. 13(8), 990–999 (2012). [DOI] [PubMed] [Google Scholar]
- Reeves K. D. & Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern. Ther. Health Med. 6(2), 68–74 (2000). [PubMed] [Google Scholar]
- Rabago D. et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann. Fam. Med. 11(3), 229–237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D. et al. Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch. Phys. Med. Rehabil. 94(11), 2075–2082 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8(5), 336–341(2010). [DOI] [PubMed] [Google Scholar]
- Altman R. D. Criteria for classification of clinical osteoarthritis. J. Rheumatol. Suppl. 27, 10–12 (1991). [PubMed] [Google Scholar]
- Roos E. M., Klassbo M. & Lohmander L. S. WOMAC osteoarthritis index. reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. western ontario and MacMaster universities. Scand. J. Rheumatol. 28(4), 210–215 (1999). [DOI] [PubMed] [Google Scholar]
- Tubach F. et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann. Rheum. Dis. 64(1), 29–33 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N. et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. consensus development at OMERACT III. J. Rheumatol. 24(4), 799–802 (1997). [PubMed] [Google Scholar]
- Bellamy N., Buchanan W. W., Goldsmith C. H., Campbell J. & Stitt L. W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 15(12), 1833–1840 (1988). [PubMed] [Google Scholar]
- Rejeski W. J. et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J. Rheumatol. 22(6), 1124–1129 (1995). [PubMed] [Google Scholar]
- Wong-Baker Faces. Available at: http://wongbakerfaces.org/. (Date of access: April 2014 (2009)).
- Higgins J. P. et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. B.M.J. 343, 5928 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. C., Schmid C. H., Lau. J. & Trikalinos T. A. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. B.M.C. Med. Res. Methodol. 9(1), 80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T. & Rothstein H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 1(2), 97–111 (2010). [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. P. T. & Altman D. G. Heterogeneity in Cochrane handbook for systematic reviews of interventions. (eds Higgins J. P. T. & Green S.) Ch.9.5, 276–282 (Wiley-Blackwell, 2008). [Google Scholar]
- Tubach F., Wells G. A., Ravaud P. & Dougados M. Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: methodological issues. J. Rheumatol. 32(10), 2025–2029 (2005). [PubMed] [Google Scholar]
- Ehrich E. W. et al. Minimal perceptible clinical improvement with the western ontario and McMaster universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J. Rheumatol. 27(11), 2635–2641 (2000). [PubMed] [Google Scholar]
- Brandt K. D., Fife R. S., Braunstein E. M. & Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the kellgren and lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 34(11), 1381–1386 (1991). [DOI] [PubMed] [Google Scholar]
- Rabago D. et al. Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern. Complement Med. 18(4), 408–414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S. V. Interpreting estimates of treatment effects: implications for managed care. P. & T. 33(12), 700 (2008). [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T. & Rothstein H. R. Identifying and quantifying heterogeneity in Introduction to meta-analysis. Ch. 16, 107–125. (John Wiley & Sons, Ltd, 2009). [Google Scholar]
- Noordzij M. et al. Sample size calculations: basic principles and common pitfalls. Nephrol. Dial. Transplant. 25(5), 1388–1393 (2010). [DOI] [PubMed] [Google Scholar]
- Banks A. A rationale for prolotherpay. J. Orthop. Med. 13(3), 54–59 (1991). [Google Scholar]
- Vora A., Borg-Stein J. & Nguyen. R. T. Regenerative injection therapy for osteoarthritis: fundamental concepts and evidence-based review. P.M. & R. 4(5, Supplement), S104–S109 (2012). [DOI] [PubMed] [Google Scholar]
- Dobson F. et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthr. Cartil. 21(8), 1042–1052 (2013). [DOI] [PubMed] [Google Scholar]
- Hoogeboom T. J. et al. Joint-pain comorbidity, health status, and medication use in hip and knee osteoarthritis: a cross-sectional study. Arthrit. Care Res. 64(1), 54–58 (2012). [DOI] [PubMed] [Google Scholar]
- Hosnijeh F. S., Runhaar J., van Meurs J. B. & Bierma-Zeinstra S. M. Biomarkers for osteoarthritis: can they be used for risk assessment? a systematic review. Maturitas. 82(1), 36–49 (2015). [DOI] [PubMed] [Google Scholar]
- Xie F. et al. Use of a disease-specific instrument in economic evaluations: mapping WOMAC onto the EQ-5D utility index. Value Health. 13(8), 873–878 (2010). [DOI] [PubMed] [Google Scholar]
- Jancso G. Neurogenic mechanisms in arthritis in Neurogenic inflammation in health and disease (eds Donaldson L. F.) Ch.4, 211–241 (Elsevier, 2009). [Google Scholar]
- Lyftogt J. Subcutaneous prolotherapy treatment of refractory knee, shoulder and lateral elbow pain. Australas Musculoskelet. Med. 12(2), 110–112 (2007). [Google Scholar]
- Qvistgaard E. et al. Guidance by ultrasound of intra-articular injections in the knee and hip joints. Osteoarthr. Cartil. 9(6), 512–517 (2001). [DOI] [PubMed] [Google Scholar]
- Guyatt G. H. et al. GRADE guidelines 6. rating the quality of evidence–imprecision. J. Clin. Epidemiol. 64(12), 1283–1293 (2011). [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. P. T. & Altman D. G. Analysing data and undertaking meta-analyses in Cochrane handbook for systematic reviews of interventions. (eds Higgins J. P. T. & Green S.) Ch.9, 243–296 (Wiley-Blackwell, 2008). [Google Scholar]
- Ioannidis J. P., Patsopoulos N. A. & Rothstein H. R. Reasons or excuses for avoiding meta-analysis in forest plots. B.M.J. 336(7658), 1413–1415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins P. T. & Rothstein H. R. Vote Counting–A new name for an old problem in Introduction to meta-analysis. 251–255 (John Wiley & Sons, 2009). [Google Scholar]
- Borenstein M., Hedges L. V., Higgins P. T. & Rothstein H. R. Criticisms of meta-analysis in Introduction to meta-analysis.377–387 (John Wiley & Sons, 2009). [Google Scholar]
- Deeks J. J., Higgins J. P. T. & Altman D. G. Identifying and measuring heterogeneity in Cochrane handbook for systematic reviews of interventions. (eds Higgins J. P. T. & Green S.) Ch. 9.5.2, 277–278 (Wiley-Blackwell, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.