Abstract
The diagnostic and prognostic criteria of acute-on-chronic liver failure (ACLF) were developed in patients with no Hepatitis B virus (HBV) cirrhosis (CANONIC study). The aims of this study were to evaluate whether the diagnostic (CLIF-C organ failure score; CLIF-C OFs) criteria can be used to classify patients; and the prognostic score (CLIF-C ACLF score) could be used to provide prognostic information in HBV cirrhotic patients with ACLF. 890 HBV associated cirrhotic patients with acute decompensation (AD) were enrolled. Using the CLIF-C OFs, 33.7% (300 patients) were diagnosed as ACLF. ACLF was more common in the younger patients and in those with no previous history of decompensation. The most common organ failures were ‘hepatic’ and ‘coagulation’. As in the CANONIC study, 90-day mortality was extremely low in the non-ACLF patients compared with ACLF patients (4.6% vs 50%, p < 0.0001). ACLF grade and white cell count, were independent predictors of mortality. CLIF-C ACLFs accurately predicted short-term mortality, significantly better than the MELDs and a disease specific score generated for the HBV patients. Current study indicates that ACLF is a clinically and pathophysiology distinct even in HBV patients. Consequently, diagnostic criteria, prognostic scores and probably the management of ACLF should base on similar principles.
Acute on chronic liver failure (ACLF) is a dynamic syndrome observed in the hospitalized cirrhotic patients either with or without an identified precipitating factor characterized by hepatic and or extrahepatic organ failures and high mortality1. The elements of the above working definition was proposed by a working group under the auspices of the European and the America association for the study of liver (European Association for the Study of the Liver/the America association for the study of liver Disease, EASL/AASLD) in 20102. Since then, large prospective studies were performed by the EASL chronic liver failure (CLIF) Consortium, the data from which suggested that the diagnosis of ACLF can be accurately made using the CLIF-Consortium Organ Failure score (CLIF-C OFs; CLIF Acute-on Chronic Liver Failure in cirrhosis (CANONIC) study) and its prognosis determined using the CLIF-Consortium acute-on chronic liver failure score (CLIF-C ACLFs)3,4. In patients with acute decompensation4 (AD, refers to the acute development within 1-month before admission of ascites, variceal hemorrhage, hepatic encephalopathy and/or bacterial infections) who did not fulfill criteria for the diagnosis of ACLF, the CLIF acute decompensation score (CLIF-Consortium acute decompensation score, CLIF-C ADs) was validated to provide prognostic information5. The North American Consortium for the Study of End Stage Liver Disease (NACSELD), made similar observations confirming high mortality rates of patients with organ failures in the hospitalized cirrhotic patients with bacterial infection6.
One of the limitations of the CLIF consortium classification is that it has only been validated in cohorts of patients from Europe and the patient populations studied had an extremely low prevalence of HBV infection, which is the commonest cause of chronic liver disease in the Eastern Pacific rim. Given that infection with HBV may be associated with disease flares, which can lead to rapid deterioration in liver function despite introduction of effective antiviral therapy, it is unclear whether criteria developed for non-HBV induced ACLF can also be applied to patients with HBV related ACLF. A Consensus definition has of necessity been developed under the auspices of the Asia Pacific association for the Study of liver disease for the definition of ACLF, which is however, based on expert opinion, rather than prospectively validated data. The definition proposed by the Asia-Pacific association for the study of liver Disease (APASL) was “acute hepatic insult manifesting as jaundice and coagulopathy, complicated within four weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease7”. As is self evident, the proposed definitions are very different8. Therefore, a World Consensus on the definition of ACLF was organized under the auspices of the World Congress of Gastroenterology, which amongst other recommendations concluded that the CLIF criteria should be validated in an Asian population that included patients with HBV infection9.
Therefore, the aims of this study was to evaluate whether the CLIF-C OF score can be used to classify patients presenting with HBV infection and AD into an ACLF and no ACLF groups with distinct clinical characteristics and outcomes. We then determined whether the CLIF-C ACLF and the CLIF-C AD scores provide prognostic information in these two patient groups in comparison with standard prognostic scores. The study also aimed to develop a HBV specific score and compare its performance with the CLIF scores. In order to achieve these aims, the EASL CLIF Consortium set up collaboration with Ren Ji Hospital, Shanghai, China and all the data were analyzed by the Data Management Centre of the EASL CLIF Consortium in a manner similar to that performed for the CANONIC study4 to allow direct comparison.
Results
Clinical characteristics of the patients at the time of hospital admission
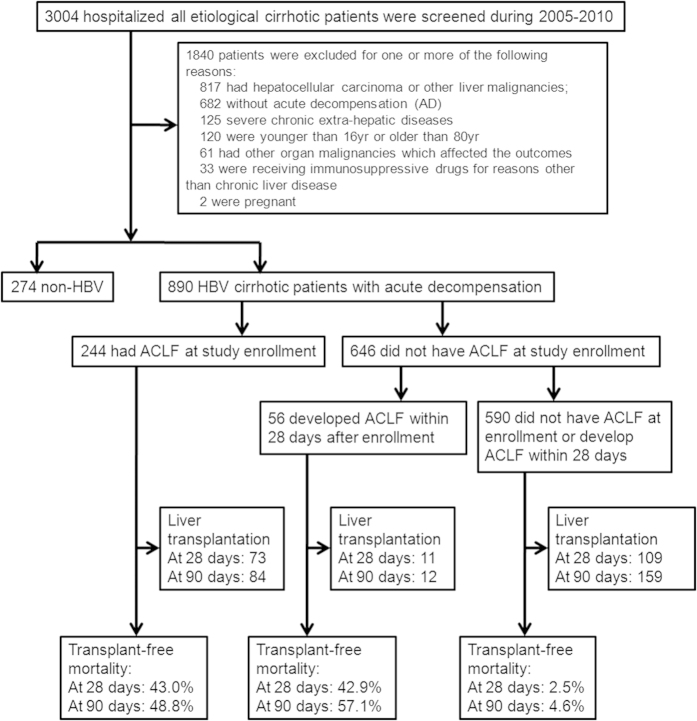
The flow chart for both screening and enrollment of patients was presented at Fig. 1. There were 3004 hospitalized cirrhotic patients screened in a single tertiary hospital (Ren Ji Hospital) in Shanghai from January 2005 to December 2010 and 890 HBV associated cirrhotic patients with AD were enrolled. Clinical characteristics of enrolled patients are summarized in Supplementary table S1. The mean age in the current series was 50 years and most patients (76.7%) were male. Seven-hundred and thirty-three patients (82.4%) presented significant HBV replication (HBV-DNA > 100 IU/ml) in concordance to the low percentage of patients (23.5%) treated with nucleotide analogs (NUCs) within 6 months prior hospitalization. Most patients had received lamivudine and none had been treated with tenofovir (a NUC with a high barrier to resistance). In 274 patients, NUCs were started after hospital admission with AD. Prior history of AD was present in only 55.1% of patients. In the vast majority of patients (90.7%) no other cause for cirrhosis other than chronic HBV was found. In the remainder patients HBV infection was associated with alcoholism (6.2%), schistosomiasis (1.2%) and other hepatic viruses (1.9%; HCV 0.8%, HEV 0.8% and CMV 0.7%).
Figure 1. Screening, enrollment, and flow of patients according to the presence or absence of ACLF according to CLIF-C OF score.
The main indication for hospital admission was ascites (56.9%) followed by GI-bleeding (26.5%), hepatic encephalopathy (11.8%) and bacterial infections (4.4%). Potential precipitating events of acute decompensation were present in 54.8% (488/890) patients. The most frequent precipitating event (PE) was bacterial infection (10.6%), followed by GI-bleeding and active alcoholism (7.5% and 7.2%, respectively). HBV reactivation was observed in only 41 patients (4.6%). In 7 patients, this was related to sudden cessation of anti-viral therapy. No cases of de novo reactivation were observed. Only 99 patients (11.1%) presented more than one PE.
Three hundred and eighty patients (42.7%) presented at least one OF. The most frequent OF was hepatic failure, which was present in 30.1% of the patients followed by coagulation failure (26.1%), renal failure (10.0%), cerebral failure (5.2%), circulatory failure (2.4%) and respiratory failure (1.2%). Renal and cerebral dysfunctions were present in 5.3% and 8.3% of patients, respectively. Mean model for end-stage liver disease (MELD) and MELD-Sodium (MELD-Na) scores were 20.2 and 22.6, respectively.
Two hundred and forty-three patients (27.3%) presented with ACLF at admission: 45 patients (5.1%) had ACLF-1, 132 (14.8%) ACLF-2 and 66 (7.4%) ACLF-3.
Clinical characteristics of patients with ACLF at admission or developing it during hospitalization
In addition to the 243 patients presenting with ACLF at admission, 57 additional patients (6.3%) developed ACLF during hospitalization. Three hundred patients, therefore, had ACLF during the study period, which represents a prevalence of 33.7%. Fifty-five patients (6.2%) had ACLF-1, 147 (16.5%) ACLF-2 and 98 (11.0%) had ACLF-3.
Table 1 shows the characteristics of patients with (300) and without (590) ACLF during the study period. Data in patients with ACLF were obtained at the time of ACLF diagnosis (at admission or during hospitalization). The most common OF in patients with ACLF was liver failure (77.7%), followed by coagulation failure (67.7%), renal failure (28.3%), cerebral failure (23.7%), circulatory failure (19%) and respiratory failure (14.3%). The prevalence rates of renal and cerebral dysfunction in patients with ACLF were 14.6% and 18.0%, respectively. The prevalence of single non-renal organ failure in patients without ACLF was extremely low; liver failure in 7.8%, coagulation failure in 9.8%, cerebral failure in 0.5% and circulatory failure in 0.3%. Renal failure by definition is ACLF and no patient without ACLF showed respiratory failure. Renal and/or cerebral dysfunctions were also very infrequent in patients without ACLF (0.4% and 2.9%, respectively).
Table 1. Characteristics and mortality of patients with and without ACLF during the study period (admission plus hospitalization).
| Characteristics | No ACLF (N = 590) | ACLF (N = 300) | p-value | ACLF grade I (N = 55) | ACLF grade II (N = 147) | ACLF grade III (N = 98) | p-value |
|---|---|---|---|---|---|---|---|
| Age (y) | 51.8 ± 10.7 | 46.5 ± 11.3 | <0.001 | 49.6 ± 11.3 | 44.8 ± 11.2 | 47.1 ± 11.0 | <0.001 |
| Male sex | 450(76.3) | 233(77.7) | 0.641 | 41(74.6) | 119(81.0) | 73(74.5) | 0.581 |
| Ascites | 482(81.7) | 229(76.6) | 0.072 | 42(76.4) | 115(78.8) | 72(73.5) | 0.235 |
| Mean arterial pressure (mm Hg) | 87 ± 11 | 84 ± 18 | 0.011 | 87 ± 18 | 87 ± 15 | 78 ± 21 | <0.001 |
| Cause of cirrhosis | 0.002 | <0.001 | |||||
| HBV alone | 544(92.3) | 263(87.7) | 47(85.6) | 130(88.5) | 86(87.7) | ||
| HBV + Alcohol | 36(6.1) | 19(6.3) | 4(7.2) | 7(4.7) | 8(8.2) | ||
| HBV + other hepatitis virus | 5(0.8) | 12(4.0) | 2(3.6) | 9(6.1) | 1(1.0) | ||
| HBV + schistosomiasis | 5(0.8) | 6(2.0) | 2(3.6) | 1(0.7) | 3(3.1) | ||
| HBV-DNA level (IU/ml): | |||||||
| ≤100 | 97 (16.4) | 60 (20.0) | 0.069 | 7(12.7) | 25(17.0) | 28(28.6) | 0.058 |
| >100–2 × 104 | 278 (47.1) | 157 (52.3) | 30(54.5) | 75(51.0) | 52(53.1) | ||
| >2 × 104–2 × 106 | 176 (29.8) | 68 (22.7) | 13(23.6) | 39(26.5) | 16(16.3) | ||
| >2 × 106 | 39 (6.6) | 15 (5.0) | 5(9.1) | 8(5.4) | 2(2.0) | ||
| HbeAg positve# | 194(33.8) | 99(35.2) | 0.678 | 14(28.6) | 53(36.3% | 32(73.2%) | 0.556 |
| Treatment with NUCs* | 114 (19.3) | 95 (31.7) | <0.0001 | 10(20) | 57(37.0) | 28(29.2) | 0.065 |
| Types of NUCs treatment before enrollment: | |||||||
| Lamivudinealone | 67/114 (58.8) | 48/95 (50.5) | 0.233 | 4/10(40.0) | 30/57(52.6) | 14/28(50.0) | 0.760 |
| Entecaviralone | 16/114 (14.0) | 23/95 (24.2) | 0.06 | 4/10 (40.0) | 14/57 (24.6) | 5/28 (17.9) | 0.391 |
| Adefoviralone | 15/114 (13.2) | 7/95 (7.4) | 0.174 | 2/10 (20.0) | 2/57 (3.5) | 3/28 (10.7) | 0.167 |
| Telbivudinealone | 3 /114(2.6) | 2 /95(2.1) | 0.803 | 0/10 (0.0) | 1/57 (1.8) | 1/28 (3.6) | 0.703 |
| Tenofoviralone | 0 /114(0.0) | 0/95 (0.0) | 1.000 | 0/10 (0.0) | 0/57 (0.0) | 0/28 (0.0) | 1.000 |
| ≥2 NUCs | 13/114 (11.4) | 15/95 (15.8) | 0.354 | 0/10 (0.0) | 10/57 (17.5) | 5/28 (17.9) | 0.161 |
| Potential precipitating events of ACLF | |||||||
| Bacterial infection*** | 35(5.9) | 59(19.7) | <0.001 | 13(23.6) | 22(15.1) | 24(24.5) | <0.001 |
| Gastrointestinal haemorrhage*** | 53(9.0) | 23(7.7) | 0.507 | 5(9.1) | 6(4.1) | 12(12.2) | 0.131 |
| Active alcoholism** | 36(6.1) | 30(10.0) | 0.036 | 4(7.3) | 18(12.2) | 8(8.2) | 0.087 |
| HBV reactivation*** | 14(2.4) | 27(9.0) | <0.001 | 5(9.1) | 16(10.9) | 6(6.1) | <0.001 |
| Superimposed by hepatitis viruses | 5(0.8) | 9(3.0) | 0.031 | 1(1.8) | 7(4.8) | 1(1.0) | 0.182 |
| Surgery** | 7(1.2) | 2(0.7) | 0.705 | 0(0.0) | 0(0.0) | 2(2.0) | 0.105 |
| Hepatotoxic drugs or herbs** | 8(1.4) | 7(2.3) | 0.284 | 1(1.8) | 1(0.7) | 5(5.1) | 0.083 |
| Portal vein thrombosis by CT/MRI*** | 50(8.5) | 9(3.0) | 0.002 | 3(5.5) | 3(2.0) | 3(3.1) | 0.486 |
| Precipitating Events(PEs) | |||||||
| No PE | 267(45.3) | 135(45.0) | 0.991 | 23(41.8) | 75(51.0) | 37(37.8) | 0.253 |
| 1 PE | 257(43.6) | 132(44.0) | 25(45.5) | 60(40.8) | 47(48.0) | ||
| >1 PE | 66(11.2) | 33(11.0) | 7(12.7) | 12(8.2) | 14(14.3) | ||
| Organ failure | |||||||
| Liver failure | 46(7.8) | 233(77.7) | <0.001 | 26(47.3) | 129(87.8) | 78(79.6) | <0.001 |
| Kidney failure | 0(0.0) | 85(28.3) | <0.001 | 23(41.8) | 16(10.9) | 46(47.0) | <0.001 |
| Cerebral failure | 3(0.5) | 71(23.7) | <0.001 | 1(1.8) | 12(8.2) | 58(59.2) | <0.001 |
| Coagulation failure | 58(9.8) | 203(67.7) | <0.001 | 4(7.3) | 120(81.6) | 79(80.6) | <0.001 |
| Circulation failure | 2(0.3) | 57(19.0) | <0.001 | 1(1.8) | 11(7.5) | 45(45.9) | <0.001 |
| Lungs failure | 0(0.0) | 43(14.3) | <0.001 | 0(0.0) | 6(4.1) | 37(37.8) | <0.001 |
| Renal dysfunction | 2(0.4) | 44(14.6) | <0.001 | 22(40.7) | 14(9.9) | 8(8.3) | <0.001 |
| Cerebral dysfunction | 17(2.9) | 54(18.0) | <0.001 | 19(34.6) | 28(19.1) | 10(10.2) | <0.001 |
| Laboratory data | |||||||
| Hematocrit (%) | 30 ± 7 | 30 ± 8 | 0.876 | 29 ± 8 | 31 ± 8 | 29 ± 8 | 0.218 |
| Platelet count (×109/L) | 73 ± 56 | 85 ± 63 | 0.007 | 92 ± 79 | 80 ± 56 | 88 ± 64 | 0.020 |
| Serum bilirubin (mg/dL) | 5.1 ± 8.5 | 26.5 ± 16.3 | <0.001 | 18.2 ± 17.8 | 29.4 ± 14.6 | 26.9 ± 16.2 | <0.001 |
| International normalized ratio | 1.6 ± 0.6 | 3.2 ± 2.1 | <0.001 | 1.8 ± 0.4 | 3.5 ± 2.6 | 3.5 ± 1.4 | <0.001 |
| Alanine aminotransferase (U/L) | 75 ± 203 | 315 ± 589 | <0.001 | 162 ± 281 | 327 ± 563 | 385 ± 730 | <0.001 |
| Aspartate aminotransferase (U/L) | 88 ± 174 | 254 ± 451 | <0.001 | 117 ± 103 | 292 ± 530 | 274 ± 430 | <0.001 |
| γ-Glutamyltransferase (U/L) | 61 ± 71 | 70 ± 98 | 0.159 | 70 ± 99 | 70 ± 103 | 69 ± 89 | 0.491 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 1.5 ± 1.5 | <0.001 | 1.9 ± 2.2 | 1.2 ± 1.1 | 1.9 ± 1.4 | <0.001 |
| Serum sodium (mmol/L) | 136 ± 9 | 130 ± 7.5 | <0.001 | 132 ± 6.2 | 130 ± 7 | 129 ± 9 | <0.001 |
| Leukocytecount (× 109/L) | 4.7 ± 3.3 | 10.3 ± 6.8 | <0.001 | 8.1 ± 5.2 | 9.5 ± 5.3 | 12.8 ± 8.7 | <0.001 |
| Previous decompensation | 0.002 | 0.015 | |||||
| No | 243(41.2) | 157(52.3) | 28(50.9) | 80(54.4) | 49(50.0) | ||
| Yes | 347(58.8) | 143(47.7) | 27(49.1) | 67(45.6) | 49(50.0) | ||
| Mortality(LT-free) | |||||||
| 28 days | 15(2.6) | 132(44.0) | <0.001 | 13(23.6) | 60(40.8) | 59(60.2) | <0.001 |
| 90 days | 27(4.6) | 150(50.0) | <0.001 | 19(34.6) | 69(46.9) | 62(63.3) | <0.001 |
| 180 days | 34(5.8) | 153(51.0) | <0.001 | 19(34.6) | 70(47.6) | 64(63.3) | <0.001 |
| 365 days | 49(8.3) | 155(51.7) | <0.001 | 21(38.2) | 70(47.6) | 64(63.3) | <0.001 |
*within 6 months prior admission; **within 3months prior admission; ***at admission; #35 patients (19 patients had ACLF) didn’t have HbeAg test.
Patients with ACLF were younger; more frequently had no prior AD episodes and had been more frequently treated with NUCs within the 6 months prior to admission than patients with AD without ACLF. The choice of therapy did not impact upon the development of ACLF and, importantly, there was no significant difference in the proportion of patients with ACLF receiving the low resistance barrier drug lamivudine compared to the high resistance barrier agent entecavir. Bacterial infections, active alcoholism, HBV reactivation and superimposed viral infections were more frequent in patients with ACLF than in those without ACLF. In contrast, there was no difference in GI-bleeding, hepatotoxic drugs/herbs and surgery between groups. Accordingly, the prevalence of 1 or more PE was more frequent in patients with ACLF. Patients with ACLF showed higher serum concentration of serum aminotransferase and lower mean arterial pressure and serum concentration of sodium than patients without ACLF.
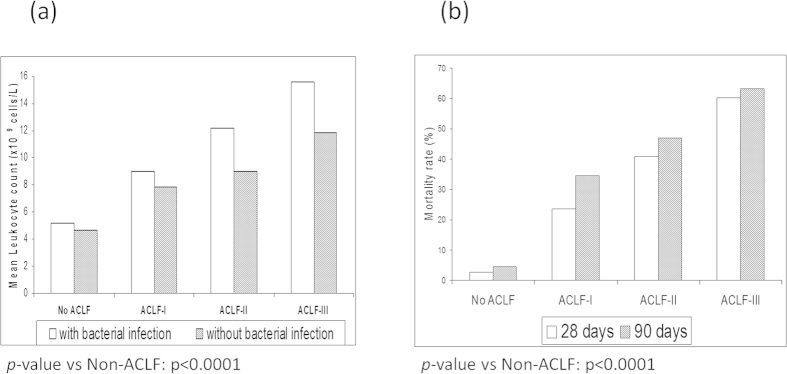
White blood cell counts (WCC) were significantly higher in patients with ACLF than in those without ACLF. This difference was observed in patients with bacterial infections (5.2 ± 2.6 vs. 12.9 ± 9.9 × 109 cells/l; p < 0.01) as well as in patients without bacterial infections (4.7 ± 3.3 vs. 9.7 ± 5.7 × 109 cells/l; p < 0.01). Figure 2A shows that there was a close direct relationship between the WCC and the grade of severity of ACLF.
Figure 2.
(A) Relationship between the severity of ACLF and the WCC.(B) Mortality rate at 28 days and 90 days according to the grade of ACLF.
Factors predicting the development of ACLF during hospitalization
Univariate analysis was performed using data obtained at admission in patients without ACLF who did or did not develop ACLF during hospitalization (Table 2). Presence of PE’s, bacterial infections and liver failure at admission were significantly more frequent, WCC, international normalized ratio (INR), alanineaminotransferase (ALT) and aspartate aminotransferase (AST) significantly higher and hematocrit and serum sodium significantly lower in patients developing ACLF during hospitalization than in those not developing the syndrome. Multivariate analysis disclosed leukocyte count (OR = 1.15; 95%CI: 1.08–1.24; p < 0,001), bilirubin (OR = 1.05; 95%CI: 1.02–1.07; p = 0,002), INR (OR = 1.66; 95%CI: 1.10–2.51; p = 0,016) and bacterial infection (OR = 3.62; 95%CI: 1.53–8.59; p = 0,004) as independent predictors of ACLF during hospitalization. There were no statistically significant differences for HBV reactivation and the level of HBV DNA between ACLF and non-ACLF groups in the univariate and multivariate analysis.
Table 2. Univariate analysis of ACLF development during hospitalization in patients without ACLF at admission.
| Characteristics | Patients not developing ACLF N = 590 | Patients developing ACLF N = 57 | p-value |
|---|---|---|---|
| Age (y) | 51.8 ± 10.7 | 51.8 ± 8.8 | 0.983 |
| Male sex | 450(76.3) | 43(75.4) | 0.888 |
| Ascites* | 482(81.7) | 46(82.1) | 0.994 |
| Mean arterial pressure (mm Hg) | 87 ± 11 | 85 ± 11 | 0.245 |
| Cause of cirrhosis | 0.025 | ||
| HBV alone | 544(92.8) | 48(85.7) | |
| HBV + Alcohol | 36(6.1) | 5(8.9) | |
| HBV + other hepatitis virus | 1(0.2) | 0(0.0) | |
| HBV + schistosomiasis | 5(0.9) | 3(5.4) | |
| HBV-DNA level (IU/ml): | 0.326 | ||
| ≤100 | 97(16.4) | 12(21.1) | |
| >100–2 × 104 | 278(47.1) | 30(52.6) | |
| >2 × 104–2 × 106 | 176(29.8) | 14(24.6) | |
| >2 × 106 | 39(6.6) | 1(1.8) | |
| Potential precipitating events of ACLF | |||
| Bacterial infection | 35(5.9) | 11(19.6) | <0.001 |
| Gastrointestinal haemorrhage | 53(9.0) | 7(12.3) | 0.412 |
| Active alcoholism** | 36(6.1) | 7(12.3) | 0.074 |
| HBV reactivation | 14(2.4) | 1(1.8) | 0.767 |
| Superimposed by hepatitis viruses | 5(0.8) | 0(0.0) | <0.001 |
| Portal vein thrombosis by CT/MRI on admission* | 50(8.5) | 3(5.3) | 0.554 |
| Surgery** | 7(1.2) | 0(0.0) | <0.001 |
| Hepatotoxic drugs or herbs** | 8(1.4) | 0(0.0) | <0.001 |
| Physiological exhaustion** | 6(1.0) | 3(5.3) | 0.043 |
| Precipitating Events(PEs) | |||
| No PE | 267(45.3) | 16(28.1) | 0.043 |
| 1 PE | 257(43.6) | 32(56.1) | |
| >1 PE | 66(11.2) | 9(15.8) | |
| Organ failures | |||
| Liver | 46(7.8) | 23(40.4) | <0.001 |
| Kidney | 0(0.0) | 0(0.0) | – |
| Cerebral | 3(0.5) | 0(0.0) | 0.589 |
| Coagulation | 58(9.8) | 6(10.5) | 0.867 |
| Circulation | 2(0.3) | 1(1.8) | 0.133 |
| Lungs | 0(0.0) | 0(0.0) | – |
| Renal dysfunction | 2(0.4) | 1(1.9) | 0.605 |
| Cerebral dysfunction | 17(2.9) | 4(7.0) | 0.092 |
| Laboratory data | |||
| Hematocrit (%) | 30 ± 7 | 27 ± 6 | 0.001 |
| Platelet count (×109/L) | 73 ± 56 | 87 ± 59 | 0.069 |
| Serum bilirubin (mg/dL) | 5.1 ± 8.5 | 14.5 ± ± 14.2 | <0.001 |
| International normalized ratio | 1.6 ± 0.6 | 1.9 ± 0.6 | <0.001 |
| Alanine aminotransferase (U/L) | 75 ± 203 | 134 ± 211 | 0.043 |
| Aspartate aminotransferase (U/L) | 88 ± 174 | 153 ± 195 | 0.011 |
| γ-Glutamyltransferase (U/L) | 61 ± 71 | 57 ± 77 | 0.751 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.054 |
| Serum sodium (mmol/L) | 136 ± 9 | 129 ± 8 | <0.001 |
| Leukocyte count (×109/L) | 4.7 ± 3.3 | 8.6 ± 4.7 | <0.001 |
| Previous decompensation | 0.696 | ||
| No | 243(41.2) | 25(43.9) | |
| Yes | 347(58.8) | 32(56.1) | |
*at admission; **within 3 months prior hospitalization.
Mortality
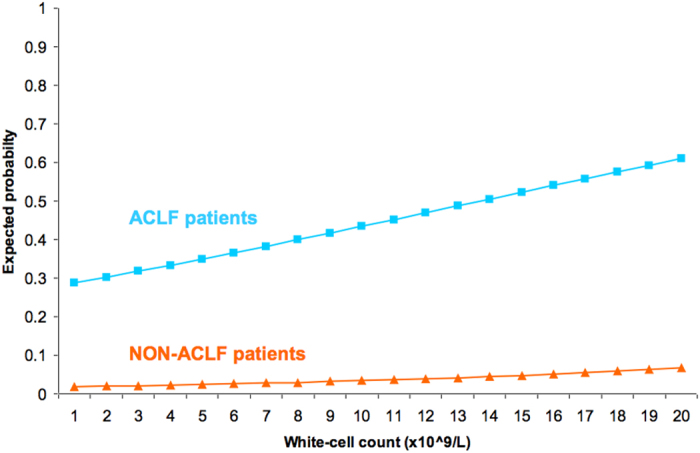
The 28-day and 90-day mortality rates were extremely low (2.6% and 4.6%, respectively) in patients without ACLF and very high (44% and 50%, respectively) in patients with ACLF. Following the first six-month period, mortality rates did not increase significantly in both groups (Table 1). Figure 2B shows that there was a close direct relationship between the severity of ACLF grade at diagnosis and the 28-day and 90-day mortality rates in patients with ACLF. The probability of death in patients with and without ACLF increased with the white-cell count (Fig. 3). However, for any given value of white-cell count, the expected death rate was significantly higher in ACLF patients. In total, 177 patients died at 90 days. The main cause of death was ACLF (125, 70.7%), followed by hypovolemic shock (24, 13.5%), septic shock (24, 13.5%) and other/unknown (4, 2.3%).
Figure 3. Relationship between the expected probability of death at 28-days, the presence or the absence of ACLF and the white cell count.
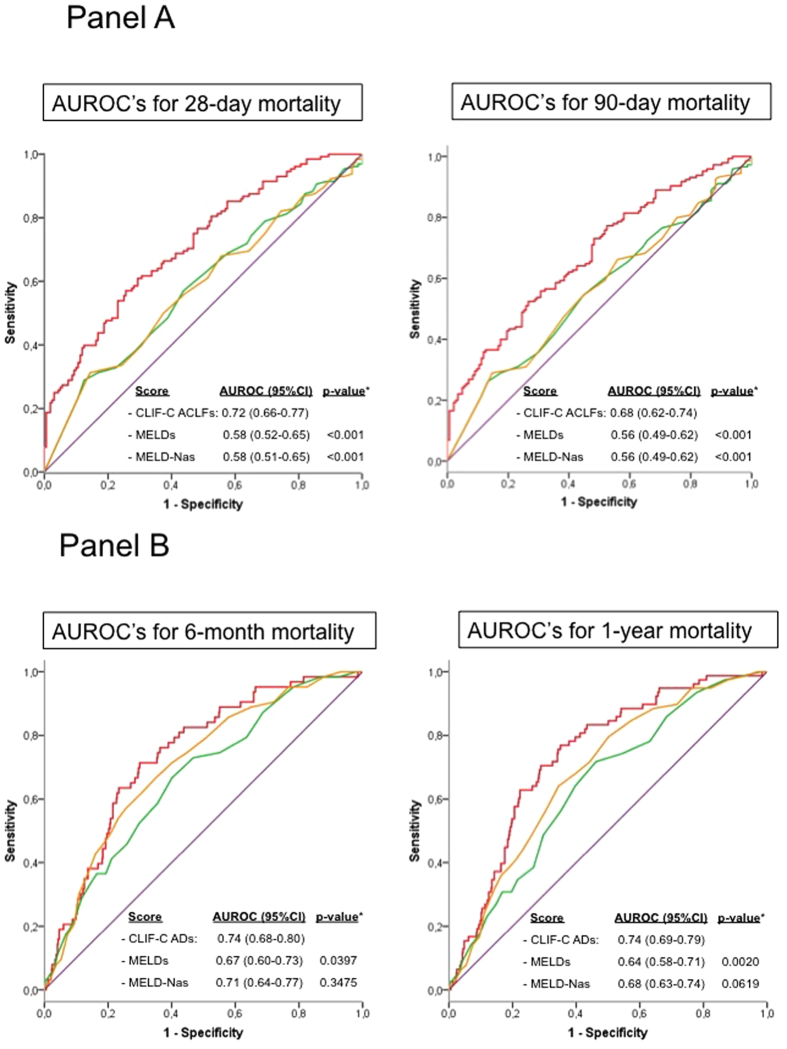
The CLIF-C ACLF score (Fig. 4, Panel A) and the CLIF-C AD score (Fig. 4, Panel B) were significantly more accurate than the MELD and MELD-Na scores in predicting short term and long term mortality in patients with and without ACLF, respectively.
Figure 4.
Panel (A): Accuracy of the CLIF-C ACLFs (red line) as compared to MELDs (green) and MELD-Nas (orange) in predicting 28-day and 90-day mortality of patients with ACLF associated to cirrhosis due to HBV infection. Comparison of the areas under the ROC curves (AUROCs) estimated for each score. The CLIF-C ACLFs showed a significantly higher predictive ability in comparison with the MELDs and MELD-Nas scores for both the 28-day and 90-day mortality. Panel (B): Accuracy of the CLIF-C ADs (red line) as compared to MELDs (green) and MELD-Nas (orange) in predicting 6-month and 1-year mortality of patients with AD (without ACLD) due to HBV associated cirrhosis. Comparison of the AUROCs estimated for each score. The CLIF-C ADs showed significantly higher predictive ability in comparison to the MELDs.
To assess whether specific prognostic scores for patients with HBV associated cirrhosis could increase the accuracy of CLIF-C ADs and CLIF-C ACLFs, two new scoring systems, the HBV-ACLFs and the HBV-ADs, were developed based on the best predictors of survival in our patients with and without ACLF. In univariate analyses age, white-cell count and creatinine were found to be significantly associated with 28-day mortality in ACLF patients, while age, bilirubin, white-cell count, INR, creatinine and serum sodium significantly predicted 1-year mortality in non-ACLF patients. The derived score coefficients were based on the best sub-sets of predictors selected in the corresponding survival models:
HBV-ADs = 0.041 age + 0.270 log (serum bilirubin) + 0.82 log (WCC)
HBV-ACLFs = 0.020 age + 0.256 log (serum creatinine) + 0.709 log (WCC)
Both scores improved the accuracy of MELD and MELD-sodium but not the accuracy of CLIF-C ADs and CLIF-C ACLFs (Table 3). In fact, CLIF-C ACLFs was significantly more accurate than the HBV-ACLFs at all main end-points.
Table 3. HBV-ACLFs and HBV-ADs comparing with c-index of CLIF-C ACLFs and CLIF-C ADs and with MELDs andMELD-Nas.
| C-index |
||||||||
|---|---|---|---|---|---|---|---|---|
| 28 days | P | 90 days | P | 180 days | P | 360 days | p | |
| Patients with ACLF in the whole study | ||||||||
| HBV-ACLFs | 0.654 (0.604–0.705) | 0.645 (0.596–0.694) | 0.644 (0.595–0.693) | 0.640 (0.591–0.688) | ||||
| CLIF-C ACLFs | 0.704 (0.661–0.748) | 0.023 | 0.685 (0.643–0.727) | 0.057 | 0.687 (0.645–0.728) | 0.041 | 0.682 (0.640–0.723) | 0.046 |
| MELDs | 0.554 (0.497–0.610) | <0.001 | 0.543 (0.490–0.596) | <0.001 | 0.543 (0.491–0.595) | <0.001 | 0.540 (0.488–0.591) | <0.001 |
| MELD-Nas | 0.549 (0.493–0.605) | <0.001 | 0.541 (0.488–0.594) | <0.001 | 0.541 (0.488–0.594) | <0.001 | 0.537 (0.486–0.589) | <0.001 |
| Patients without ACLF in the whole study | ||||||||
| HBV-ADs | 0.737 (0.659–0.814) | 0.716 (0.650–0.781) | 0.720 (0.659–0.782) | 0.721 (0.666–0.775) | ||||
| CLIF-C ADs | 0.733 (0.662–0.803) | 0.92 | 0.724 (0.663–0.784) | 0.796 | 0.728 (0.671–0.784) | 0.783 | 0.728 (0.677–0.779) | 0.788 |
| MELDs | 0.667 (0.575–0.759) | 0.08 | 0.653 (0.580–0.725) | 0.056 | 0.657 (0.589–0.724) | 0.042 | 0.639 (0.577–0.700) | 0.003 |
| MELD-Nas | 0.719 (0.643–0.796) | 0.653 | 0.710 (0.646–0.773) | 0.886 | 0.701 (0.640–0.762) | 0.54 | 0.682 (0.626–0.738) | 0.164 |
Differences between ACLF in HBV associated cirrhosis and ACLF in cirrhosis due to other etiologies included in the CANONIC study4
Results obtained in the current study in patients with ACLF associated to HBV infection (HBV-ACLF) were compared to those previously reported by the CANONIC Study in patients with ACLF associated with cirrhosis of other etiologies (CANONIC-ACLF) (Table 4). Age was significantly lower (46.5 (11.3) vs. 55.8 (11.7); p < 0.001) and male gender significantly more frequent in patients with HBV-ACLF, but these features were also observed in patients with AD without ACLF (data not shown). The prevalence of liver failure and coagulation failure was significantly higher and the prevalence of renal failure was significantly lower in HBV-ACLF. There were no differences in the prevalence of other OFs between groups. Accordingly, liver function tests (serum bilirubin, ALT and AST, and INR) were significantly worse in patients with HBV-ACLF infection while serum creatinine, gamma-glutamyltranferase and serum sodium, were significantly higher in patients with CANONIC-ACLF. There were no differences in WCC between groups. Importantly, the incidence of a prior history of AD was significantly lower in patients with HBV-ACLF indicating that a higher proportion of patients developed ACLF at the same time as the first AD of cirrhosis.
Table 4. Differences of clinical characteristics between patients with ACLF included in the current study and patients from the CANONIC study.
| Characteristics | Chinese Patients N = 300 | CANONIC patients N = 417 | p-value |
|---|---|---|---|
| Age (y) | 46.5 ± 11.3 | 55.8 ± 11.7 | <0.001 |
| Male sex | 233(77.7) | 267(64.0) | <0.001 |
| Previous decompensation | <0.001 | ||
| No | 157(52.3) | 98(24.9) | |
| Yes | 143(47.7) | 295(75.1) | |
| Ascites | 229(76.6) | 324(78.5) | 0.7346 |
| Mean arterial pressure(mmHg) | 84 ± 18 | 81 ± 13 | 0.0142 |
| Cause of cirrhosis | |||
| Alcohol,noHCV | – | 233(58.4) | – |
| No Alcohol,HCV | – | 59(14.8) | – |
| Alcohol+HCV | – | 37(9.3) | – |
| Other Etiologies* | – | 70(17.5) | – |
| HBV alone | 263(87.7) | – | – |
| HBV+Alcohol | 19(6.3) | – | – |
| HBV+other hepatitis virus | 12(4.0) | – | – |
| HBV+schistosomiasis | 6(2.0) | – | – |
| Organ failures | |||
| Liver | 233(77.7) | 157(37.7) | <0.001 |
| Kidney | 85(28.3) | 217(52.0) | <0.001 |
| Cerebral | 71(23.7) | 94(22.5) | 0.7925 |
| Coagulation | 203(67.7) | 118(28.3) | <0.001 |
| Circulation | 57(19.0) | 78(18.7) | 0.9977 |
| Lungs | 43(14.3) | 47(11.3) | 0.2684 |
| Renal dysfunction | 44(14.7) | 64(15.4) | 0.801 |
| Cerebral dysfunction | 54(18.0) | 130(31.3) | <0.001 |
| Laboratory data | |||
| Hematocrit(%) | 30 ± 8 | 28 ± 9 | 0.0018 |
| Leucocytes(×109/L) | 10.3 ± 6.8 | 9.5 ± 6.0 | 0.1033 |
| Platelet count(×109/L) | 85 ± 63 | 103 ± 70 | <0.001 |
| Serum bilirubin(mg/dL) | 27 ± 16 | 11 ± 11 | <0.001 |
| International normalized ratio | 3.2 ± 2.1 | 2.0 ± 0.8 | <0.001 |
| Alanine aminotransferase(U/L) | 315 ± 589 | 69 ± 122 | <0.001 |
| Aspartate aminotransferase(U/L) | 254 ± 451 | 202 ± 991 | 0.3454 |
| γ-Glutamyltransferase(U/L) | 70 ± 98 | 154 ± 207 | <0.001 |
| Serum creatinine(mg/dL) | 1.5 ± 1.5 | 2.0 ± 1.5 | <0.001 |
| Serum sodium(mmol/L) | 130 ± 7 | 133.5 ± 7 | <0.001 |
| Site of hospitalization | <0.001 | ||
| Intensive care unit | 0(0.0) | 98(23.6) | |
| Ward | 300(100.0) | 318(76.4) | |
| ACLF grades: | <0.001 | ||
| ACLF-I | 55(18.3) | 214(51.3) | |
| ACLF-II | 147(49.0) | 147(35.3) | |
| ACLF-III | 98(32.7) | 56(13.4) | |
| Mortality | |||
| 28 days | 129(43.0) | 125(30.0) | <0.001 |
| 90 days | 151(50.3) | 178(42.7) | 0.0624 |
| 180 days | 154(51.3) | 204(48.9) | 0.6358 |
| 365 days | 156(52.0) | 228(54.7) | 0.4709 |
*Hepatitis B-associated cirrhosis in 21 patients (5.2%).
The CANONIC data is reproduced with permission from Moreau et al. Gastro2013.
The prevalence of ACLF-1 was significantly higher in CANONIC-ACLF. In contrast, the prevalence of ACLF-2 and ACLF-3 was significantly more frequent in patients with HBV-ACLF. Accordingly, the 28-day and 90-day mortality was significantly higher in patients with HBV-ACLF.
Discussion
The results of this study validate the diagnostic ability of CLIF-C OFs to differentiate hospitalized HBV patients with decompensated cirrhosis into those with ACLF and those without. As observed in the CANONIC study4, the 28-day mortality rate for our patients without ACLF was very low (2.6%) compared with a 44% mortality in those classified as ACLF. Moreover, the data demonstrated the significance of OFs in determining survival in this HBV population; mortality rates increased progressively with the number of OFs. The CLIF-C ACLF and the CLIF-C AD scores performed better at predicting prognosis than MELD and MELD-Na scores3,5. Specific score developed from the data of patients with HBV associated ACLF (HBV-ACLFs) was less accurate than the previously defined CLIF-C ACLF score in predicting outcome. From the pathophysiological perspective, the putative importance of inflammation in the pathogenesis of ACLF was confirmed in the HBV population, as white cell count correlated with the presence and grade of ACLF and remained an independent predictor of mortality4. Taken together, the data suggest that the CLIF Consortium criteria for the diagnosis and prognosis of hospitalized cirrhotic patients that was developed in Europe in non-HBV patients can also be used in Chinese patients with HBV infection with a high degree of accuracy.
Despite the many similarities in the diagnostic and prognostic criteria for ACLF in HBV patients with the CANONIC cohort, important differences were observed in the Chinese cohort with HBV compared with the European non-HBV patients suggesting that ACLF due to hepatitis B confers unique characteristics to the syndrome.
One of the most intriguing observations was the main organ failures in HBV cohort was ‘Liver’ and ‘Coagulation’, which tended to occur together and more frequently (78.7 and 68.4% respectively) accounting for ACLF gradation of ACLF-2. As the proportion of patients with ACLF-2 was over represented while the proportion of patients in the ACLF-1 cohort was low in the HBV population, the 28-days mortality of the HBV cohort was significantly higher than that observed in the non-HBV, CANONIC cohort (43% vs. 30%)4. A possible explanation for the observation of increased ‘Liver’ and ‘Coagulation’ failure is likely to be the pathological characteristics of HBV ACLF patients. Our previous study10 explored that submassive hepatic necrosis accompanied with cholestasis and/or ductular bilirubinostasis (sepsis) were the major hepatic pathological features of HBV related ACLF patients in contrast to mainly inflammation and bilirubinostasis in the alcohol related ACLF patients11,12. Lacking prothrombin biosynthesis caused by extensive parenchymal hepatocyte destruction and severe cholestasis around residual cirrhotic nodules in HBV submassive hepatic necrosis liver are, therefore, reasonable explanations for prolonged INR and hyperbilirubinemia. For precipitating events, more than 80% patients were not receiving NUC’s and therefore, it was not surprising to note that they had detectable levels of HBV DNA at the time of hospital admission but surprisingly, less than 5% patients showed clinical and virological evidence of HBV reactivation13. There was no significant difference in the distribution of HBV-DNA levels in patients who did, or did not, develop ACLF suggesting that HBV associated hepatitis was not related to the development of liver dysfunction14,15. However it is well established that changes in HBV-DNA may precede liver injury and given that the transaminase levels were increased in patients with ACLF compared to those who did not develop ACLF it is impossible to exclude an effect of HBV. As no other precipitating event was detected in about 60% of the patients, it is possible that there may well have been ‘HBV flares’ accounting for the development of ACLF but this hypothesis will need to be studied further16,17. It was also notable that other risk factors for ACLF such as infection with other hepatitis viruses were over represented in those HBV patients that developed ACLF. Not surprisingly, alcohol abuse on the background of HBV cirrhosis was also associated with the occurrence of ACLF more frequently. As in the CANONIC cohort, the clinical course of ACLF and the associated mortality were not related to the presence and type of precipitating events. This indicates that, although precipitating events are important in the development of ACLF, once it develops the clinical course and mortality depend more on the severity of systemic inflammation and number of OFs.
Another interesting observation was that, in contrast to the CANONIC study, which comprised non-HBV patients, kidney failure rate in the HBV patients was significantly lower (52% vs. 28.6%). The mechanism of lower rates of renal failure in the HBV ACLF population is not clear but may suggest different mechanisms of injury. In the alcoholic cirrhosis population, which formed the majority of patients in the CANONIC study, gut bacterial translocation is pathophysiologically important and has been shown to possibly ‘prime’ the kidneys by up-regulation of toll-like receptor 4 to the effect of a superimposed inflammatory insult18,19,20. Alternatively, or in addition, this difference may reflect that a ‘hepatic’ insult such as HBV flares/reactivation compared with ‘extrahepatic’ insult such as bacterial infection or alcohol abuse, which were the predominant precipitating illnesses in the CANONIC cohort4, was an important mechanism of ACLF in our series. It is therefore, interesting to note that in HBV patients with infections or alcoholism as the precipitating event, the rate of renal failure was 19.6%, (30/153 patients), which was significantly higher than in patients without these PE’s (7.5% (55/737) patients) (P < 0.001).
It is also interesting to note that white cell count was an independent predictor of mortality in both the cohorts indicating the strong and the very important influence of inflammation in driving the pathogenesis of HBV related ACLF, as was the case in the CANONIC patients. As illustrated in Fig. 2A, the white cell count was significantly higher in all the ACLF cohorts and increased progressively with more severe grades of ACLF. Remarkably, the presence of bacterial infection at each stage of ACLF was associated with markedly higher WCC count, which was absent in the non-ACLF cohort. Moreover, as illustrated in Fig.3, the correlation between leucocyte count and 28-day mortality showed that for any given value of white-cell count and (presumably) inflammation, the probability of death was significantly higher in patients with ACLF than in those without the syndrome. Bacterial infection as a precipitating event was significantly more commonly associated with ACLF and its presence was associated with more severe grades of ACLF in this HBV cohort as was the case with the CANONIC patients4. These observations, which confirm the results from the CANONIC study, suggest that in addition to systemic inflammation, altered host response to injury accounts for organ failure even in the HBV patients who develop ACLF1. Further studies are needed to define the underlying mechanisms.
HBV patients with ACLF were significantly younger than patients in the European cohort but in both cohorts, the ACLF patients were younger than the patients with AD. Severity of ACLF also correlated inversely with age. Additionally, 52.3% patients with HBV related ACLF had no previous history of decompensation, a proportion that was significantly higher than in patients with HBV infections without ACLF. Taken together, the data show clearly that HBV-ACLF is pathophysiologically similar to the CANONIC patients as in both cohorts ACLF was more commonly associated with younger age, lack of previous decompensation and inflammation21,22.
The CLIF-C ACLF score was developed to provide sequential prognostic information in ACLF patients in the non-HBV cohort and validated independently in a similar population3. Its performance in predicting mortality was shown to be of similar accuracy in the HBV-ACLF patients. Importantly, its performance was significantly better than the best scoring systems that are currently used to allocate organs for liver transplantation, the MELD23 and the MELD-Na21 scores providing a further independent validation of the CLIF-C ACLF score for HBV-ACLF patients. Using the dataset, a bespoke scoring system for the HBV ACLF patients, the HBV-ACLF score was developed. It is important to note that the performance of this etiology specific scoring system was significantly less accurate compared with the CLIF-C ACLF score confirming that this score is not be constrained by etiology. As the 1-year mortality rate was extremely low in the non-ACLF patients (49 patients; 8.3%), assessment of the CLIF-C AD score as a prognostic model for the non-ACLF patients5 is likely to be underpowered and therefore the data should be interpreted with caution. Nevertheless, the data show that the score performs significantly better than the MELD score at all the time points and does not significantly improve the performance of MELD-Na score. A bespoke HBV scoring system for the non-ACLF HBV cohort was developed from the data. Its performance was shown to be similar to that of CLIF-C AD score confirming its validity in the HBV non-ACLF population.
There are two further points that should be emphasized. Firstly, NUCs were not covered by nation wide medical insurance in China before 2009, the persistence of antiviral therapy for HBV was paid by the patient’s themselves. This can explain the reason why only a low percentage of enrolled patients (23.5%) were treated by NUCs before enrollment (patients’ data collected between 2005 and 2010). Secondly, a recent study24 from another HBV high endemic region in China has reported the rate of both flare-up and exacerbation of HBV were 35.8% in ACLF patients, while our HBV reactivation rate was 9.0%. Different definition about HBV reactivation and flare-up/exacerbation caused the significant gap in these two studies. HBV reactivation was defined as both ALT > 3NL and HBV-DNA > 100 U/L due to NUC resistance and cessation of the antiviral treatment in current study. However, In the study by Shi Y et al.’s study HBV ‘flare-up’ was defined as more than twice of the baseline value with HBV DNA positive within one month before admission and ‘exacerbation’ defined as ALT > 5NL with HBV DNA positive24.
There are still lack of clarity amongst Asian hepatologists in understanding whether acute decompensation (AD) of cirrhotic patients is related to precipitating factors or disease complications. In current study, 52% of HBV ACLF patients had new occurrence of decompensation (Table 4) which reflects that a precipitating event is the cause of AD in more than half the ACLF patients.
In current study multiple organ failure cirrhotic patients may include some chronic liver failure patients, However the liver specific multiple organ failure score (CLIF-OF) has clearly demonstrated that it can distinguish high mortality from low mortality patients not only in Eastern type (HBV) but also in Western type (Alcoholic) cirrhotic AD patients. This is the key point of this study to exhibit that a uniform diagnostic criterion could be applied for distinguishing ACLF from both HBV and alcoholic cirrhotic AD patients.
The study may be criticized because of its retrospective nature and the lack of a head to head comparative population with a non-HBV cohort. Nonetheless, because this study was based in a single hospital with access to all the patient records and biochemistry, there were very few missing data making the observations robust and increasing the validity of conclusions. Another potential criticism of this study is the management of the patients in a ward-based rather than ICU environment. It is possible that this may affect the results of the analysis but the similarities to the outcomes obtained and observations made in the CANONIC study, makes this is unlikely. Other groups have shown that the CLIF sequential organ failure assessment score (SOFA), which was first used in the CANONIC study to define ACLF, can be used to accurately classify patients further confirming the fidelity of the data and the validity of the observations from the present study25,26,27,28,29,30,31.
In summary, although ACLF in HBV patients (present study) and in patients with mainly alcohol related or hepatitis C virus (CANONIC study) exhibited differences in clinical features, the data in this study demonstrates that ACLF is a clinically and pathophysiologically distinct entity independent of the underlying etiology. First, age, previous decompensations and the presence or type of precipitating events were similar in the disease course and prognosis in both populations. Second, a systemic inflammatory (white cell count) response independently correlated with the presence and grade of ACLF and was an independent predictor of mortality. Third, the liver specific multiple organ failure score (CLIF-C OFs) had the equal diagnostic ability to differentiate ACLF from AD patients and the number of OFs determined short term mortality of the patients. Finally, the prognostic score designed in a non-HBV cohort was equally accurate for patients with HBV and could not be improved significantly increased by a bespoke disease specific score.
In conclusion, ACLF is by far the most frequent cause of death in patients with acute decompensation due to HBV infection, significantly more frequent than in patients with alcoholic and/or HCV infection. The data presented here confirm the validity of the CLIF criteria in the diagnosis and prognosis of patients with ACLF. The study also identified key clinical differences that should be the subject of future studies.
Methods
Study and patients characteristics
The study consisted in a retrospective analysis of 890 patients admitted to Ren Ji Hospital, Shanghai Jiao Tong University, from January 2005–December 2010 from a total of 3004 patients admitted to the hospital (Supplementary table S1). Patients with chronic hepatitis B virus infection and cirrhosis (hepatitis B surface antigen (HBsAg) +ve and antibody to hepatitis B core antigen (anti-HBc) +ve in the absence of other potential cause of cirrhosis; chronicity was defined by the presence of HBsAg +ve for > 6 months) who had either ascites, hepatic encephalopathy, variceal hemorrhage and/or bacterial infections, or other clinical, ultrasonography and/or laboratory data compatible with the diagnosis of cirrhosis were recruited in the analysis. The criterion of bacterial infection in current study was the same as CANONIC Study4 and included spontaneous bacterial peritonitis, spontaneous bacteremia (positive blood culture), urinary tract infection, pneumonia and cellulitis. Two hundred and sixty-four patients received an orthotopic (87%) or living donor (13%) liver transplant (LT) within 90-days following admission and in 95% of these patients the presence of cirrhosis was confirmed by liver histology. Thirteen patients had advanced fibrosis histologically. Informed consent had been obtained from all patients and/or their relatives about usage of their clinical and pathological data. The ethics committee of the Ren Ji Hospital approved the study and methods (Ethics Number EC (2014) 148K). Methods were carried out in accordance with the approved guidelines.
Data collection
Data collection was performed in December 2013. Demographic data, prior history of a decompensation, main cause of admission, precipitating events (PE) associated with the acute decompensation or ACLF, other clinical and exploratory data, laboratory tests, mean arterial pressure, pulse oximetry, markers of HBV infection, and antiviral treatment given within 6-months prior to and during hospitalization were obtained from patient medical records or the hospital database. Clinical and laboratory data given in the article are those obtained at the time of admission into the hospital or, when indicated, at the time of diagnosis of ACLF. Survival rates at 28-days, 90-days, 6-months and 1-year following enrollment were obtained through patient medical records or by direct contact with the patients or their families.
Diagnostic criteria for ‘reactivation’ of HBV infection
Reactivation of hepatitis B32,33,34 was diagnosed if patients presented with at least one of the following criteria:
Absolute increase in both HBV-DNA (>1000 copies/ml; [>200 IU/ml]) and ALT > 3 times normal in a patient on continuous treatment with nucleotide analogs (NUCs) due to the development of NUC resistance OR
An acute increase in HBV-DNA and ALT in a patient on continuous treatment with NUCs following cessation of the antiviral treatment OR
Acute increase in ALT associated with HBsAg reappearance in 2 consecutive tests 5 days apart and increase in HBV-DNA (>105 copies/ml; [>2 × 104 IU/ml]) in a patient with prior negative HBsAg and positive anti-HBc antibody.
Diagnostic criteria of ACLF and prognostic scores
Definitions, diagnostic criteria and methodology used to analyze the study data were those proposed by the investigators of the CLIF Consortium in the CANONIC Study3 (Supplementary Figure S2). The term ACLF defines a syndrome characterized by AD of cirrhosis associated to organ failure (OF) and poor short-term probability of survival3,5. CLIF-C OFs was used for the diagnosis of organ failures (liver failure: serum bilirubin ≥12 mg/dl; renal failure: serum creatinine ≥2 mg/dl or renal support therapy; cerebral failure: grade III-IV hepatic encephalopathy; coagulation failure: INR ≥2.5; respiratory failure: PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤214; circulatory failure: vasoconstrictor requirements to maintain arterial pressure). In addition to organ failure, renal dysfunction (as defined by a serum creatinine of 1.5–1.9 mg/dl) and/or cerebral dysfunction (grade 1–2 hepatic encephalopathy) were also used for the diagnosis of ACLF in patients with single non-renal organ failure. (Supplementary Figure S2).
Based on the presence of organ failure and renal and/or cerebral dysfunction the following groups of patients were either excluded or included from the diagnosis of ACLF: 1. Excluded: (a) No organ failure; (b) Single non-renal organ failure without renal and/or cerebral dysfunction. Included: (A) Single renal failure (ACLF grade I, ACLF-1); (B) Single non-renal organ failure plus renal dysfunction and/or cerebral dysfunction (ACLF-1); (C) 2 organ failures (ACLF-2); (D) 3–6 OFs (ACLF-3)4.
CLIF-C ACLF score (based on CLIF-C OF score plus age and white blood cell count, WCC), CLIF-C AD score (based on serum creatinine, INR, serum sodium, age and WCC) were used to assess risk of mortality in patients with ACLF and AD3,5, respectively and compared to MELD23, and MELD-Sodium (MELD-Na) scores21.
An online application to compute CLIF Consortium scores and estimate the correspondingpredicteddeath rate at time ‘t’ is available at the CLIF-Consortium website: http://www.clifconsortium.com/.
Statistics
All the data was analyzed at the Data Management Centre of the CLIF Consortium, Barcelona in line with the analysis of the previously published CANONIC study. All the variables used in statistical analysis were obtained at the time of hospital admission or, when indicated, at the time of diagnosis of ACLF. Data are presented as mean ± standard deviation for continuous parameters or frequencies and percentages for categorical variables. Mortality rates were estimated as transplant-free (patients who received a liver transplant were considered as lost to follow-up). Univariate analysis using Chi-square, Student’s t test or one way analysis of variance were performed to assess differences between groups or the association between all potential factors and outcomes. Two specific scores for HBV patients with and without ACLF (HBV-ACLF and HBV-AD scores) were estimated by fitting two Cox proportional-hazards models for 28-day and 1-year mortality, respectively. The initial model included all the risk factors found to be significantly associated with mortality in univariate analyses. Each score coefficients were based on the best independent sub-sets of predictors selected by means of the stepwise-forward method based on changes in model Likelihood Ratio (p-in = p-out = 0.05).
Harrell’s concordance index (C-index) was used to assess the discrimination ability of each severity score35.
Statistical comparison of C-index estimates was carried out for the main study time-points using the standardized normal approximation. A confirmatory analysis was carried out to assess the discrimination ability of the prognostic scores by estimating and comparing the corresponding Areas under the ROC curves (AUROCs). A univariate analysis was also carried out to identify the main baseline factors associated to the development of ACLF during the hospitalization in patients without ACLF at admission. A Logistic Regression model was fitted including all the significant potential predictors from this analysis. The best independent predictors of ACLF development in those without ACLF at presentation was selected by means of the stepwise-forward method based on changes in the model Likelihood Ratio (p-in = p-out = 0.05). In all statistical tests, the significance level was set at p < 0.05.
Additional Information
How to cite this article: Li, H. et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci. Rep. 6, 25487; doi: 10.1038/srep25487 (2016).
Supplementary Material
Acknowledgments
The authors thank Dr. Ji-Dong Jia from Beijing You-Yi Hospital, Beijing, China and Dr. Hong-lei Weng from Medical Faculty Mannheim, Heidelberg University, Germany for advices and discussion. The study was supported by National Natural Science Foundation of China (NSFC), grant number: 30770962, 30971333, 81170421, 81470869 and Chinese High Technology “863” programme, grant number: 2006AA02A411 (Hai.Li); The CLIF Consortium Data Management Center is supported by an unrestricted grant from Grifols.
Footnotes
There are potential competing financial interests as follows. Rajiv Jalan received research funding from Vital Therapies, has served on Scientific Advisory Board for Conatus Pharma, and received lecture fees from Gambro and has on-going research collaboration with Gambro, Grifols and is the Principal Investigator of an Industry sponsored study (Sequana Medical). He is also inventor for a drug, L-ornithine phenyl acetate which UCL has licensed to Ocera Therapeutics. Vicente Arroyo has research grants from Grifols.
Author Contributions H.L., R.J. and V.A. designed and draftedthe article. L.-Y.C., S.-T.L., N.-n.Z., B.Z., M.P., À.N., Q.X., F.X., X.M., J.H., L.S. and D.-k.Q. collected, analysed and interpreted patients’ data. Q.X. and the corresponding author made a frame of protocol of current studying and obtained advice or opinions during the whole studying period. G.F., R.M. and P.G., G.D. and R.P.M. reviewed and edited the article critically.
References
- Jalan R. et al. Acute-on chronic liver failure. J Hepatol 57, 1336–1348, doi: 10.1016/j.jhep.2012.06.026 (2012). [DOI] [PubMed] [Google Scholar]
- Olson J. C. et al. Intensive care of the patient with cirrhosis. Hepatology 54, 1864–1872, doi: 10.1002/hep.24622 (2011). [DOI] [PubMed] [Google Scholar]
- Jalan R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 61, 1038–1047, doi: 10.1016/j.jhep.2014.06.012 (2014). [DOI] [PubMed] [Google Scholar]
- Moreau R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437, 1437.e1421-1429, doi: 10.1053/j.gastro.2013.02.042 (2013). [DOI] [PubMed] [Google Scholar]
- Jalan R. et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 62, 831–840, doi: 10.1016/j.jhep.2014.11.012 (2015). [DOI] [PubMed] [Google Scholar]
- Bajaj J. S. et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 60, 250–256, doi: 10.1002/hep.27077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 3, 269–282, doi: 10.1007/s12072-008-9106-x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J. S. Defining acute-on-chronic liver failure: will East and West ever meet? Gastroenterology 144, 1337–1339, doi: 10.1053/j.gastro.2013.04.024 (2013). [DOI] [PubMed] [Google Scholar]
- Jalan R. et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 147, 4–10, doi: 10.1053/j.gastro.2014.05.005 (2014). [DOI] [PubMed] [Google Scholar]
- Li H. et al. Submassive hepatic necrosis distinguishes HBV-associated acute-on chronic liver failure from cirrhotic patients with acute decompensation. J Hepatol, doi: 10.1016/j.jhep.2015.01.029 (2015). [DOI] [PubMed] [Google Scholar]
- Mookerjee R. P. et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol 55, 1103–1111, doi: 10.1016/j.jhep.2011.02.021 (2011). [DOI] [PubMed] [Google Scholar]
- Katoonizadeh A. et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut 59, 1561–1569, doi: 10.1136/gut.2009.189639 (2010). [DOI] [PubMed] [Google Scholar]
- Singal A. K. & Fontana R. J. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther 35, 674–689, doi: 10.1111/j.1365-2036.2011.04990.x (2012). [DOI] [PubMed] [Google Scholar]
- Wai C. T. et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. J Viral Hepat 12, 192–198, doi: 10.1111/j.1365-2893.2005.00581.x (2005). [DOI] [PubMed] [Google Scholar]
- Dao D. Y. et al. Use of nucleoside (tide) analogues in patients with hepatitis B-related acute liver failure. Dig Dis Sci 57, 1349–1357, doi: 10.1007/s10620-011-2013-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrillo R. P. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120, 1009–1022 (2001). [DOI] [PubMed] [Google Scholar]
- Dao D. Y. et al. Two distinct subtypes of hepatitis B virus-related acute liver failure are separable by quantitative serum immunoglobulin M anti-hepatitis B core antibody and hepatitis B virus DNA levels. Hepatology 55, 676–684, doi: 10.1002/hep.24732 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawale J. M. et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int 30, 725–732, doi: 10.1111/j.1478-3231.2009.02182.x (2010). [DOI] [PubMed] [Google Scholar]
- Shah N. et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 33, 398–409, doi: 10.1111/liv.12047 (2013). [DOI] [PubMed] [Google Scholar]
- Jalan R. et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 60, 1310–1324, doi: 10.1016/j.jhep.2014.01.024 (2014). [DOI] [PubMed] [Google Scholar]
- Kim W. R. et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 359, 1018–1026, doi: 10.1056/NEJMoa0801209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D. S. & Soares M. P. Disease tolerance as a defense strategy. Science 335, 936–941, doi: 10.1126/science.1214935 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath P. S. & Kim W. R. The model for end-stage liver disease (MELD). Hepatology 45, 797–805, doi: 10.1002/hep.21563 (2007). [DOI] [PubMed] [Google Scholar]
- Shi Y. et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 62, 232–242, doi: 10.1002/hep.27795 (2015). [DOI] [PubMed] [Google Scholar]
- Theocharidou E. et al. The Royal Free Hospital score: a calibrated prognostic model for patients with cirrhosis admitted to intensive care unit. Comparison with current models and CLIF-SOFA score. Am J Gastroenterol 109, 554–562, doi: 10.1038/ajg.2013.466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. E. et al. Single-centre validation of the EASL-CLIF Consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int, doi: 10.1111/liv.12597 (2014). [DOI] [PubMed] [Google Scholar]
- Pan H. C. et al. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure - sequential organ failure assessment score (CLIF-SOFA). Aliment Pharmacol Ther 40, 1056–1065, doi: 10.1111/apt.12953 (2014). [DOI] [PubMed] [Google Scholar]
- McPhail M. J. et al. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol, doi: 10.1016/j.cgh.2014.08.041 (2014). [DOI] [PubMed] [Google Scholar]
- Lee M. et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int 35, 46–57, doi: 10.1111/liv.12683 (2015). [DOI] [PubMed] [Google Scholar]
- Dhiman R. K., Agrawal S., Gupta T., Duseja A. & Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol 20, 14934–14941, doi: 10.3748/wjg.v20.i40.14934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Duseja A., Gupta T., Dhiman R. K. & Chawla Y. Simple organ failure count versus CANONIC grading system for predicting mortality in acute-on-chronic liver failure. J Gastroenterol Hepatol 30, 575–581, doi: 10.1111/jgh.12778 (2015). [DOI] [PubMed] [Google Scholar]
- Lok A. S. & McMahon B. J. Chronic hepatitis B. Hepatology 45, 507–539, doi: 10.1002/hep.21513 (2007). [DOI] [PubMed] [Google Scholar]
- Hui C. K. et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 131, 59–68, doi: 10.1053/j.gastro.2006.04.015 (2006). [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H. Reactivation of hepatitis B. Hepatology 49, S156–165, doi: 10.1002/hep.22945 (2009). [DOI] [PubMed] [Google Scholar]
- Harrell F. E. Jr., Lee K. L. & Mark D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15, 361–387, doi: (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.