Abstract
Background
Of the 1.5 million people diagnosed with pleural effusion annually in the U.S., approximately 178,000 undergo thoracentesis. While it is known that malignant pleural effusion portends a poor prognosis, mortality of patients with nonmalignant effusions has not been well studied.
Methods
This prospective cohort study evaluated 308 patients undergoing thoracentesis. Chart review was performed to obtain baseline characteristics. The etiology of the effusions was determined using standardized criteria. Mortality was determined at 30-days and 1-year.
Results
247 unilateral and 61 bilateral thoracenteses were performed. Malignant effusion had the highest 30-day (37%) and 1 year (77%) mortality. There was substantial patient 30-day and 1-year mortality with effusions due to multiple benign etiologies (29% and 55%), CHF (22% and 53%), and renal failure (14% and 57%). Patients with bilateral pleural effusion, relative to unilateral, were associated with higher risk of death at 30 days and 1 year (17% versus 47%; HR 2.58 CI [1.44–4.63] and 36% versus 69%; HR 2.32 CI [1.55–3.48]).
Conclusions
Patients undergoing thoracentesis for pleural effusion have high short and long-term mortality. Patients with malignant effusion had the highest mortality followed by multiple benign etiologies, CHF and renal failure. Bilateral pleural effusion is distinctly associated with high mortality.
Introduction
Pleural effusions are diagnosed annually in over 1.5 million people in the United States (1) and are caused by various medical conditions. The most common causes of pleural effusion are, in descending order, congestive heart failure (CHF), pleural infection, and malignancy (2). CHF alone is responsible for about one-third of all cases in the United States each year (1). Although malignant pleural effusions are associated with high mortality, there is little in the literature regarding mortality of patients with non-malignant pleural effusion.
Bilateral pleural effusions are common, accounting for 15% of the cases in non-critically ill patients, and up to 55% of those in the intensive care population. The etiology of bilateral pleural effusion includes CHF, post CABG, fluid overload, liver and renal failure, as well as malignancy (3, 4). To our knowledge, the association between bilateral pleural effusion and mortality has not been previously reported.
In this study we determined 30-day and 1-year mortality for patients with pleural effusions of differing etiologies. We also compared the mortality rates between patients with unilateral and bilateral pleural effusion.
Methods
This prospective observational cohort study, approved by the Institutional Review Board (IRB), was performed at Yale-New Haven Hospital (YNHH), a tertiary care academic hospital with over 1000 beds. All patients who underwent a thoracentesis between December 2010 and December 2011 by the interventional pulmonary service were approached for enrollment. In addition to informed consent for the procedure, IRB-approved informed consent for the study was obtained from each participant. The interventional pulmonary service at YNHH performs the majority of the thoracenteses on both inpatients and outpatients, with a minority performed by interventional radiology, surgical services and others. Chart review and patient interviews were performed to identify baseline patient characteristics and demographics, including medical comorbidities.
All thoracenteses were performed using ultrasound guidance (SonoSite S-ICU, Bothell, WA) in a sterile fashion using the Safe-T-Centesis kit (Cardinal Health, Dublin, OH). Fluid was aspirated manually and routinely sent for analysis including cell count with differential, total protein, lactate dehydrogenase (LDH), glucose, pH, cultures (bacterial, fungal and acid-fast bacilli), and cytology. Flow cytometry was requested if lymphoma was a reasonable concern. Light’s criteria were used to classify each effusion as transudate or exudate (5). When bilateral effusions were present, concurrent bilateral thoracenteses were always performed (3).
Table 1 provides the definitions used to categorize the etiology of the pleural effusion. Patients were classified as having a certain etiology if they met one of the characteristics listed in a category. Mortality was determined at 30-day and 1-year using chart review.
Table 1.
Categories of pleural effusion etiologies and their corresponding definitions.
| Definitions for Pleural Effusion Etiology |
|---|
| Congestive heart failure Echocardiogram within 1 month or at the time of the procedure documenting any one or more of the following:
or
|
Renal failure:
|
Liver failure:
|
Pneumonia/infection:
|
Multiple benign etiologies
|
Malignant:
|
Paramalignant:
|
CXR= chest X-ray, EKG= electrocardiogram, WBC= white blood cell
Statistical Analysis
The demographics and occurrence of common comorbidities were described. Unadjusted 30-day and 1-year mortalities were calculated and diagrammed for subgroups according to the following hierarchy: laterality (bilateral versus unilateral) and malignancy (yes versus no). Non-malignant effusion was further classified by type of effusion (exudate versus transudate and mismatch, or dissimilar between sides, for some bilateral). Multivariable proportional hazards Cox models evaluated associations between short term (30-day) and long term (1-year) mortality and the following explanatory variables: age in years, male sex (referent to female), nonwhite race, number of comorbidities, bilateral thoracentesis (referent to unilateral) and indicators for each of the following etiologies (referent to malignancy): CHF, infectious disease, liver failure, renal failure, multiple benign etiologies, and paramalignant. Kaplan-Meier plots of adjusted survival probabilities were created for 30-day and 1-year mortality and stratified for bilateral versus unilateral thoracentesis. All analyses were performed using SAS V9.3 (SAS Institute, Cary, North Carolina), and P<0.05 (2-tailed) was used to denote statistical significance.
Results
A total of 320 patients over the study period were enrolled and 308 patients were included in the analysis. Twelve participants were excluded for multiple reasons including incomplete clinical data or insufficient pleural fluid for thoracentesis after enrollment in the study. Baseline patient characteristics are presented in Table 2. The majority of thoracenteses were performed on inpatients and patients with a history of cancer or a cardiac condition (53% and 80%). A total of 247 unilateral and 61 bilateral thoracenteses were performed. Overall mortality across all groups was 21% (65 of 308 patients) at 30-days and 51% (158 of 308 patients) at one year, with no procedure-related deaths.
Table 2.
Baseline characteristics and demographics.
| Patient Demographics (N=308) | |
|---|---|
| Characteristic | n (%) |
| Age in years, mean [sd] | 67.6 [14.4] |
| Male | 155 (50.3) |
| Non-white race | 48 (16.1) |
| Inpatient | 258 (83.8) |
| Number of Comorbidities, mean [sd] | 4.5 [1.9] |
| Arthritis | 163 (53.3) |
| Diabetes | 86 (27.9) |
| GI conditions* | 148 (48.4) |
| Mental issues* | 187 (60.7) |
| Liver failure/ Cirrhosis | 38 (12.3) |
| Renal failure | 67 (21.8) |
| Obesity | 59 (19.3) |
| Autoimmune disease | 76 (24.8) |
| Cancer | 162 (52.6) |
| Neurologic disease* | 52 (16.8) |
| Pulmonary disease* | 81 (26.3) |
| Sleep apnea | 25 (8.1) |
| Heart condition* | 247 (80.2) |
GI = Digestive problems (such as gastroesophageal reflux disease, Crohn’s Disease, Ulcerative Colitis or Irritable Bowel Syndrome)
Mental = Anxiety, depression or memory problems
Neurologic = Stroke, ministroke, seizure
Pulmonary = Asthma, COPD, ILD
Heart condition = MI, angina, coronary disease, arrhythmia, hypertension, hyperlipidemia, CHF, pacemaker
Missing data: Race (n=10), Arthritis (n=2), GI (n=2), Obesity (n=2), Autoimmune (n=2)
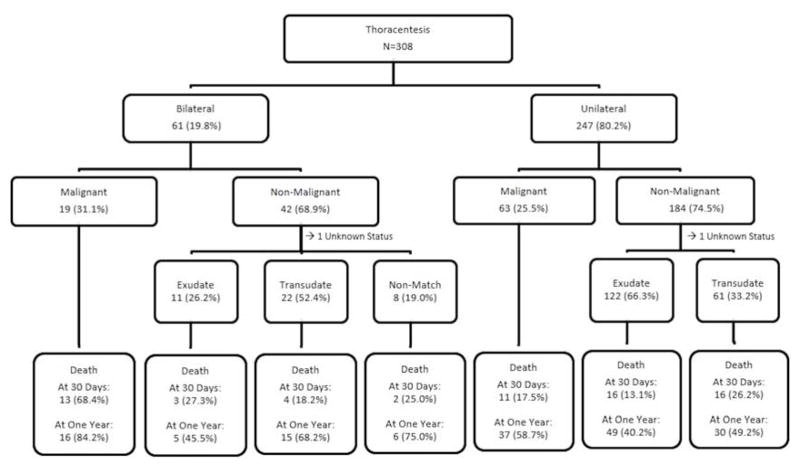
Figure 1 depicts the pleural effusion characteristics and corresponding unadjusted mortality at 30-days and 1-year. Malignant pleural effusion was identified in 27% of patients. The percentage of patients with malignancy as their etiology was similar in patients with unilateral and bilateral effusion. Pleural fluid type of non-malignant effusion differed by whether it was bilateral; most unilateral non-malignant cases were exudates whereas most bilateral non-malignant cases were transudates. As calculated from Figure 1, thirty-day and one-year mortality of patients with unilateral effusion was 17% and 47%, compared with 36% and 69% in patients with bilateral effusion. Relative to patients with unilateral effusion, unadjusted mortality of patients with bilateral effusion was higher at thirty-days (p=0.0003) and at one-year (p=0.0001).
Figure 1.
Pleural Effusions and Mortality
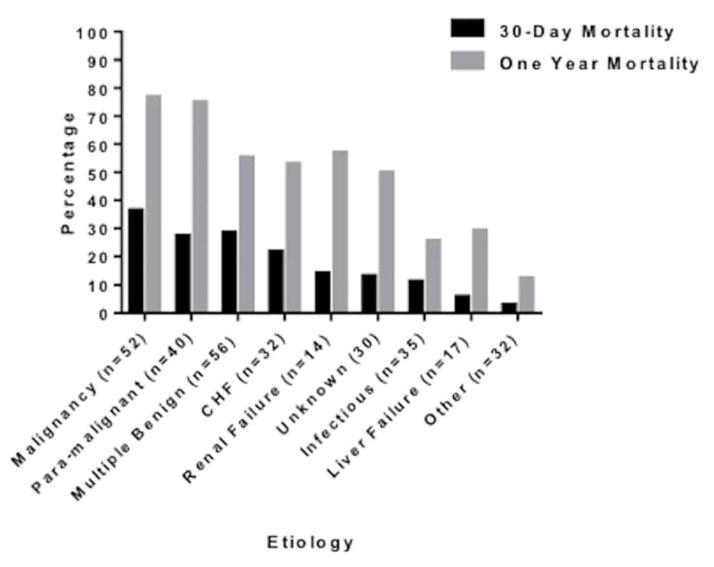
Figure 2 depicts mortality based on the etiology of the pleural effusion at 30-days and 1-year. Patients with malignant and paramalignant pleural effusion had the highest 30-day (37% and 28% respectively) and one-year mortality (77% and 75% respectively). Patients whose effusion was attributed to multiple benign etiologies also had high mortality at 30-days and 1-year (29% and 55%), as did patients with pleural effusion singularly due to CHF (22% and 53%). Patients with infection, including parapneumonic effusion and empyema, also exhibited substantive 30-day and 1-year mortality (11% and 26%).
Figure 2.
Percentage of Mortality within Etiologic Category
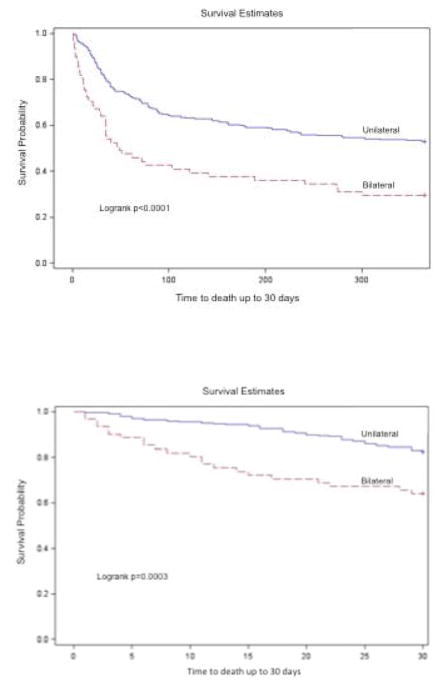
Figure 3 contrasts the Kaplan-Meier survival curves of patients with unilateral and bilateral pleural effusion at 30-days and 1-year. In both time periods, the presence of bilateral pleural effusion was associated with significantly lower survival probability.
Figure 3.
Table 3 presents associations between the following explanatory variables and short term (30-day) and long-term (1-year) mortality: age, sex, race, number of comorbidities, etiology (relative to malignancy) and bilateral (relative to unilateral). Number of comorbidities was the only covariate significantly associated with mortality at both 30-days and 1-year (HR 1.21 [1.05–1.39] p=0.009 and HR 1.19 [1.09–1.30] p=0.0002, respectively). Even after adjustment for these covariates, the presence of bilateral effusion, relative to unilateral effusion, was significantly associated with higher 30-day and 1-year mortality (HR 2.58 [1.44–4.63] p=0.002 and HR 2.32 [1.55–3.48] p<0.001, respectively).
Table 3.
Adjusted associations between characteristics of thoracentesis/pleural effusion and mortality among patients undergoing thoracentesis.
| 30 Day Mortality | 1 Year Mortality | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio (95%CI) | p-value | Hazard Ratio (95%CI) | p-value |
| Thoracentesis* | ||||
| Bilateral | 2.58 (1.44, 4.63) | 0.002 | 2.32 (1.55, 3.48) | <0.001 |
| Etiology † | ||||
| CHF | 0.33 (0.13, 0.85) | 0.021 | 0.28 (0.15, 0.54) | 0.001 |
| Infectious Disease | 0.20 (0.09, 0.45) | <0.001 | 0.20 (0.12, 0.33) | <0.001 |
| Liver disease | 0. 09 (0.01, 0.71) | 0.022 | 0.17 (0.07, 0.45) | 0.003 |
| Multiple Benign Etiologies | 0.43 (0.20, 0.90) | 0.024 | 0.36 (0.21, 0.61) | <0.001 |
| Paramalignant | 0.59 (0.28, 1.25) | 0.164 | 0.87 (0.54, 1.40) | 0.568 |
| Renal disease | 0.24 (0.05, 1.07) | 0.061 | 0.37 (0.17, 0.81) | 0.013 |
| Covariates | ||||
| Age in years | 0.99 (0.97, 1.01) | 0.430 | 1.01 (0.97, 1.02) | 0.361 |
| Male sex | 0.96 (0.58, 1.60) | 0.877 | 1.07 (0.77, 1.40) | 0.686 |
| Nonwhite race | 0.63 (0.28, 1.42) | 0.262 | 0.98 (0.61, 1.58) | 0.937 |
| Number of comorbidities | 1.21 (1.05, 1.39) | 0.009 | 1.19 (1.09, 1.30) | <0.001 |
referent to unilateral;
referent to malignant
Discussion
Encountered frequently by chest physicians and other medical sub-specialists, pleural effusion is a significant cause of dyspnea and patient suffering. Therapeutic thoracentesis may provide symptomatic relief, and care directed at the underlying etiology often determines whether the effusion recurs. This study is the first to evaluate both short- and long-term mortality in patients who have undergone thoracentesis, and whose pleural effusion is attributed to a variety of etiologies.
The short-term mortality in patients undergoing thoracentesis for pleural effusion is high, with over 20% of patients dying within 30- days. The Centers for Medicare & Medicaid Services (CMS) and Hospital Quality Alliance (HQA) began publically reporting 30-day mortality measures for acute myocardial infarction and CHF in 2007 and for pneumonia in 2008. Hospitals may be penalized for high readmission and mortality rates and rewarded for significant improvements in such measures. It stands to reason that patients with cardiac disease and pneumonia develop pleural effusion and that a heightened awareness of pleural effusion’s role in these diseases may influence treatment decisions. Recognizing that pleural effusion is associated with high mortality may prompt more aggressive treatment of the disease process driving its manifestation.
Unilateral versus bilateral effusion
The study is novel in evaluating mortality associated with bilateral effusions. We demonstrated a significant positive association with mortality at both 30-days and 1-year in patients with bilateral versus unilateral pleural effusion. Because each group had similar percentages of malignant effusions, that single etiology does not fully account for the difference. A large study of patients with community-acquired pneumonia demonstrated that patients with bilateral pleural effusion had significantly higher 30-day mortality than those with unilateral (26% versus 5.9% respectively) (6). We believe this is not attributable to the bilateral nature of the intervention, but rather an indication of seriously advanced disease.
Pleural effusion in malignancy
It is well known that dissemination of malignant cells into the pleural space is a marker of advanced malignancy. Survival of patients with malignant pleural effusion differs by type of primary tumor, with the shortest survival associated with lung cancer (7). Numerous studies, including this one, demonstrate that malignant pleural effusion is associated with poor outcomes (8–11). In this study, malignant and paramalignant effusion had similar mortality outcomes at both 30-days and 1-year, justifying our classification of paramalignant as those that are cytologically negative but most likely secondary to cancer. In our study, the highest mortality associated with pleural effusion was observed in patients with underlying malignancy. One-third of these patients died within thirty days of thoracentesis and two-thirds within one year. Patients with bilateral malignant pleural effusion are unique and have high 30-day mortality. These patients, in particular, may be better suited for symptom management via therapeutic thoracentesis than for more invasive procedures, such as pleurodesis (7). For these patients, palliation and consideration of hospice care may be more appropriate.
Pleural effusion in congestive heart failure
In the U.S., annual overall mortality for patients with CHF ranges from 10–16% (12, 13). The patients in this study with pleural effusion due to CHF had short term and annual mortality that was nearly triple that reported in other studies. Our results align with recent data reporting annual mortality of 33% in patients hospitalized with acute decompensation of chronic heart failure (14). Data was recently published indicating that pleural effusion in acute decompensated CHF does not correlate with increased mortality at 6 months (15). Notably in that study, the majority of the pleural effusion cases were mild, identified only by CXR and did not necessitate thoracentesis. We believe that pleural effusion due to CHF should be viewed as further evidence of acute decompensation and, in order to prevent readmission and death, advocate more aggressive therapy.
Infectious pleural effusion
Pleural effusion in the setting of pulmonary infection is common, occurring in up to 9% of patients with pneumonia, and is a poor prognostic sign, particularly if the effusion is moderate to large in size, bilateral and/or associated with empyema (6, 16–18). In our study, patients with infection contributing to their effusion had similar mortality as prior studies with 30-day and 1-year mortality of 11% and 26%. The treatment of complicated pleural space infection with tissue plasminogen activator and DNase improves outcome (19). Appropriate management of these cases includes early identification of empyema via thoracentesis, treatment with appropriate antibiotics, chest tube drainage, and surgery when indicated.
Pleural effusion in renal failure
A small number of patients in this study had pleural effusion due to renal failure, which is most often due to hypervolemia but can also be secondary to exudative uremic pleuritis (20). Sometimes they are also occur via communication between the peritoneum and thorax through the diaphragm in peritoneal dialysis patients (21). Mortality associated with effusion due to kidney disease has not been well studied. A small study of patients undergoing peritoneal dialysis demonstrated that patients with exudative pleural effusion, likely from uremic pleuritis, had high mortality (60% at 3 years) (22). In our small cohort of 14 patients with renal failure as the single cause of their effusion, their 30-day and 1-year mortality was 14% and 57%, respectively.
Pleural effusion associated with multiple benign etiologies
No other study has characterized patients with pleural effusion due to multiple benign etiologies and their mortality. Their high mortality is likely multifactorial. Common benign conditions contributing to development of pleural effusion include cardio-renal disease and hepato-renal disease. Underlying malnutrition and hypoalbuminemia associated with these multi-organ disease states likely contributes to increased mortality. Pleural effusion in these patient populations may be a marker of their illness severity. In the setting of multiple benign etiologies, these results provide evidence that multi-organ dysfunction is worse than single organ failure.
Study Strengths and Limitations
Although this study is a well-characterized cohort of medical patients undergoing thoracentesis, it has general applicability to the broader inpatient medical population. The patient sample size is, to our knowledge, the largest published to date that evaluates mortality by etiology of pleural effusion. It is also the first study to report the association between bilateral pleural effusion and mortality.
Limitations of this study include that it was performed at a single academic institution and the majority of patients had diseases with effusion secondary to their medical etiology. Because the majority of thoracenteses in our hospital are performed by dedicated proceduralists, patients who had their procedures performed by other providers were not enrolled. Because this cohort is limited to patients who underwent thoracentesis, we cannot say anything about mortality in patients with pleural effusion who did not undergo thoracentesis. A recent paper attempted to retrospectively address this question (23) by identifying all patients who had pleural effusion per chest imaging at time of hospital admission. That study determined 30-day and 1-year mortality of patients who had thoracentesis performed versus those who did not. Thirty-day mortality trended lower in the thoracentesis subgroup (9% versus 16%), and trended higher at 1 year (36% versus 31%). However, neither of these trends was statistically significant as this was a small, underpowered retrospective study. Mortality rates were on a whole lower in this study compared to the current study, also likely a reflection of the small sample size of eleven patients that underwent thoracentesis in a one-month period.
Pleural effusion serves as a marker of disease and is influenced by many factors. In CHF for example, multiple variables are involved that subsequently influence its course, including medication regimens, patient compliance, ventricular assist devices, co-presence of coronary artery disease and ischemia, and arrhythmia. Similar variability is encountered in patients with renal failure, infection and malignancy. Importantly, this study does not evaluate the influence on mortality of different treatment regimens to control effusion. In this sense, the pleural disease serves more as a marker of severe disease, but does not guide a specific way to decrease mortality. Rather, given the degree of mortality seen in patients with pleural effusions, the requirement of undergoing a thoracentesis for diagnostic or therapeutic purposes should indicate that the patient’s underlying disease process is advanced and that maximal therapy aimed at treating the underlying etiology is paramount.
Conclusions
Patients who undergo thoracentesis as a treatment for pleural effusion are encountered across all aspects of medicine. In addition to malignant effusion, paramalignant effusion, effusion due to multiple benign etiologies, and bilateral effusion all have considerable 30-day and 1-year mortality. Malignant effusion had the highest 30-day (37%) and 1 year (77%) mortality, followed by effusions due to multiple benign etiologies (29% and 55%), CHF (22% and 53%), and renal failure (14% and 57%). Patients with bilateral pleural effusions had a significantly higher risk of death than patients with unilateral effusions at both time points. Caregivers encountering patients with pleural effusion should be aware of their heighted mortality risks, and aggressive management of the underlying etiology is warranted.
Acknowledgments
Funding information: Yale Claude D. Pepper Older Americans Independence Center (P30AG21342).
Footnotes
This study shows malignant, bilateral and effusion due to multiple benign etiologies have significant mortality.
References
- 1.Light RW. Pleural Diseases. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Light RW. Pleural Effusions. Medical Clinics of North America. 2011;95(6):1055–70. doi: 10.1016/j.mcna.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Puchalski JT, Argento AC, Murphy TE, Araujo KL, Oliva IB, Rubinowitz AN, et al. Etiologies of bilateral pleural effusions. Respir Med. 2013;107(2):284–91. doi: 10.1016/j.rmed.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalomenidis I, Rodriguez M, Barnette R, Gupta R, Hawthorne M, Parkes KB, et al. Patient with bilateral pleural effusion: are the findings the same in each fluid? Chest. 2003;124(1):167–76. doi: 10.1378/chest.124.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 6.Hasley PB, Albaum MN, Li YH, Fuhrman CR, Britton CA, Marrie TJ, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med. 1996;156(19):2206–12. [PubMed] [Google Scholar]
- 7.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 8.Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol. 2010;5(10):1544–50. doi: 10.1097/JTO.0b013e3181e95cb8. [DOI] [PubMed] [Google Scholar]
- 9.Ozyurtkan MO, Balci AE, Cakmak M. Predictors of mortality within three months in the patients with malignant pleural effusion. Eur J Intern Med. 2010;21(1):30–4. doi: 10.1016/j.ejim.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000;117(1):79–86. doi: 10.1378/chest.117.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362–8. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 12.Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of Carvedilol on Survival in Severe Chronic Heart Failure. New England Journal of Medicine. 2001;344(22):1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JG, Daubert J, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. New England Journal of Medicine. 2005;352(15):1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 14.Lassus JP, Siirila-Waris K, Nieminen MS, Tolonen J, Tarvasmaki T, Peuhkurinen K, et al. Long-term survival after hospitalization for acute heart failure - differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168(1):458–62. doi: 10.1016/j.ijcard.2012.09.128. [DOI] [PubMed] [Google Scholar]
- 15.Davutoglu V, Yildirim C, Kucukaslan H, Yuce M, Sari I, Tarakcioglu M, et al. Prognostic value of pleural effusion, CA-125 and NT-proBNP in patients with acute decompensated heart failure. Kardiol Pol. 2010;68(7):771–8. [PubMed] [Google Scholar]
- 16.Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis. 2007;45(11):1480–6. doi: 10.1086/522996. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson AD, Prescott RJ, Selkon JB, Watson D, Swinburn CR. The clinical course and management of thoracic empyema. Qjm. 1996;89(4):285–9. doi: 10.1093/qjmed/89.4.285. [DOI] [PubMed] [Google Scholar]
- 18.Davies CW, Kearney SE, Gleeson FV, Davies RJ. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1682–7. doi: 10.1164/ajrccm.160.5.9903002. [DOI] [PubMed] [Google Scholar]
- 19.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural Use of Tissue Plasminogen Activator and DNase in Pleural Infection. New England Journal of Medicine. 2011;365(6):518–26. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 20.Bakirci T, Sasak G, Ozturk S, Akcay S, Sezer S, Haberal M. Pleural effusion in long-term hemodialysis patients. Transplant Proc. 2007;39(4):889–91. doi: 10.1016/j.transproceed.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Lew SQ. Hydrothorax: pleural effusion associated with peritoneal dialysis. Perit Dial Int. 2010;30(1):13–8. doi: 10.3747/pdi.2008.00168. [DOI] [PubMed] [Google Scholar]
- 22.Kwan BC, Chow KM, Pang WF, Leung CB, Li PK, Szeto CC. Unexplained exudative pleural effusion in chronic peritoneal dialysis patients. Perit Dial Int. 2010;30(5):534–40. doi: 10.3747/pdi.2009.00135. [DOI] [PubMed] [Google Scholar]
- 23.Kookoolis AS, Puchalski JT, Murphy TE, Araujo KL, Pisani MA. Mortality of Hospitalized Patients with Pleural Effusions. J Pulm Respir Med. 2014;4(184) doi: 10.4172/2161-105X.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]