Abstract
Curcumin exerts beneficial effects on cardiovascular diseases, including hypertension. However, its mechanisms are unknown. We propose that curcumin prevents the development of hypertension by regulating AT1 receptor (AT1R) expression in arteries. The present study examined how curcumin regulates AT1R expression in vascular smooth muscle cells and investigated the physiological significance of this regulation in angiotensin (Ang) II-induced hypertension. The results showed that curcumin decreased AT1R expression in a concentration- and time-dependent manner in vascular smooth muscle cells. Using luciferase reporters with an entire AT1 or a mutant AT1R in A10 cells, the AT1R promoter activity was inhibited by 10−6 M curcumin, and the proximal element (from −61 to +25 bp) of the AT1R promoter was crucial for curcumin-induced AT1R down-regulation. An electrophoretic mobility shift assay showed that curcumin decreased specificity protein 1 (SP1) binding with the AT1R promoter in A10 cells. Curcumin treatment reduced Ang II-induced hypertension in C57Bl/6J mice, which was accompanied by lower AT1R expression in the arteries and decreased Ang II-mediated vasoconstriction in the mesenteric artery. These findings indicate that curcumin down-regulates AT1R expression in A10 cells by affecting SP1/AT1R DNA binding, thus reducing AT1R-mediated vasoconstriction and subsequently prevents the development of hypertension in an Ang II-induced hypertensive model.
The renin-angiotensin system (RAS) is one of the most important regulators of arterial blood pressure, and RAS activation is related to the pathogenesis of hypertension1. Angiotensin II (Ang II), the principal RAS effector peptide, binds to two distinct receptors: the Ang II type-1 receptor (AT1R) and the Ang II type-2 (AT2R) receptor. Most Ang II actions are transmitted via AT1R, including vasoconstriction, reduction of vascular compliance, cardiac contractility, cellular dedifferentiation and proliferation. Therefore, AT1R antagonists decrease blood pressure in patients with hypertension2,3.
Turmeric (prepared from the root of Curcuma longa) belongs to the ginger family. Curcumin, which is the main active constituent of turmeric, exerts anti-oxidant, anti-bacterial, anti-fungal, anti-viral, anti-inflammatory, anti-proliferative, pro-apoptotic and anti-atherosclerotic effects. Curcumin has been used in traditional Indian medicine for the treatment of many diseases, such as wounds, arthritis, trauma, ulcers, fever and skin diseases, over the past 60 years4,5. Furthermore, curcumin exerts beneficial effects on cardiovascular disease, although the molecular targets are multiple. Curcumin might regulate DNA polymerase, protein kinase C, lipoxygenase, growth factors, transcription factors, inflammatory cytokines, adhesion molecules and apoptosis-related proteins6. Recently, curcumin was reported to prevent the development of hypertension and vascular remodeling and to protect against cadmium-induced vascular dysfunction and hypertension7,8,9,10. We propose that curcumin might prevent the development of hypertension by regulating AT1R in arteries. The present study examined how curcumin regulates AT1R expression in A10 cells, a rat thoracic aorta-derived smooth muscle cell line, and investigated the physiological significance of this regulation in Ang II-induced hypertension. The results shows that, via affecting specificity protein 1 (SP1)/AT1R DNA binding, curcumin down-regulated AT1R expression in A10 cells, reduced AT1R-mediated vasoconstriction, and subsequently prevented the development of hypertension in an Ang II-induced hypertensive model.
Results
The inhibitory effect of curcumin on AT1R expression at the transcriptional level in A10 cells
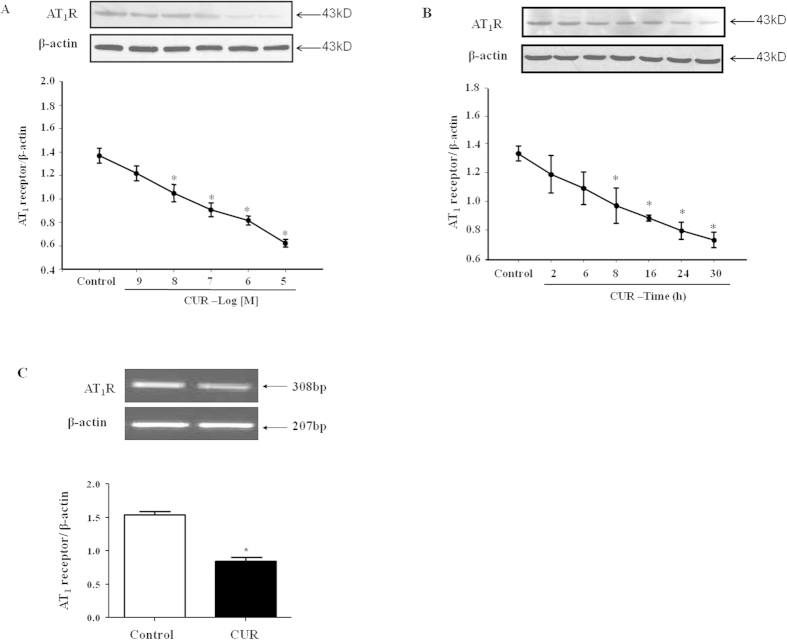
A10 cells were incubated with curcumin at different concentrations and for different time periods. Curcumin decreased the AT1R protein expression in a concentration (10−5–10−9 M, 24 h)- and time (10−6 M, 2–30 h)-dependent manner. Western blot analysis revealed that curcumin decreased AT1R protein levels significantly at 10−8 mol/L compared with the control level, and the reduction reached a maximum at 10−5 mol/L (Fig. 1A). Curcumin also down-regulated AT1R protein levels in a time-dependent manner; the reduction reached a maximum at 30 h (Fig. 1B). The expression of AT1R mRNA, as determined by RT-PCR, was significantly reduced by curcumin (10−6 M) at 24 h compared with the control (Fig. 1C). Therefore, the regulation of curcumin on AT1R expression may occur at the transcriptional or post-transcriptional level.
Figure 1. The inhibitory effect of curcumin on AT1R protein and mRNA expression in A10 cells.
Curcumin decreased the AT1R protein expression in a concentration- (for 24 hrs) (A) and time- (at 10−6 M) (B) dependent manner. (C) AT1R mRNA expression was also inhibited by curcumin (CUR, 10−6 M/24 hrs) compared with the control (C). Cropped gels/blots were used in the figure; the AT1R expression was normalized against β-actin. The values are expressed as the mean ± SEM (*P < 0.05 vs. control, n = 6).
To determine whether the regulation of curcumin on AT1R occurs at the protein degradation level, A10 cells were treated with cycloheximide (10−6 M) to block de novo protein synthesis. Cycloheximide had no effect on the down-regulation of curcumin on AT1R protein expression (Fig. 2A), which demonstrates that AT1R degradation is not involved in the mechanism. We then examined whether curcumin affects the stability of AT1R mRNA. Actinomycin D (ActD, 5 μg/ml), an inhibitor of de novo mRNA synthesis, was used in this study. Actinomycin D had no effect on the down-regulation of curcumin on AT1R expression (Fig. 2B). These results indicate that curcumin does not regulate AT1R protein expression at the protein or mRNA degradation levels, suggesting that curcumin regulates AT1R expression at the transcriptional level.
Figure 2. Effect of cycloheximide and actinomycin D on curcumin-induced AT1R downregulation.
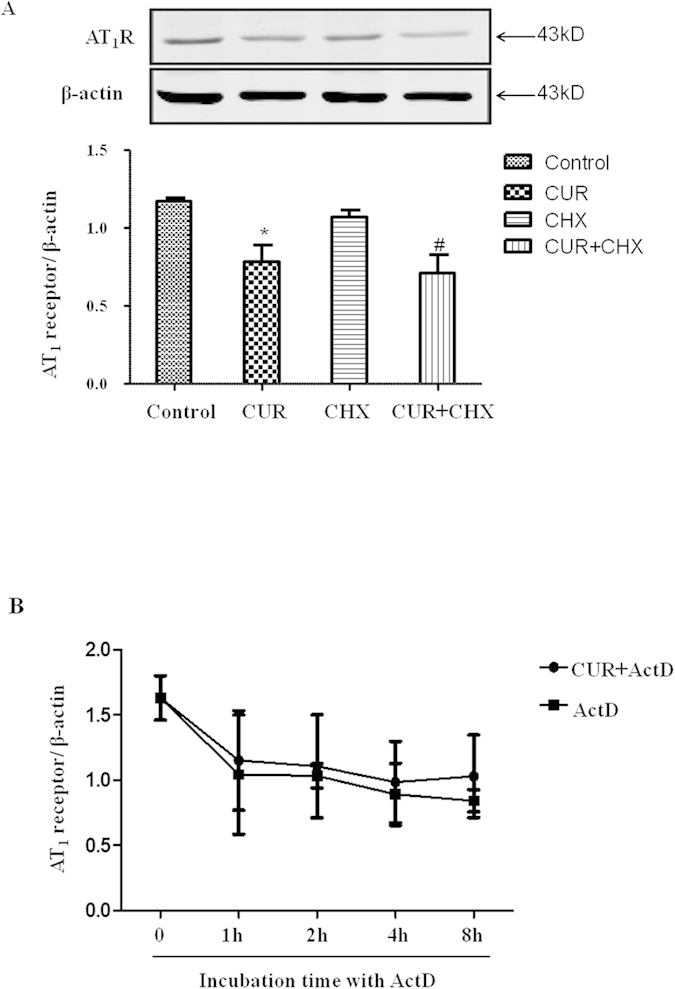
(A) The effect of cycloheximide (CHX) on curcumin (CUR)-induced AT1R protein down-regulation was examined by Western blot analysis; cropped blots are shown. The AT1R expression level was normalized against β-actin (*P < 0.05 vs. control, #P < 0.05 vs. CHX, n = 7). (B) The effect of actinomycin D (ActD) on CUR-induced AT1R mRNA down-regulation was examined by RT-PCR analysis. The AT1R expression was normalized against β-actin. The values are expressed as the mean ± SEM (*P < 0.05 vs. control, n = 7).
Role of the transcription factor SP1 in curcumin-induced down-regulation of AT1R expression in A10 cells
To elucidate the molecular mechanism underlying the curcumin-induced down-regulation of AT1R gene expression, five AT1R promoter-luciferase DNA constructs were transfected into A10 cells (Fig. 3A). The deletion mutants of the AT1R promoter resulted in decreased promoter activity. AT1R promoter activity was inhibited by 10−6 M curcumin in all constructs (Fig. 3B), suggesting that curcumin inhibited AT1R expression at the transcriptional level and that the proximal element (from −61 to +25 bp) of the AT1R promoter was crucial for curcumin-induced AT1R down-regulation.
Figure 3. Effect of curcumin on AT1R mRNA transcription.
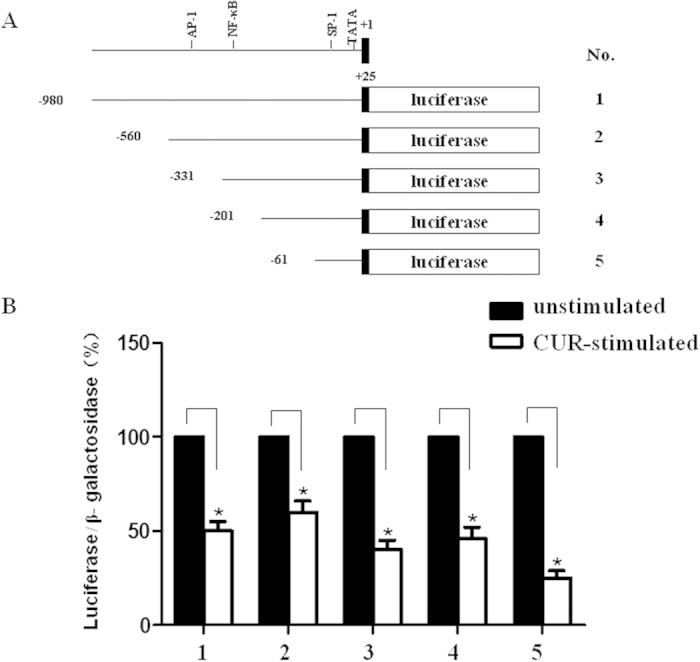
(A) Schematic of deletion mutants of the AT1R promoter/luciferase fusion DNA construct. (B) The bar graphs indicate luciferase activity normalized against β-galactosidase activity derived from the corresponding deletion. The data indicate the luciferase activity of curcumin-stimulated A10 cells (white bars) relative to unstimulated (black bars) in each group. The values (mean ± SEM) are expressed as a percent (*P < 0.05 vs. unstimulated, n = 6).
The ubiquitous transcription factor SP1 is important for the regulation of AT1R expression11,12. We detected DNA-binding protein bound to the SP1 site using an electrophoretic mobility shift assay (Fig. 4). When nuclear extracts from curcumin-stimulated A10 cells (lanes 3) were used, DNA-binding protein (arrow) was decreased compared with unstimulated A10 cells (lanes 2). No nuclear extracts and 50 times of unlabeled probe were added to the reaction mixture as controls (lanes 1 and 4, respectively), and these controls did not exhibit this band, confirming the binding specificity.
Figure 4. Effect of curcumin on the binding ability of SP1 at the AT1R gene promoter in A10 cells.

The binding activity of the AT1R gene promoter (−40 bp to −6 bp), which contains an Sp1 site, was examined in the nuclear protein from unstimulated (lane 2) or curcumin (10−6 M)-stimulated (lane 3) A10 cells by electrophoretic mobility shift assay; DNA-binding protein (arrow) was decreased compared with unstimulated A10 cells. No nuclear extracts or 50 times of unlabeled probe were added to the reaction mixture as controls (lanes 1 and 4, respectively).
Inhibition of AT1R expression by Curcumin and its function in arteries from Ang II-induced hypertensive C57Bl/6J mice
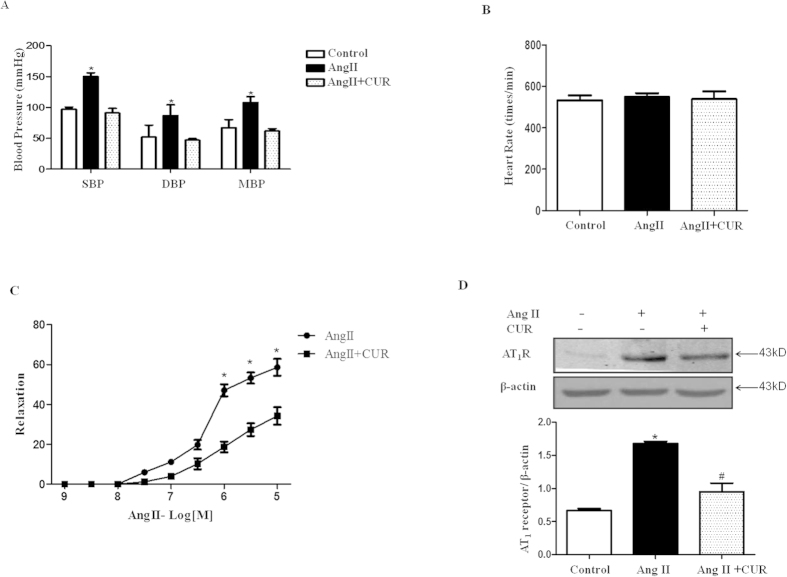
To investigate the physiological significance of the regulation of curcumin on AT1R expression, we established an Ang II-induced hypertension model in C57Bl/6J mice. Eight-week-old mice (weight 20 g ± 0.89) were divided into three groups: control, Ang II (490 ng/min/kg), and Ang II (490 ng/min/kg) + curcumin (300 mg/kg/d). Ang II was administered subcutaneously via an osmotic minipump (Model 1004, ALZET Osmotic Pumps, CA) for one week. Curcumin was suspended in normal saline, and the mice were given the suspension by oral gavage once per day; normal saline was used as a control. After one week, the blood pressure and heart rate were measured using the tail-cuff method. The blood pressures of the Ang II-treated mice (SBP: 150 ± 6.24 mmHg, DBP: 86 ± 17.61 mmHg) were significantly higher than the Ang II + curcumin group (SBP: 91 ± 7.55 mmHg, DBP: 47 ± 2.64 mmHg) (Fig. 5A). However, the heart rate was not significantly different between the individual groups (Fig. 5B).
Figure 5. Effect of curcumin on AT1R expression and function in arteries from Ang II-induced hypertensive C57Bl/6J mice.
The blood pressure (A) and heart rate (B) were examined in Ang II-induced hypertensive C57Bl/6J mice with or without curcumin treatment (*P < 0.05 vs. control, n = 5). The Ang II-mediated vasoconstriction in the third-order mesenteric arteries (B) and the AT1R expression in the aorta (D) were examined in these mice (*P < 0.05 vs. others, n = 7). Cropped blots were used in this figure; the AT1R expression was normalized against β-actin. The values are expressed as the mean ± SEM (*P < 0.05 vs. control group, #P < 0.05 vs. Ang II group, n = 7).
To determine how curcumin exerts its function in Ang II-related hypertension, we studied AT1R function in third-order mesenteric arteries from the mice of the Ang II and Ang II + curcumin groups. Isolated mesenteric arteries from normal C57Bl/6J mice were used to measure the contractile responses to different concentrations of phenylephrine and KCl and the relaxant response to acetylcholine (Ach). The effect of curcumin (10−6 M) alone on mesenteric arteries was compared. The mesenteric arteries were incubated with curcumin for 60 min before starting the experiment. The changes in contractile and relaxant responses to phenylephrine, KCl and Ach were also measured. Our results showed that curcumin slightly decreased the contractile response of aortic rings to phenylephrine and KCl (Supplemental Fig. 1A), whereas it did not affect the relaxant response to Ach (Supplemental Fig. 1B). We found that Ang II (10−9–10−5 M) induced concentration-dependent vasoconstriction in the third-order mesenteric arteries from the mice; the vasoconstrictive effect of Ang II was lowered significantly by curcumin (Fig. 5C). Further experiments were performed to examine AT1R expression in the arteries, and curcumin reduced the increased AT1R protein levels in the aortas of Ang II-induced hypertensive C57Bl/6J mice (Fig. 5D).
Discussion
Previous studies have demonstrated that curcumin possesses strong antioxidant and cardioprotective properties. Therefore, curcumin is considered to be a new therapeutic candidate for cardiovascular diseases, including idiopathic pulmonary arterial hypertension, glomerular hypertension and L-NAME-induced hypertension7,8,9,10,13,14. However, the role of curcumin in hypertension and its associated complications remains unknown. In this study, we determined that curcumin suppressed AT1R expression in in vitro and in vivo experiments. To our knowledge, this is the first report demonstrating the down-regulation of vascular AT1R by curcumin.
The beneficial effects of curcumin on L-NAME-induced vascular dysfunction are associated with increased eNOS protein expression, reduced oxidative stress, and replenished antioxidant glutathione with partial restoration of normal redox status7. As one of the major regulatory factors of blood pressure, we were interested in whether curcumin regulated AT1R expression. Our present study showed that curcumin lowers the blood pressure in Ang II-induced hypertensive C57Bl/6J mice, and this anti-hypertensive effect is partially attributed to the inhibition of AT1R expression in arteries. Moreover, curcumin-regulated AT1R expression in the arteries is of physiological significance because the Ang II-mediated vasoconstrictive effect is lower in Ang II-induced hypertensive C57Bl/6J mice after treatment with curcumin. The anti-oxidative effect of curcumin has been shown in hypertensive rats7,10,15,16; increased ROS increases AT1R expression in the kidney17,18,19. However, whether ROS is involved in the regulation of curcumin on arterial AT1R expression remains unknown and needs to be determined in the future.
The underlying mechanisms regulating AT1R expression are complicated and might involve several levels, including transcriptional and post-transcriptional levels13,17,20,21. To determine whether the regulation occurs at the post-transcriptional or post-translational levels, we treated cells with cycloheximide to block de novo protein synthesis or actinomycin D to block de novo mRNA synthesis; cycloheximide and actinomycin D had no effect on the regulation of curcumin on AT1R expression in A10 cells, which demonstrated that the regulation of curcumin on AT1R protein expression did not occur at the protein or mRNA degradation levels and that the regulation of curcumin on AT1R expression occurs at the transcriptional level. The deletion mutant analysis of the AT1R gene promoter (from −980 bp to +25 bp) revealed that the inhibition of AT1R expression by curcumin depends on the most proximal promoter region (from −61 bp to +25 bp), which contains an SP1 binding site (GC box). A gel shift assay showed that DNA-binding protein bound to the SP1 site was decreased in curcumin-stimulated A10 cells, indicating a crucial role of the SP1 site in curcumin-induced AT1R down-regulation.
This study demonstrated that curcumin suppressed AT1R protein expression by inhibiting SP1 activity. The ability of curcumin to inhibit AT1R expression might be of clinical significance. Whether or not there is a synergistic or additional effect between curcumin and other anti-hypertensive medicines, including ARBs and ACE inhibitors, needs to be identified in the future.
Methods
Materials
The rabbit polyclonal AT1R antibody was purchased from Santa Cruz Biotechnology (sc-1173, Dallas, TX). Curcumin was obtained from Sigma (c7727, St. Louis, MO); Ang II was purchased from Calbiochem (4474-91-3, San Diego, CA); Dulbecco’s modified eagle medium (DMEM, Ca# 11995065,) and fetal bovine serum (FBS, Ca# 16000044) were obtained from Life Technologies (Carlsbad, CA). All other chemicals for various buffers were of the highest purity available and were purchased from Sigma or Gibco (Grand Island, NY).
Cell Culture
Embryonic thoracic aortic smooth muscle cells (passage 10–20) from normotensive Berlin-Druckrey IX22 (A10; American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% FBS at 37 °C in a 95% air-5% CO2 atmosphere and were used for the experiments.
Western Blot Analysis
The expression of AT1R was detected by Western blot analysis, as described previously23. The specific band was scanned with an imaging analyzer, and β-actin was used as a loading control. The expression level of AT1R was indicated as a ratio of AT1R to β-actin.
RT-PCR for AT1R
A total of 2 μg of total RNA extracted from A10 cells was used to synthesize cDNA and served as a template for the amplification of AT1R and β-actin, which served as the house-keeping gene control. The AT1R mRNA expression levels were normalized to β-actin mRNA. The primers for β-actin included forward primer 5′-CCACTGCCGCATCCTCTT-3′ and reverse primer 5′-GTCAGCAATGCCTGGGTA-3′ (GenBank accession No. NM_031144, 251 bp). The primers for AT1R included forward primer 5′-AAATTGAGTGGCTGTATG-3′ and reverse primer 5′-CTTGACCTCCCATCTCCT-3′ (GenBank accession No. NM_031009, 160 bp).
Transfection of AT1R Promoter-Luciferase Fusion DNA Construct into VSMCs
Five deletion mutants of the rat AT1R gene promoter region were ligated to the luciferase gene, as described previously24. A10 cells were prepared in a 6-cm tissue culture dish. After 48 hours, 5 μg of the AT1R promoter luciferase fusion DNA construct and 2 μg of the LacZ gene driven by the SV40 promoter-enhancer sequence were transfected into the A10 cells. In addition to the AT1R promoter luciferase fusion DNA construct (980 bp, 2 μg) and the LacZ gene (2 μg), vector plasmid (3 μg) was introduced concomitantly into the A10 cells. These cells were stimulated with 10−6 M25,26,27 curcumin and cultured in DMEM with 10% FBS for 48 hours. The luciferase activity was then measured and normalized against the β-galactosidase activity.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assay
An EMSA was performed with a Light-shift Chemiluminescent EMSA Kit (Ca# 20158, Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommendations28. Using a non-isotopic method to detect DNA–protein interactions, this system includes an enhanced luminol substrate for horseradish peroxidase with optimized blocking and washing buffers that produce sensitivity equivalent to radioactive (32P) systems. A synthetic DNA double-stranded oligonucleotide probe containing the sequence of the rat AT1R gene promoter between nucleotides −40 and −6 bp (5′-GGAACCTGCAGAGCAGCGA CGCCCCCTAGGCT ATA-3′, 3′-CCTTGGACGTCTCGTCGCTGCGGGGGATCCGATAT-5′, both of which contain an Sp1 site) was labeled with biotin and incubated with the nuclear extracts. After the reaction, the DNA–protein complexes were subjected to 6% native polyacrylamide gel electrophoresis and transferred onto a nylon membrane (Millipore Corp. Lincoln Park, NJ). After transfer, the membrane was immediately cross-linked for 15 min on a UV transilluminator. A chemiluminescent detection method using a luminal/enhancer solution and stable peroxide solution (Thermo Fisher Scientific, Waltham, MA) was used, as described by the manufacturer, and the membranes were exposed to X-ray films for 30 s to 5 min before development.
Animal Experiment
Eight-week-old C57Bl/6J mice, weighing approximately 20 g, were purchased from the Laboratory Animal Center of Daping Hospital of the Third Military Medical University. The mice were divided into the following experimental groups: control, Ang II (490 ng/min/kg), and Ang II (490 ng/min/kg) + curcumin (300 mg/kg/d). Curcumin was suspended in normal saline, and the mice were administered the suspension by oral gavage once per day for one week. The estimated dose of curcumin was 300 mg/kg/d29,30. In the Ang II group, 490 ng/min/kg of Ang II was administered subcutaneously via an osmotic minipump (Model 1004, ALZET Osmotic Pumps, CA). After one week, the blood pressure and heart rate were measured using the tail-cuff method (UR-5000, UEDA, Japan); the mice were then sacrificed under pentobarbital anesthesia (60 mg/kg). The thoracic aorta was removed quickly, minced into small pieces, lysed in a lysis buffer, sonicated, placed on ice for 1 h and centrifuged at 15000 rpm for 30 min at 4 °C. The supernatants were stored at −70 °C until being subjected to Western blot analysis for AT1R and β-actin.
All study procedures were approved by the Third Military Medical University Animal Use and Care Committee. All experiments conformed to the guidelines of the ethical use of animals, and all efforts were made to minimize animal suffering and to reduce the number of animals used.
Mesenteric Artery Study
Each mouse was anesthetized with pentobarbital. The total mesenteric bed was removed carefully and placed in an ice-cold physiological salt solution (PSS, containing NaCl 119 mM, KCl 4.7 mM, CaCl2·H2O 2.5 mM, MgSO4·H2O 1.17 mM, NaH2CO3 25 mM, KH2PO4 1.18 mM, EDTA 0.027 mM, and glucose 5.5 mM, adjusted to pH 7.4). The surrounding fat and connective tissues of the mesenteric artery were carefully and quickly dissected. Third-order branches of the superior mesenteric artery were cut into rings approximately 2 mm in length and mounted on 25 μm tungsten wires in an isometric Mulvany-Halpern small-vessel myograph (model 91 M610, J.P. Trading, Science Park, Aarhus, Denmark). The rings were maintained in ice-cold PSS at 37 °C and continuously bubbled with oxygen (95%) and carbon dioxide (5%) (Carbogen). All procedures were performed carefully to protect the endothelium from any damage. In the first set of experiments, the rings were contracted with high-potassium PSS (KPSS, 70 mM) to obtain the maximal response31,32. After obtaining the maximal response to KPSS, the response curves to Ang II were measured by a cumulative concentration-dependent protocol (10−9 to 10−5 M). To test the curcumin-induced inhibition of vasoconstriction, the mice were administered curcumin by oral gavage once per day for 1 week before the Ang II treatment.
Statistical Analysis
Statistical analysis was performed with performed 1- or 2-way ANOVA and Fisher’s test, if appropriate. The data are shown as the mean ± SEM. A value of P < 0.05 was considered significant.
Additional Information
How to cite this article: Yao, Y. et al. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 6, 25579; doi: 10.1038/srep25579 (2016).
Supplementary Material
Acknowledgments
These studies were partly supported by grants from the National Natural Science Foundation of China (31430043, 81570379), the Natural Science Foundation Project of Chongqing (cstc2015jcyjA10060), the National International Technology Special Grant (2014DFA31070) and the National Basic Research Program of China (2012CB517801).
Footnotes
Author Contributions Y.Y., J.Y. and C.Z. conceived and designed the experiments. Y.Y., W.W., M.L., C.C. and J.W. performed the experiments. Y.Y., H. R. and W.E.W. analyzed the data. Y.Y., H. R. and W.W. contributed reagents/materials/tools. Y.Y. prepared the first draft of the manuscript. J.Y. and C.Z. provided substantial input for manuscript writing. All authors read and approved the final manuscript.
References
- Moon J. Y. Recent Update of Renin-angiotensin-aldosterone System in the Pathogenesis of Hypertension. Electrolyte Blood Press 11, 41–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajbegovic S. et al. Valsartan in the treatment of hypertension. Med Arh 67, 174–177 (2013). [DOI] [PubMed] [Google Scholar]
- Tzemos N., Lim P. O. & MacDonald T. M. Valsartan improves endothelial dysfunction in hypertension: a randomized, double-blind study. Cardiovasc Ther 27, 151–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. W., Fu M., Gao S. H. & Liu J. L. Curcumin and Diabetes: A Systematic Review. Evid Based Complement Alternat Med 2013, 636053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher H., Planalp R., Cho J., Torti F. M. & Torti S. V. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65, 1631–1652 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Beevers C. S. & Huang S. The targets of curcumin. Curr Drug Targets 12, 332–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakmareong S. et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol 383, 519–529 (2011). [DOI] [PubMed] [Google Scholar]
- Hlavackova L. et al. Spice up the hypertension diet - curcumin and piperine prevent remodeling of aorta in experimental L-NAME induced hypertension. Nutr Metab (Lond) 8, 72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukongviriyapan U. et al. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients 6, 1194–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakmareong S. et al. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens Res 35, 418–425 (2012). [DOI] [PubMed] [Google Scholar]
- Imayama I. et al. Liver X receptor activator downregulates angiotensin II type 1 receptor expression through dephosphorylation of Sp1. Hypertension 51, 1631–1636 (2008). [DOI] [PubMed] [Google Scholar]
- Kubo T., Kinjyo N., Ikezawa A., Kambe T. & Fukumori R. Sp1 decoy oligodeoxynucleotide decreases angiotensin receptor expression and blood pressure in spontaneously hypertensive rats. Brain Res 992, 1–8 (2003). [DOI] [PubMed] [Google Scholar]
- Khajehdehi P. et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr 22, 50–57 (2012). [DOI] [PubMed] [Google Scholar]
- Bronte E. et al. Role of curcumin in idiopathic pulmonary arterial hypertension treatment: a new therapeutic possibility. Med Hypotheses 81, 923–926 (2013). [DOI] [PubMed] [Google Scholar]
- Boonla O. et al. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide 42, 44–53 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal 25, 615–629 (2013). [DOI] [PubMed] [Google Scholar]
- Chugh G., Lokhandwala M. F. & Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol 300, F133–138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banday A. A. & Lokhandwala M. F. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol 295, F698–706 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banday A. A. & Lokhandwala M. F. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 57, 452–459 (2011). [DOI] [PubMed] [Google Scholar]
- Li H. et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118, 2180–2189 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banday A. A., Siddiqui A. H., Menezes M. M. & Hussain T. Insulin treatment enhances AT1 receptor function in OK cells. Am J Physiol Renal Physiol 288, F1213–1219 (2005). [DOI] [PubMed] [Google Scholar]
- Chen K. et al. Role of GRK4 in the regulation of arterial AT1 receptor in hypertension. Hypertension 63, 289–296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C. et al. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 99, 494–500 (2006). [DOI] [PubMed] [Google Scholar]
- Takeda K., Ichiki T., Funakoshi Y., Ito K. & Takeshita A. Downregulation of angiotensin II type 1 receptor by all-trans retinoic acid in vascular smooth muscle cells. Hypertension 35, 297–302 (2000). [DOI] [PubMed] [Google Scholar]
- Yang X. et al. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol 26, 85–90 (2006). [DOI] [PubMed] [Google Scholar]
- Grabowska W. et al. Curcumin induces senescence of primary human cells building the vasculature in a DNA damage and ATM-independent manner. Age (Dordr) 37, 9744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska A. et al. Curcumin induces oxidation-dependent cell cycle arrest mediated by SIRT7 inhibition of rDNA transcription in human aortic smooth muscle cells. Toxicol Lett 18, 233, 227–238 (2015). [DOI] [PubMed] [Google Scholar]
- Oltra E., Pfeifer I. & Werner R. Ini, a small nuclear protein that enhances the response of the connexin43 gene to estrogen. Endocrinology 144, 3148–3158 (2003). [DOI] [PubMed] [Google Scholar]
- Rungseesantivanon S., Thengchaisri N., Ruangvejvorachai P. & Patumraj S. Curcumin improves prostanoid ratio in diabetic mesenteric arteries associated with cyclooxygenase-2 and NF-kappaB suppression. Diabetes Metab Syndr Obes 3, 421–429 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungseesantivanon S., Thengchaisri N., Ruangvejvorachai P. & Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med 10, 57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. et al. Impaired dopamine D1 receptor-mediated vasorelaxation of mesenteric arteries in obese Zucker rats. Cardiovasc Diabetol 13, 50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjerning J. et al. Histamine-dependent prolongation by aldosterone of vasoconstriction in isolated small mesenteric arteries of the mouse. Am J Physiol Heart Circ Physiol 304, H1094–1102 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.