Abstract
Welding has been associated with neurobehavioral disorders. Welding fumes contain several metals including copper (Cu), manganese (Mn), and iron (Fe) that may interact to influence welding-related neurotoxicity. Although welding-related airborne Fe levels are about 10-fold higher than Mn, previous studies have focused on Mn and its accumulation in the basal ganglia. This study examined differences in the apparent transverse relaxation rates [R2* (1/T2*), estimate of Fe accumulation] in the basal ganglia (caudate nucleus, putamen, and globus pallidus) between welders and controls, and the dose–response relationship between estimated Fe exposure and R2* values. Occupational questionnaires estimated recent and lifetime Fe exposure, and blood Fe levels and brain magnetic resonance imaging (MRI) were obtained. Complete exposure and MRI R2* and R1 (1/T1: measure to estimate Mn accumulation) data from 42 subjects with welding exposure and 29 controls were analyzed. Welders had significantly greater exposure metrics and higher whole-blood Fe levels compared with controls. R2* in the caudate nucleus was significantly higher in welders after controlling for age, body mass index, respirator use, caudate R1, and blood metals of Cu and Mn, whereas there was no difference in R1 values in the basal ganglia between groups. The R2* in the caudate nucleus was positively correlated with whole-blood Fe concentration. This study provides the first evidence of higher R2* in the caudate nucleus of welders, which is suggestive of increased Fe accumulation in this area. Further studies are needed to replicate the findings and determine the neurobehavioral relevance.
Key words: welders, iron, R2*, basal ganglia, caudate nucleus
Welding has been associated with neurobehavioral disorders (Cersosimo and Koller, 2006). Welding fumes contain several metals originating from the electrodes and base metals, and previous studies suggest that these metal mixtures may interact to cause welding-related toxicity, especially for welders with chronic exposure (Lu et al., 2005). Iron (Fe), copper (Cu), and manganese (Mn) are among the major elements in many types of welding fumes (Burgess and Burgess, 1995). Although airborne Fe concentrations are about ten-fold greater than those of Mn (Ellingsen et al., 2006; Flynn and Susi, 2009) and whole blood Fe levels are much higher than those of Mn (Lu et al., 2005), past welding-related studies have focused on Mn accumulation in brain (Choi et al., 2007; Lee et al., 2015) with few studies examining brain deposition of Fe in welders or mine workers (Criswell et al., 2015; Long et al., 2014).
The major route of inhaled Fe into the brain is via the blood-brain-barrier (BBB), although Fe also can enter the brain via the blood–cerebrospinal fluid (CSF) barrier and perhaps olfactory pathways (Yokel, 2006). Influx of Fe into the brain is carrier-mediated, but Fe efflux is not fully understood (Ward et al., 2014). Fe uptake into brain is affected by several factors including particle size and the presence of other metals such as Mn and Cu. For example, Fe and Mn may compete for common transporters [e.g., transferrin and/or divalent metal transporter-1 (DMT1)] (Erikson et al., 2004), and Cu overload may decrease Fe brain uptake (Crowe and Morgan, 1996). Thus, increased levels of welding-related metals other than Fe (e.g., Mn, Cu, etc.) may influence its uptake into the brain and vice versa. Brain Fe distribution has regional specificity, with the highest concentrations found in the extrapyramidal system, less in the cerebral cortex, and least in the prefrontal cortex. Among the structures in the extrapyramidal system, the basal ganglia have the highest Fe concentration (primarily the globus pallidus [GP]), followed by putamen (PUT) and caudate nucleus (CN) (Hallgren and Sourander, 1958).
Similar to Mn, Fe is an essential element that can be neurotoxic at higher doses (Sipe et al., 2002). It has long been known that brain deposition of Fe increases with aging (Hallgren and Sourander, 1958), and Fe overload in the brain is associated with neurodegenerative disorders (Wallis et al., 2008; Ward et al., 2014). Indeed, pathological studies have demonstrated increased Fe accumulation in the substantia nigra of Parkinson’s patients (Dexter et al., 1991; Sofic et al., 1991).
In magnetic resonance imaging (MRI), Fe has paramagnetic characteristics and shortens the apparent transverse relaxation time (T2*; Haacke et al., 2005). Thus, one of the commonly used methods to assess Fe accumulation in human brain is measurement of the T2* relaxation rate [R2* (1/T2*)]. Indeed, several neuroimaging studies (Gelman et al., 1999; Peran et al., 2010) established associations between R2* values in selected brain areas (including the basal ganglia) and brain Fe content estimated from the postmortem data of Hallgren and Sourander (1958). Other neuroimaging studies have shown increased R2* in the substantia nigra of Parkinson’s patients (Du et al., 2011; Ulla et al., 2013). R2* may be, however, affected by the presence of other paramagnetic metals (e.g., Cu and Mn) that are common in welding (Vymazal et al., 1993). For example, increased R1 and R2 values occurred when there was elevated Mn exposure without additional Fe (Fitsanakis et al., 2010).
Studies examining Fe brain accumulation in welding or Mn exposure are sparse and inconsistent. Long et al. (2014) assessed Fe concentration via T2* and, when compared to controls, found full-time welders had lower T2* in the frontal cortex, but no difference in selected subcortical regions of interests (ROIs; e.g., GP, thalamus, and hippocampus). A recent study in deceased mine workers reported no differences in Fe tissue concentrations in the basal ganglia compared to controls (Criswell et al., 2015), although increased Fe tissue concentration was reported in the basal ganglia of Mn-exposed monkeys (Olanow et al., 1996).
In the present study, two hypotheses were tested based on the associations between high welding exposure and disorders of the basal ganglia. First, in welders with chronic, low level exposure, there will be higher R2* values in the basal ganglia structures (CN, PUT, and GP) when compared to controls. Second, if the first hypothesis is true, the increased R2* values will be positively correlated with increased Fe exposure assessed by exposure metrics and blood Fe levels. We also explored the associations between R2* and other welding-related metals such as Cu and Mn.
METHODS
Subjects
Eighty-one subjects were recruited from meetings of regional unions in Philadelphia and Harrisburg, PA, USA, and from the community around the Penn State Hershey Medical Center (HMC). Welders were defined as subjects who had welded at any point during their lifetime and controls were those who did not. Detailed demographic information was obtained from all subjects. This included age, education, history of smoking, and current and/or past major medical/neurological disorders. All subjects were examined and ascertained to be free of any obvious neurological and movement deficits using the Unified Parkinson’s Disease Rating Scale-motor scores (UPDRS-III) with threshold score of <15 (Lee et al., 2015). All subjects had normal blood calcium and magnesium levels, and no Fe deficiency. All welders underwent an orbital radiograph to rule out any metal fragments around the eye. Written informed consent was obtained from all subjects in accordance with guidelines approved by the Internal Review Board/Human Subjects Protection Office of the Penn State HMC.
One welder failed to complete the MRI acquisition. Six welders and three controls had poor quality R2* images and thus the data from these subjects were excluded. The final data set included 29 controls without history of welding and 42 welders (see demographic information in Table 1-A).
TABLE 1.
Summary Statistics for Demographic and Exposure Metrics (A) and Blood Metals (B) in Welders and Controls
| Welders (N = 42) | Controls (N = 29) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | P values | |
| A. Demographic and exposure metrics | |||
| Age (years) | 48.1 ± 11.2 | 44.0 ± 11.5 | .14 |
| Education (years) | 13.0 ± 1.7 | 16.2 ± 2.4 | <.001*** |
| HrsW (hours) | 229 ± 184 | 0 ± 0 (0) | <.001*** |
| YrsW (years) | 25.8 ± 11.1 | 0 ± 0 (0) | <.001*** |
| ALT (IU/l) | 39.4 ± 17.5 | 37.3 ± 16.7 | .23 |
| BMI (kg/m2) | 28.9 ± 5.1 | 26.2 ± 3.4 | .04* |
| UPDRS-III | 2.1 ± 2.5 | 1.6 ± 2.2 | .24 |
| B. Whole blood metals | |||
| Whole Cu (ng/mL) | 899 ± 113 | 754 ± 139 | <.001*** |
| Whole Mn (ng/mL) | 10.8 ± 3.2 | 8.5 ± 2.1 | .013* |
| Whole Fe (μg/mL) | 560 ± 49 | 496 ± 77 | <.001*** |
| Plasma blood levels | |||
| Plasma Fe (μg/dl) | 104 ± 38 | 105 ± 32 | .97 |
| Total_IBC (μg/dl) | 324 ± 66 | 321 ± 52 | .81 |
| Transferrin saturation (%) | 33 ± 13 | 33 ± 10 | .85 |
| Hemoglobin (μg/dl) | 15.2 ± 1.0 | 14.8 ± 0.8 | .07 |
| RBC (μg/dl) | 5.0 ± 0.4 | 5.0 ± 0.3 | .28 |
ALT, alanine aminotransferase; IBC, iron binding capacity.
*P < 0.05, **P < 0.01, ***P < 0.001.
Welders represented several different trades and industry groups (e.g., boilermakers, pipefitters, pile drivers, railroad welders, and a variety of different manufacturing jobs). To explore the nature of Fe accumulation in basal ganglia, the 42 welders were subdivided into two subgroups: welders with recent exposure (Welding-Recent; N = 35) who had welded in the 90 days prior to the MRI acquisition and welders without exposure in the 90 days prior to the MRI (Welding-Not-Recent; N = 7). Controls were age-matched volunteers from the community of the same region with various occupations.
Exposure assessment
Exposures were assessed by two questionnaires. The work history (WH; Lee et al., 2015) questionnaire was designed to collect job information for the individual’s working lifetime, with an emphasis on characterizing welding and other jobs that may be associated with welding exposure. An additional supplementary exposure questionnaire (SEQ) focused on the three-month period prior to the MRI, and determined the time spent on welding, the type of metal welded, and the various types of welding performed. Information on respirator use, confined space work, and use of ventilation also was collected. The primary exposure metric derived from the SEQ was hours welding, brazing, or soldering [HrsW = (weeks worked) × (hrs/week) × (fraction of time worked that is directly related to welding); Lee et al., 2015] in the 90 day period preceding the MRI and blood draw. Responses to the WH enabled an estimate of the cumulative lifetime years welding (YrsW = the Years spent welding during the subjects’ life; Lee et al., 2015).
Blood analysis
Whole blood Cu, Fe, and Mn levels were assessed as described in Lee et al. (2015). Plasma Fe, total Fe binding capacity (TIBC; maximum amount of Fe needed to saturate plasma transferrin), and percent transferrin saturation [measure of Fe that is actually bound to transferrin; (plasma Fe/TIBC) × 100] were measured at the Penn State HMC Clinical Laboratory using a standardized protocol based on the absorbance of ferrozine against known standards.
MRI image acquisition and analysis
All images were acquired using a Siemens 3T scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) with an 8-channel head coil. First, high-resolution T1-weighted (T1W) and T2-weighted (T2W) images were acquired for anatomical segmentation. T1W images were collected using an MPRAGE sequence with Repetition Time (TR) = 1540 ms, Echo Time (TE) = 2.3 ms, Field of View (FoV ) = 256 × 256 mm, matrix = 256 × 256 mm, slice thickness = 1 mm, slice number = 176 (with no gap), and voxel spacing 1 × 1 × 1 mm. T2W images were collected using a fast-spin-echo sequence with TR/TE = 2500/316, and the same spatial resolution as the T1W images. A multigradient-echo sequence was used to estimate the apparent transverse relaxation rate: R2* (1/T2*). Five echoes with TE ranging from 8 to 40 ms and an interval of 8 ms were acquired with TR = 51 ms, flip angle = 15°, FoV = 230 mm × 230 mm, matrix = 256 × 256, slice thickness = 1.6 mm, and slice number = 88 with bandwidth of 35.9 kHz. The middle slice of the image was placed on the line between the anterior and posterior commissures.
Defining brain regions of interest
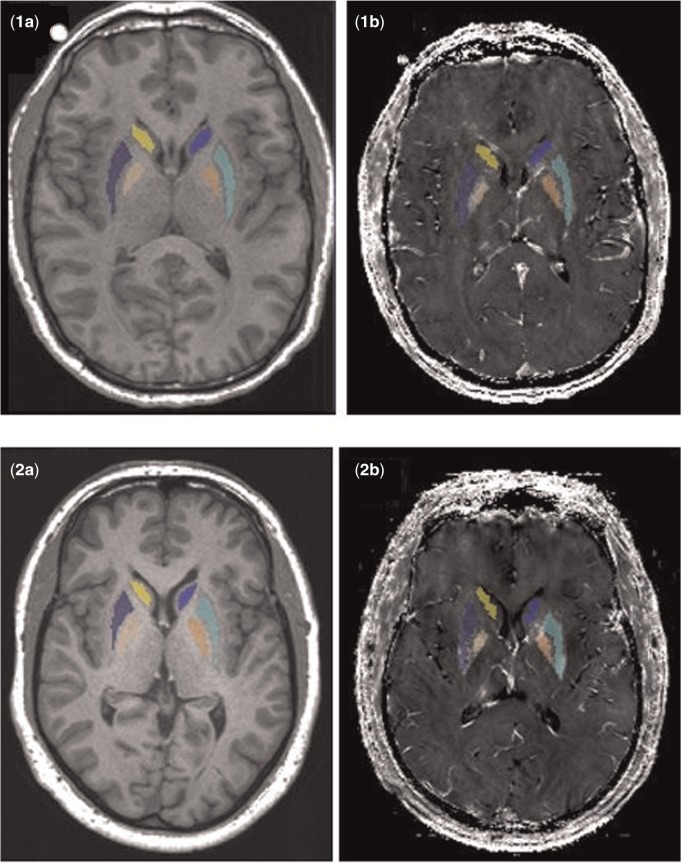
Bilateral basal ganglia structures [GP, PUT, CN] were selected as ROIs (Dorman et al., 2006). ROIs were defined for each subject using automatic segmentation software (AutoSeg; Gouttard et al., 2007) and then were eroded by 1 voxel using a morphological operation in order to make sure the segmented ROIs were within the anatomical ROIs (Figure 1). The quality of the segmentation then was visually confirmed for all subjects by a reviewer blinded to group assignment.
FIG. 1.
Automatically segmented basal ganglia ROI (CN, PUT, and GP) on T1-weighted MPRAGE images after erosion by 1 voxel for one representative welder (1a) and one control (2a). ROIs co-registered and overlaid onto the R2* maps for welder (1b) and control (2b).
Estimation of R2* values
The magnitude images of multigradient-echo images were used to generate R2* maps by using a voxel-wise linear least-squares fit to a monoexponential function with free baseline using in-house MATLAB (The MathWorks, Inc., Natick, MA) tools (see Supplementary Figure 1 for quality control information of R2* maps). The automatically segmented ROIs that were on the T1W images first were co-registered onto T2W images, and then the ROIs on T2W image space were co-registered again onto the R2* maps using an affine registration implemented in 3D Slicer (www.slicer.org, accessed January 27, 2016; Rueckert et al., 1999). The last 3-4 inferior slices on the transverse plane were manually deleted for all subjects in order to ensure that the co-registered ROIs did not go beyond the lower boundary of the anatomical ROIs (Supplementary Figure 2). The R2* values of each ROI were calculated as 1/T2* in each voxel and averaged over the entire ROI using a trimmed mean (5%–95%) to reduce possible segmentation error and imaging noise.
Estimation of R1 values
First, whole brain T1 time images were generated by the scanner. ROIs were co-registered onto the T1 maps using an affine registration implemented in 3D Slicer (www.slicer.org; Rueckert et al., 1999). The R1 values in each ROI were calculated as 1/T1 in each voxel and averaged over the entire ROI using a trimmed mean (5%–95%), the same method as used for the T1W intensities (Lee et al., 2015).
Statistical analysis
All error terms in the text, figures, and tables are standard deviations. SAS 9.3 was used to perform all statistical analyses. Right- and left hemisphere MRI data were averaged within each subject. One-way analysis of covariance (ANCOVA) with t-distributed errors was used for any group comparisons in order to account for outliers (Lange, 1989). For the between-group comparisons of MRI R2*s or R1s, the analyses were adjusted by using age, body mass index (BMI), respirator use, R1 or R2* values for the corresponding ROI, and whole blood metal levels of Mn and Cu as covariates. Because multiple brain areas were compared, the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) was used to control the false discovery rate (FDR) at the q = 0.05 level. We report raw p-values and indicate whether the tests were significant at a FDR level of 0.05.
Welding-Recent and Welding-Not-Recent subjects were examined using ANCOVA. Subgroup analyses were corrected for multiple group (i.e., three) comparisons using the Benjamini-Hochberg method. To explore any relationships between R2* and exposure metrics, both Pearson (to examine a linear relationship) and Spearman (to account for outliers) correlation analyses were performed. Both correlations were performed with adjustment of age in welders only because controls had 0 HrsW and YrsW. To explore the relationships between R2* and whole blood Fe levels, Pearson and Spearman partial correlations were performed with adjustment for age, R1 values for the corresponding ROI, and whole blood Mn and Cu levels for subjects in the entire cohort and then for welders and controls separately. Statistical significance was defined as α = 0.05. Due to the exploratory nature of these analyses, they were not corrected for multiple comparisons.
RESULTS
Group Comparisons between All Welders and Controls
Demographic, exposure metrics, and blood Fe levels
As shown in Table 1-A, there were no significant group differences in age, liver function (ALT: alanine aminotransferase), or UPDRS-III scores. Welders had a higher BMI (p = 0.04) and controls had more years of education than welders (p < 0.001). Welders had significantly greater HrsW, a short-term exposure metric, and YrsW, a long-term exposure metric (p < 0.001; Table 1-A). Whole blood Fe (560 ± 49 vs 496 ± 77 μg/mL; p < 0.001), Cu (899 ± 113 vs 754 ± 139 ng/mL; p < 0.001), and Mn (10.8 ± 3.2 vs 8.5 ± 2.1 ng/mL; p = 0.014) levels also were significantly higher in welders compared with controls (Table 1-B). There were no significant group differences in plasma Fe, TIBC, and transferrin saturation levels (Table 1-B).
MRI R2* and R1 measurements in basal ganglia
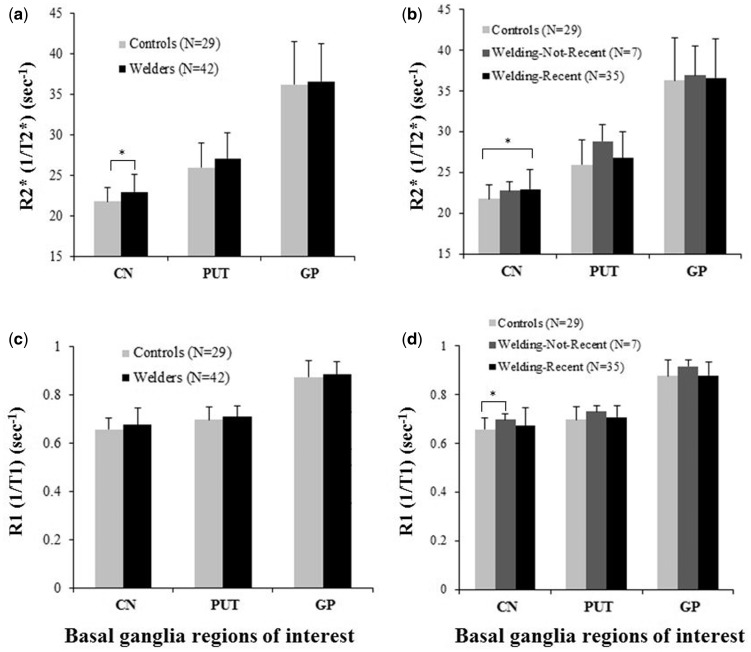
As shown in Figure 2a, R2* in the CN was significantly greater in welders compared to controls (p = 0.004) after controlling for age, BMI, respirator use, R1 in the CN, and whole blood levels of Cu and Mn. This CN difference remained significant after correction for multiple comparisons. Comparison of R2* values in the PUT and GP between welders and controls did not reach statistical significance, with p = 0.12 for PUT and p = 0.48 for GP (Figure 2-A). As seen in Figure 2c, there were no group differences in R1 values of any ROI after controlling for age, BMI, respirator use, R2* value of the corresponding ROI, and blood metal levels of Cu and Fe (ps > 0.18).
Fig. 2.
(A) MRI apparent transverse relaxation rates (R2*) in basal ganglia ROI (CN, PUT, and GP) for welders and controls, and (B) for controls, Welding-Not-Recent, and Welding-Recent subgroups. (C) MRI longitudinal relaxation rates (R1) in basal ganglia ROI for welders and controls, and (D) for controls, Welding-Not-Recent, and Welding-Recent subgroups. Values are raw R2* means ± SD. * indicates significance (p < 0.05) after correction for multiple group comparisons using the Benjamin-Hochberg method.
The association of R2* measures in the basal ganglia with Fe exposure estimates
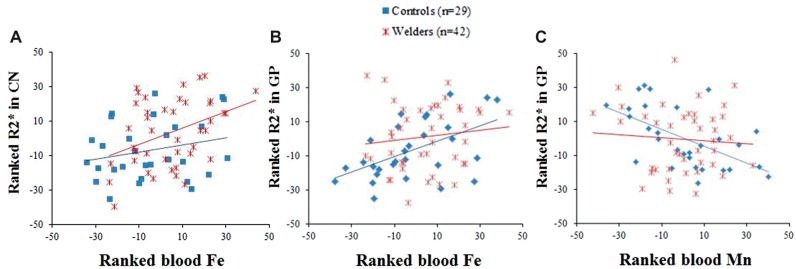
The welder group showed no correlations between R2* values in any ROI and HrsW or YrsW after controlling for age (ps > 0.39). On the other hand, R2* values in the CN and GP were correlated positively with whole blood Fe levels among all subjects (Spearman; R = 0.35, p = 0.004 for CN and R = 0.30, p = 0.015 for GP) after controlling for age, the R1 value for the corresponding ROI, and whole blood Cu and Mn levels. Within welders, R2* in the CN was correlated positively with whole blood Fe level (Spearman; R = 0.37, p = 0.021; Table 2-B and Figure 3-A).
TABLE 2.
Pearson (A) and Spearman (B) Correlation Coefficients of R2* Values in Basal Ganglia Regions with Exposure Metrics and Whole Blood Metals
| Among All Subjects (N = 71) |
Welders (N = 42) |
Controls (N = 29) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R2* CN | R2* PUT | R2* GP | R2* CN | R2* PUT | R2* GP | R2* CN | R2* PUT | R2* GP | |
| A. Pearson correlations | |||||||||
| HrsW | −0.12 | −0.09 | −0.04 | ||||||
| YrsW | 0.07 | 0.14 | −0.07 | ||||||
| Cu | −0.02 | −0.02 | −0.11 | −0.20 | −0.16 | −0.20 | −0.24 | −0.07 | −0.13 |
| Fe | 0.19 | −0.02 | 0.15 | 0.29 | 0.01 | −0.04 | −0.04 | −0.19 | 0.41* |
| Mn | −0.15 | 0.06 | −0.16 | −0.15 | 0.15 | 0.06 | −0.38 | −0.20 | −0.57** |
| B. Spearman correlations | |||||||||
| HrsW | −0.19 | −0.20 | −0.03 | ||||||
| YrsW | 0.21 | 0.25 | 0.21 | ||||||
| Cu | −0.10 | −0.07 | −0.12 | −0.25 | −0.15 | −0.16 | −0.14 | −0.08 | −0.10 |
| Fe | 0.36** | 0.10 | 0.30* | 0.37* | 0.03 | 0.21 | −0.10 | −0.15 | 0.51* |
| Mn | −0.22 | −0.06 | −0.30* | −0.10 | 0.11 | −0.11 | −0.40* | −0.27 | −0.62** |
Correlations between R2* values and exposure metrics were conducted with adjustments for age. Correlations between R2* values and whole blood Fe levels were conducted with adjustments for age and whole blood metals Cu and Mn. For R2* values and blood Mn level correlations, blood Cu and Fe levels were included in the analysis as covariates. For R2* values and blood Cu level correlations, blood Fe and Mn levels were used as covariates.
*p < 0.05, **p < 0.01
FIG. 3.
Scatter plots show (A) ranked R2* in the CN (y-axis) versus ranked blood iron (Fe: x-axis) for controls and welders; (B)ranked R2* in the GP (y-axis) versus ranked blood Fe (x-axis). R2* and blood Fe values were adjusted for age and whole Cu and Mn levels for (A) and (B); (C) ranked R2* in the GP (y-axis) and ranked blood Mn (x-axis). R2* and blood Mn values were adjusted for age and blood levels of Cu and Fe.
For controls, R2* values in the GP were correlated positively with blood Fe levels after adjustments for age, GP R1, and blood levels of Cu and Mn (for both Pearson and Spearman, Rs > 0.41, ps < 0.045), whereas R2* values in the CN and GP were correlated negatively with blood Mn levels after controlling for age, R1 values in the corresponding ROI, and blood levels of Cu and Fe (Spearman; R = −0.40, p = 0.047 for CN and for both Pearson and Spearman: Rs < −0.57, ps < 0.003 for GP; Table 2 and Figure 3b-c).
Subgroup Comparisons among Controls, Welding-Not-Recent, and Welding-Recent
Demographic, exposure metrics, and blood metal levels
As seen in Table 3, both welding subgroups had greater YrsW compared to controls (ps < 0.001). The Welding-Not-Recent subgroup was older and had last welded between 5-180 months before participation. The Welding-Not-Recent subgroup also had higher whole blood Cu (898 ± 99 vs 754 ± 139 ng/mL; p = 0.029) and Fe (543 ± 20 vs 496 ± 77 μg/mL; p = 0.005) levels than controls. The Welding-Recent subgroup had greater whole blood Fe levels (563 ± 53 vs 496 ± 77 μg/mL; p < 0.001), along with increased whole blood Cu (900 ± 117 vs 754 ± 139 ng/mL; p < 0.001) and Mn (10.6 ± 2.9 vs 8.5 ± 2.1 ng/mL; p = 0.01) levels compared with controls.
TABLE 3.
Summary Statistics for Demographic and Exposure Metrics (A) and Blood Metals (B) in the 3 Subgroups
| A. Controls (N = 29) |
B. Welding-Not-Recent (N = 7) |
C. Welding-Recent (N = 35) |
|||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Significant Comparisons | ||
| A. Demographic and exposure metrics | |||||
| Age (years) | 44.0 ± 11.5 | 53.1 ± 7.5 | 47.1 ± 11.6 | A < B*; B > C | |
| Education (years) | 16.2 ± 2.4 | 12.3 ± 0.5 | 13.1 ± 1.8 | A > B*; A > C* | |
| HrsW (hours) | 0 ± 0 | 0 ± 0 | 274 ± 168 | A < C*; B < C* | |
| YrsW (years) | 0 ± 0 | 27.1 ± 11.4 | 25.5 ± 11.2 | A < C*; B < C* | |
| ALT (IU/l) | 37.3 ± 16.7 | 41.4 ± 12.8 | 38.9 ± 18.5 | ||
| BMI | 26.2 ± 3.4 | 29.6 ± 1.4 | 28.7 ± 5.6 | A < B | |
| Hemoglobin | 14.8 ± 0.8 | 14.9 ± 0.7 | 15.3 ± 1.1 | A < B; A < C | |
| UPDRS-III | 1.6 ± 2.2 | 2.0 ± 2.2 | 2.1 ± 2.5 | ||
| B. Blood Metals | |||||
| Mn (ng/mL) | 8.5 ± 2.1 | 11.7 ± 4.7 | 10.6 ± 2.9 | A < C* | |
| Fe (μg/mL) | 496 ± 77 | 543 ± 20 | 563 ± 53 | A < B*; A < C* | |
| Cu (ng/mL) | 754 ± 139 | 898 ± 99 | 900 ± 117 | A < B*; A < C* | |
*Indicates significance (p < 0.05) after correction for multiple group comparisons using the Benjamin–Hochberg method.
MRI R2* and R1 measurements in basal ganglia
As seen in Figure 2-B, R2* values in the CN were significantly greater in the Welding-Recent subgroup compared to controls (p = 0.0007) after controlling for age, BMI, respirator use, CN R1, and whole blood levels of Cu and Mn. This CN difference remained significant after correction for multiple group comparisons. Comparisons of R2* values among controls and welding subgroups in the PUT and GP revealed no group differences (ps > 0.16 for PUT and ps > 0.51 for GP). Although R2* values in the CN seemed to be higher in the Welding-Not-Recent group, this comparison did not reach statistical significance after controlling for age, BMI, respirator use, CN R1, and whole blood levels of Cu and Mn.
As seen in Figure 2d, R1 in the CN for the Welding-Not-Recent subgroup was greater than that of controls (p = 0.015); this remained significant after correction for multiple group comparisons. Comparisons of R1 values among controls and welding subgroups in the PUT and GP revealed no differences after controlling for age, BMI, respirator use, the R2* value of the corresponding ROI, and blood metal levels of Cu and Fe (ps > 0.095).
The association of R2* measures in the basal ganglia with Fe exposure estimates
There were no significant associations between R2* measurements and exposure metrics in either welding subgroup in any of the ROIs after controlling for age (ps > 0.17). R2* values in the CN were, however, correlated positively with whole blood Fe levels for the Welding-Recent subgroup (Spearman; R = 0.40, p = 0.026). No other correlations within the Welding-Recent subgroup were significant (ps > 0.15). Moreover, there were no significant associations between R2* measurements and blood Fe level in the Welding-Not-Recent subgroup in any ROI (ps > 0.099).
DISCUSSION
Using R2* as a surrogate for Fe accumulation, our study of PA-based cohort of welders and controls found that welders had greater exposure metrics, higher whole blood Fe levels, and higher R2* values in the basal ganglia compared to controls. In addition, the imaging changes were selective for the CN, not other regions of the basal ganglia. Whereas past welding-related studies focused on Mn accumulation in the basal ganglia (predominantly in the GP), the present study is the first human study to delineate increased R2* values in the basal ganglia (predominantly in the CN), which suggests higher Fe accumulation in this region, and its association with blood Fe levels in asymptomatic welders. Follow-up studies that provide insight into a possible role of Fe in welding-related neurobehavioral disorders are clearly important.
Increased Fe Exposure in Welders with Chronic, Low Level Exposure
Our welders averaged 229 welding hours in the past 90 days, approximately equivalent to spending nearly half of their worktime on welding (Lee et al., 2015). They also had an average of 25.8 lifetime welding years. Thus, our welders have the characteristics of chronic but low-level welding exposure, different from most previous studies that focused on high-level welding exposure.
Despite relatively modest exposure, higher Fe exposure was evidenced by both greater exposure metrics and higher whole blood Fe levels, and even the Welding-Not-Recent subgroup who had not welded for at least five months prior to the study visit showed higher blood Fe (543 ± 20 μg/mL) compared to controls (496 ± 77 μg/mL). This result is consistent with previous reports that both airborne and blood Fe concentrations were much higher than those of Mn in many types of welding (Ellingsen et al., 2006), and underscore the importance of studying the role of Fe in welding-related toxicity in relatively low-exposed welders.
Blood Fe Homeostasis in Welders with Chronic, Low Level Exposure
Interestingly, although blood Fe levels were greater in welders, plasma Fe levels were comparable to controls. This result is consistent with previous studies suggesting whole blood metal levels may be a better exposure biomarker than plasma or serum metal levels (Costa and Aschner, 2014; Spahr et al., 1996), especially when exposure is relatively low. This is probably because the majority of Fe in the body (approximately 80%) is bound to hemoglobin in red blood cells (RBC) and only a fraction is found in plasma (Collings et al., 2013). In addition, the finding of comparable plasma Fe levels may suggest that the body is able to regulate Fe overload. This is important because Fe content in plasma (rather than RBCs) influences what is transported to other organs including the brain via the BBB or choroid plexus (Yokel, 2006).
Higher R2* Values in Selective Basal Ganglia Structures in Welders
Along with increased exposure metrics and whole blood Fe levels, welders also demonstrated significantly higher R2* values, suggestive of elevated Fe accumulation, in the basal ganglia after controlling for confounders including R1 values and blood levels of Cu and Mn. R2* values were highest in the GP (followed by PUT and CN) for both controls and welders, consistent with the general Fe brain distribution patterns based on postmortem data (Hallgren and Sourander, 1958). A significantly increased R2* value in welders, however, was present only in the CN, not the PUT or GP. The seemingly greater R2* value in the PUT of welders failed to be significant perhaps because the increase is due to aging rather than welding (Hallgren and Sourander, 1958). Also, note that the GP is the region where welding-related Mn predominantly accumulates (Erikson et al., 2004; Kim et al., 1999). Our data are consistent with Long et al. (2014) who did not find significant difference in T2* (1/R2*) in the GP, but unfortunately did not include the CN as an ROI and also did not control for possible Mn brain accumulation effects on T2*.
It is unclear why higher R2* values were found in the CN and not in the PUT and GP. A PET study reported that occupational welders had decreased dopamine transporter density, particularly in the CN but not the PUT (Criswell et al., 2011), suggesting that dopaminergic terminal loss may share a similar pattern of Fe accumulation. It also is possible that welding-related Fe increases may be detected more easily in the CN than in the PUT or GP because Fe levels in the CN are generally lower than those in the PUT and GP (Hallgren and Sourander, 1958).
Previous studies have reported welding-induced executive function and working memory deficits (Bowler et al., 2006; Park et al., 2009). Note that the frontal cortex (Long et al., 2014) and CN [regions reported as having increased welding-related Fe brain deposition suggested by lower T2* and higher R2* values] are areas often associated with similar types of cognitive performance (Grahn et al., 2008). Future studies are needed to investigate any potential connections between the cognitive decline in welders and increased Fe deposition in these areas.
It is important to note that no differences in R1 values were found between welders and controls. Subgroup analyses indicated that only the Welding-Not-Recent subgroup showed higher R1 values in the CN compared to controls, probably because Mn may not accumulate in brain if the exposure level is low (Lee et al., 2015). Note that the Welding-Recent subgroup had an average 274 welding hours (past 90 days), indicating low-level exposure. The Welding-Not-Recent subgroup may have higher R1 values because subjects may have higher exposure level when they welded.
Dose–Response Relationship between Estimated Fe Exposure and R2* Values
We demonstrated that higher R2* values in the CN were correlated positively with blood Fe levels after controlling for age, R1 in the CN, and blood Mn and Cu levels. Along with the absence of increased R1 values in any ROIs for welders, this suggests that increased R2* may be associated with increased exposure specifically to Fe. In addition, increased blood Fe levels were observed in both Welding-Recent and Welding-Not-Recent subgroups, suggesting that the blood Fe levels may reflect lingering long-term exposure effects. Although the Welding-Not-Recent subgroup showed higher R2* values in the CN than controls, this increase was not significant probably due to relatively small sample size. The correlation between R2* values and YrsW was also not significant. Although it is possible that the YrsW metric is a poor measure of long-term exposure, it is unclear whether the higher R2* values in the CN are associated with long-term or short-term exposure effects. Further studies with larger sample sizes and better exposure metrics (such as cumulative lifetime hours of welding and on-site airborne Fe measures) are needed to determine the exact nature of the R2* increase observed in welders.
Roles of Other Welding-related Metals (e.g., Cu and/ or Mn) in R2* Measures
We explored the associations between R2* values in the basal ganglia and other welding-related metals such as Mn and/or Cu. We found that R2* in the CN and GP was correlated negatively with blood Mn, whereas R2* in the GP was correlated positively with blood Fe in controls after adjustment for confounders including R1 values and other blood metal levels. These results suggest potential attenuation of Fe brain accumulation due to Mn for those who have a well-regulated balance of Mn and Fe concentrations in blood and brain. It is interesting that the negative correlations between R2* values and blood Mn are lacking in welders. It is possible that in welders, physiological handling is disrupted or more complicated, since all welding-related metals (e.g., Cu, Fe, and Mn) were elevated simultaneously. Previous animal studies reported complex interactions between Mn and Fe when content of both metals was increased (Fitsanakis et al., 2010; Zhang et al., 2009), and Fitsanakis et al. (2010) reported increased levels in both R1 and R2 values for rats exposed to Mn without additional Fe exposure. The R2 values were even greater when rats were exposed simultaneously to both Mn and Fe, although brain Fe concentrations in those rats showed no change (e.g., striatum) or even decreases (e.g., cerebellum and cortex). More studies are needed to investigate the interactions among multiple metal mixtures, their brain deposition, and effects on MRI changes.
CONCLUSION
The present study demonstrated increased R2* values in the CN concomitant with increased Fe exposure in chronic, low-exposure, asymptomatic welders. The current findings of higher R2* values in welders compared to controls after adjustment for confounders (including R1 values and blood Cu and Mn levels), suggest the Fe exposure–R2* association. Although it is impossible to measure actual brain metal content of the study cohort in order to directly link Fe exposure and R2*, vast body of literature unequivocally supports such a relationship. Considering the involvement of the CN in neurobehavioral functions, this result may suggest a potential role of Fe in welding-related neurobehavioral changes. These findings may guide future studies and the development of occupation- and public health-related polices involving welding exposure.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (R01 ES019672) and National Institute of Neurological Disease and Stroke (NS082151).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all the volunteers who participated in this study. In addition, we are indebted to many individuals who helped make this study possible including Melissa Santos and Susan Kocher for subject coordination, recruitment, blood sample handling, and data entry; Pam Susi and Pete Stafford of CPWR; Mark Garrett, John Clark, and Joe Jacoby of the international Brotherhood of Boilermakers; Fred Cosenza and all members of the Safety Committee for the Philadelphia Building and Construction Trades Council; Ed McGehean of the Steamfitters Local Union 420; Jim Stewart of the Operating Engineers; Sean Gerie of the Brotherhood of Maintenance of Way Employees Division Teamsters Rail Conference; and Terry Peck of Local 520 Plumbers, Pipefitters and HVAC. The authors do not have any financial conflicts of interest to disclose.
References
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- Bowler R. M., Koller W., Schulz P. E. (2006). Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology 27, 327–332. [DOI] [PubMed] [Google Scholar]
- Burgess W. A., Burgess W. (1995). Recognition of Health Hazards in Industry: A Review of Materials and Processes. Wiley, New York. [Google Scholar]
- Cersosimo M. G., Koller W. C. (2006). The diagnosis of manganese-induced parkinsonism. Neurotoxicology 27, 340–346. [DOI] [PubMed] [Google Scholar]
- Choi D. S., Kim E. A., Cheong H. K., Khang H. S., Ryoo J. W., Cho J. M., Sakong J., Park I. (2007). Evaluation of MR signal index for the assessment of occupational manganese exposure of welders by measurement of local proton T1 relaxation time. Neurotoxicology 28, 284–289. [DOI] [PubMed] [Google Scholar]
- Collings R., Harvey L. J., Hooper L., Hurst R., Brown T. J., Ansett J., King M., Fairweather-Tait S. J. (2013). The absorption of iron from whole diets: a systematic review. Am. J. Clin. Nutr. 98, 65–81. [DOI] [PubMed] [Google Scholar]
- Costa L. G., Aschner M. (2014). Manganese in Health and Disease. Royal Society of Chemistry, Cambridge, UK. [Google Scholar]
- Criswell S., Perlmutter J., Videen T., Moerlein S., Flores H., Birke A., Racette B. (2011). Reduced uptake of [18F] FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology 76, 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell S. R., Nelson G., Gonzalez-Cuyar L. F., Huang J., Shimony J. S., Checkoway H., Simpson C. D., Dills R., Seixas N. S., Racette B. A. (2015). Ex vivo magnetic resonance imaging in South African manganese mine workers. Neurotoxicology 49, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A., Morgan E. H. (1996). Iron and copper interact during their uptake and deposition in the brain and other organs of developing rats exposed to dietary excess of the two metals. J. Nutr. 126, 183–194. [DOI] [PubMed] [Google Scholar]
- Dexter D., Carayon A., Javoy-Agid F., Agid Y., Wells F., Daniel S., Lees A., Jenner P., Marsden C. (1991). Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114, 1953–1975. [DOI] [PubMed] [Google Scholar]
- Dorman D. C., Struve M. F., Wong B. A., Dye J. A., Robertson I. D. (2006). Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 92, 219–227. [DOI] [PubMed] [Google Scholar]
- Du G., Lewis M. M., Styner M., Shaffer M. L., Sen S., Yang Q. X., Huang X. (2011). Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov. Disord. 26, 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen D. G., Dubeikovskaya L., Dahl K., Chashchin M., Chashchin V., Zibarev E., Thomassen Y. (2006). Air exposure assessment and biological monitoring of manganese and other major welding fume components in welders. J. Environ. Monit. 8, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Erikson K. M., Syversen T., Steinnes E., Aschner M. (2004). Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J. Nutr. Biochem. 15, 335–341. [DOI] [PubMed] [Google Scholar]
- Fitsanakis V. A., Zhang N., Avison M. J., Erikson K. M., Gore J. C., Aschner M. (2010). Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging (MRI). Toxicol. Sci. 120, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M. R., Susi P. (2009). Manganese, iron, and total particulate exposures to welders. J. Occup. Environ. Hyg. 7, 115–126. [DOI] [PubMed] [Google Scholar]
- Gelman N., Gorell J. M., Barker P. B., Savage R. M., Spickler E. M., Windham J. P., Knight R. A. (1999). MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 210, 759–767. [DOI] [PubMed] [Google Scholar]
- Gouttard S., Styner M., Joshi S., Smith R. G., Hazlett H. C., Gerig G. (2007). Subcortical structure segmentation using probabilistic atlas priors, pp. 3746. Medical Image Computing and Computer Assisted Intervention. Workshop on 3D Segmentation in the ClinicA Grand Challenge, October 2007, Brisbane, Australia. [Google Scholar]
- Grahn J. A., Parkinson J. A., Owen A. M. (2008). The cognitive functions of the caudate nucleus. Prog. Neurobiol. 86, 141–155. [DOI] [PubMed] [Google Scholar]
- Haacke E. M., Cheng N. Y., House M. J., Liu Q., Neelavalli J., Ogg R. J., Khan A., Ayaz M., Kirsch W., Obenaus A. (2005). Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 23, 1–25. [DOI] [PubMed] [Google Scholar]
- Hallgren B., Sourander P. (1958). The effect of age on the non-haemin iron in the human brain. J. Neurochem. 3, 41–51. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Chang K. H., Chi J. G., Cheong H. K., Kim J. Y., Kim Y. M., Han M. H. (1999). Sequential change of MR signal intensity of the brain after manganese administration in rabbits: correlation with manganese concentration and histopathologic findings. Invest. Radiol. 34, 383–393. [DOI] [PubMed] [Google Scholar]
- Lange K. L., Little R. J A., Taylor J. M. G. (1989). Robust statistical modeling using the t distribution. J. Am. Stat. Assoc. 84, 881–896. [Google Scholar]
- Lee E. Y., Flynn M. R., Du G., Lewis M. M., Fry R., Herring A. H., Van Buren E., Van Buren S., Smeester L., Kong L., et al. (2015) T1 relaxation rate (R1) indicates nonlinear Mn accumulation in brain tissue of welders with low-level exposure. Toxicol. Sci. 146, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z., Jiang Y. M., Li X. R., Fadel W., Xu J., Yeh C. L., Long L. L., Luo H. L., Harezlak J., Murdoch J. B., et al. (2014). Vulnerability of welders to manganese exposure—a neuroimaging study. Neurotoxicology 45, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhang L. L., Li G. J., Guo W., Liang W., Zheng W. (2005). Alteration of serum concentrations of manganese, iron, ferritin, and transferrin receptor following exposure to welding fumes among career welders. Neurotoxicology 26, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow C., Good P., Shinotoh H., Hewitt K., Vingerhoets F., Snow B., Beal M., Calne D., Perl D. (1996). Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology 46, 492–498. [DOI] [PubMed] [Google Scholar]
- Park R. M., Bowler R. M., Roels H. A. (2009). Exposure-response relationship and risk assessment for cognitive deficits in early welding-induced manganism. J. Occup. Environ. Med. 51, 1125–1136. [DOI] [PubMed] [Google Scholar]
- Peran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., Celsis P., Rascol O., Demonet J. F., Stefani A., et al. (2010). Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 133, 3423–3433. [DOI] [PubMed] [Google Scholar]
- Rueckert D., Sonoda L. I., Hayes C., Hill D. L., Leach M. O., Hawkes D. J. (1999). Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18, 712–721. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Lee P., Beutler E. (2002). Brain iron metabolism and neurodegenerative disorders. Dev. Neurosci. 24, 188–196. [DOI] [PubMed] [Google Scholar]
- Sofic E., Paulus W., Jellinger K., Riederer P., Youdim M. (1991). Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J. Neurochem. 56, 978–82. [DOI] [PubMed] [Google Scholar]
- Spahr L., Butterworth R. F., Fontaine S., Bui L., Therrien G., Milette P. C., Lebrun L. H., Zayed J., Leblanc A., Pomier-Layrargues G. (1996). Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 24, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Ulla M., Bonny J. M., Ouchchane L., Rieu I., Claise B., Durif F. (2013). Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One 8, e57904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vymazal J., Bulte J. W., Frank J. A., Chiro G. D., Brooks R. A. (1993). Frequency dependence of MR relaxation times I. Paramagnetic ions. J. Magn. Reson. Imaging 3, 637–640. [DOI] [PubMed] [Google Scholar]
- Wallis L. I., Paley M. N., Graham J. M., Grünewald R. A., Wignall E. L., Joy H. M., Griffiths P. D. (2008). MRI assessment of basal ganglia iron deposition in Parkinson's disease. J. Magn. Reson. Imaging 28, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Ward R. J., Zucca F. A., Duyn J. H., Crichton R. R., Zecca L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel R. A. (2006). Blood-brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J. Alzheimers Dis. 10, 223–254. [DOI] [PubMed] [Google Scholar]
- Zhang N., Fitsanakis V. A., Erikson K. M., Aschner M., Avison M. J., Gore J. C. (2009). A model for the analysis of competitive relaxation effects of manganese and iron in vivo. NMR Biomed. 22, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.