Abstract
Background
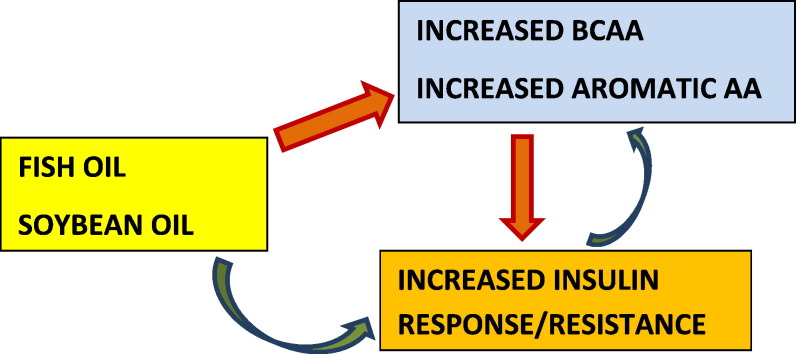
Both fish (FO) and flaxseed oils (FLX) are n-3 polyunsaturated fatty acids (PUFA). Fish oil contains long chain while FLX contains essential n-3 PUFA. We demonstrated that FO altered insulin secretion and resistance in polycystic ovary syndrome (PCOS) women but FLX did not. Surprisingly, the effects of FO were similar to those of the n-6 PUFA-rich soybean oil (SBO). Since increased branched chain (BCAA) and aromatic amino acids (AA) affect insulin secretion and resistance, we investigated whether FO, FLX and /or SBO affect plasma metabolites, especially AA.
Methods and findings
In this six-week, randomized, 3-parallel arm, double-blinded study, 54 women received 3.5 g/day FO, FLX or SBO. In 51 completers (17 from each arm), fasting plasma metabolites were measured at the beginning and at the end.
As compared to FLX, FO and SBO increased insulin response and resistance as well as several BCAA and aromatic AA. Pathway analysis indicated that FO exerted the largest biochemical impact, affecting AA degradation and biosynthesis, amine, polyamine degradation and alanine, glycine, l-carnitine biosynthesis and TCA cycle, while FLX had minimal impact affecting only alanine biosynthesis and l-cysteine degradation.
Conclusion
Effects of FO and SBO on plasma AA were similar and differed significantly from those of the FLX. The primary target of dietary PUFA is not known. Dietary PUFA may influence insulin secretion and resistance directly and alter plasma AA indirectly. Alternatively, as a novel concept, dietary PUFA may directly affect AA metabolism and the changes in insulin secretion and resistance may be secondary.
Keywords: n-3 Polyunsaturated fatty acids, Plasma metabolites, Branched chain amino acids, Insulin resistance, PCOS
Graphical abstract
Highlights
-
•
Increased serum branched chain amino acids (BCAA) and aromatic amino acids are associated with insulin resistance and type 2 diabetes.
-
•
Although both fish oil (FO) and flaxseed oil (FLX) are n-3 PUFA, FO contains the long chain, while FLX contains the essential n-3 PUFA.
-
•
We compared the effects of different PUFAs on plasma metabolites in women with insulin resistance.
-
•
Fish oil, but not FLX, increased plasma BCAA, and insulin resistance and secretion, indicating differential effects of essential vs. long chain n-3 PUFA.
-
•
It is possible that effects of FO on insulin resistance and secretion may have been indirect, through its actions on BCAA metabolism.
1. Introduction
Polyunsaturated fatty acids (PUFA) are powerful modulators of lipid and glucose metabolism. Their actions are considered to be class specific as the PUFA from omega-3 (n-3) vs. n-6 have different and usually opposing actions. For example, n-3 PUFA lower serum triglycerides while n-6 PUFA lower total cholesterol and low density lipoprotein (LDL)-cholesterol [1]. Experimental studies suggest that n-3 PUFA decrease, whereas n-6 PUFA increase insulin secretion; and both n-3 and n-6 PUFA increase insulin sensitivity [2]. Although the findings of experimental vs. clinical studies agree on lipid metabolism, they vary a great deal regarding glucose homeostasis.
Significant gaps in knowledge exist regarding the biological effects of PUFA. For example, no distinction has been made between the effects of long-chain vs. essential n-3 PUFA. Flaxseed oil is a rich source of the essential n-3 PUFA α-linolenic acid (ALA, 18:3) while fish oil contains the long chain n-3 PUFA eicosapentanoic acid (EPA, 20:5) and docosahexanoic acid (DHA, 22:6). Although ALA can be converted to EPA in vivo, this is very inefficient in humans [3]. Despite that, in clinical practice these oils are used interchangeably. However, the choice of the exact type of oil used is important because ALA is metabolized differently than EPA and DHA. While ALA competes with the n-6 essential PUFA linoleic acid (LA, 18:2) for Δ6 desaturase and interferes with production of arachidonic acid (AA, 20:4), EPA and DHA compete at more distal steps at the cyclooxygenase and lipooxygenase [4].
We had compared the effects fish, flaxseed and soybean oils on glucose homeostasis in polycystic ovary syndrome (PCOS) [5]. This syndrome provides an excellent clinical model because the affected young women are insulin resistant; at least 50% of PCOS patients have metabolic syndrome; one third of patients have glucose intolerance; and one out of five develops type 2 diabetes before the age of 40 years. Hyperinsulinemia aggravates reproductive dysfunction by stimulating ovarian androgen production, by reducing sex hormone binding globulin (SHBG); thus by increasing bioavailable testosterone. Treatment of insulin resistance increases fertility in PCOS [7].
We assessed insulin resistance and secretion using oral and frequently sampled intravenous glucose tolerance tests (OGTT and FS-IGT, respectively). Unexpectedly, fish and soybean oils caused similar changes in glucose homeostasis; furthermore, their effects were distinctly different than those of flaxseed oil [6]. These observations challenged the widely accepted concept that “the biological effects of PUFA are specific To their omega class” The similarities between the effects of n-3 PUFA rich fish oil and n-6 PUFA rich soybean oil suggested complex regulatory mechanisms.
Several studies employing the metabolomics technology suggest perturbations of branched chain amino acid (BCAA) and aromatic AA metabolism in insulin resistance and obesity [8]. Obese, insulin resistant individuals demonstrated a characteristic increase in fasting plasma concentrations of BCAA: (valine, isoleucine, leucine) and their catabolic byproducts such as glutamate, α-ketoglutarate, C3 and C5 acylcarnitines [9], [10], [11], [12]. We also found direct correlations between serum BCAA ad insulin resistance parameters in women with metabolic syndrome [13]. Similar findings were observed in a subpopulation of the Framingham Cohort where a few AA (leucine, isoleucine, valine, phenylalanine, tyrosine) could predict the five-fold increase in type 2 DM [12]. Therefore, we investigated the effects of fish, flaxseed and soybean oils on BCAA and other primary metabolites in PCOS.
2. Research design and methods
2.1. Subjects
The study was approved by the Institutional Review Board of University of California, Davis and registered with the NIH. The subjects were recruited between September 2007 and February 2010, and were included in the study after signing the written informed contents. Clinical characteristics of the patients, the study protocol, clinical studies and the results have been reported previously [6].
Women between the ages 20 and 45 years and with a body mass index (BMI) of 25–45 kg/m2, fulfilling the NIH criteria for PCOS by having ovarian dysfunction (amenorrhea; no periods for > 6 months, or oligomenorrhea: < 6 periods/year; clinical or laboratory evidence for hyperandrogenemia; total testosterone > 54 ng/dl or free testosterone > 9.2 pg/ml), along with the absence of any confounding clinical pathology (i.e. Cushing's disease, 21 hydroxylase deficiency or prolactinoma) were recruited [7]. Patients were excluded if they used oral contraceptives, anti-androgenic medications, insulin sensitizers, d-chiro inositol, lipid lowering drugs during the preceding two months; had diabetes mellitus, untreated hypothyroidism or thyroid disease, and any other systemic illness such as renal, hepatic, and gastrointestinal disease; smoke; or drink > 2 alcoholic drinks per week.
2.2. Consort statement
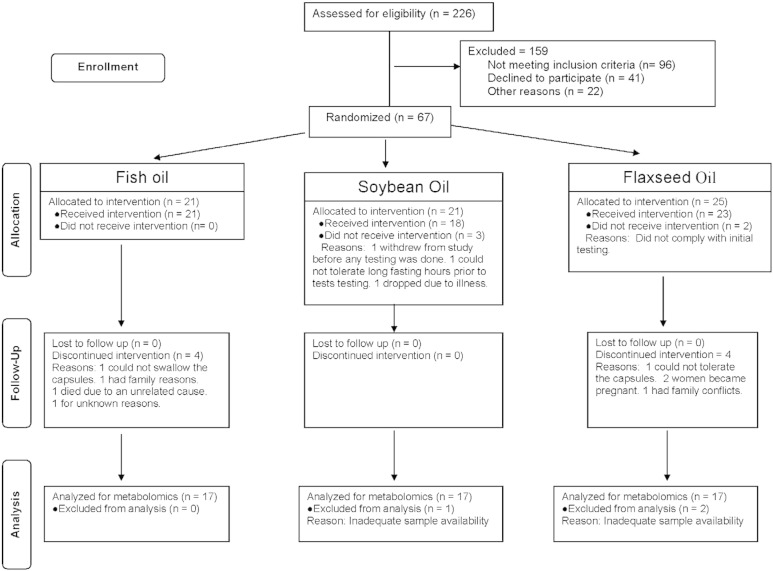
As shown in Fig. 1, two hundred and twenty-six PCOS patients were assessed for eligibility; 159 subjects either failed to meet the inclusion/exclusion criteria (n = 96), refused to participate (n = 41) or had other reasons preventing participation (n = 22). Remaining 67 patients were randomized using the http://www.randomizer.org/form.htm. Five subjects left before receiving any intervention; the remaining received either fish oil (n = 21), soybean oil (n = 18) or flaxseed oil (n = 23). Fifty-four patients completed the 6-week study (17 in the fish oil arm, 18 in the soybean oil arm and 19 in the flaxseed oil arm). Plasma samples from fifty-one patients (17 in each arm; 35 White, 5 African American, 5 Hispanic, 4 Asian, 1 American Indian and 1 other) were analyzed for the plasma metabolites and reported in this manuscript.
Fig. 1.
Consort diagram.
2.3. Study design
This was a six-week long, randomized, 3-parallel arm, double-blinded study. Fish oil and flaxseed oil supplements contained approximately the same amount of total n-3 PUFA in 6 capsules (3.5 g). Each flaxseed oil capsule contained 545 mg ALA (Barleans Organic Oils, Ferndale, WA); fish oil contained 358 mg EPA plus 242 mg DHA (Nordic Naturals, Watsonville, CA). Soybean oil (Nordic Naturals, Watsonville, CA), which was recommended as placebo by the Product Quality Working Group (PQWG) of the NCCAM, contained 200 mg oleic acid, 429 mg LA, 57 mg ALA and very small amounts of palmitic and stearic acids.
2.4. Data collection
Data were obtained at the beginning and at the end of the study at the CTSC-CCRC of the University of California, Davis. The methodologies for the collection of nutritional intake, anthropometrics measurements, OGTT, IVGTT, biochemical measurements and calculations have been reported in detail previously [6].
2.5. Metabolomic studies (GC-TOF MS-based)
Pyridine, acetonitrile, isopropanol, methoxyamine hydrochloride, N-methyl-N-trimethylsiliyl trifluoroacetamide (MSTFA) and all the standards were obtained from Sigma Aldrich, USA.
20 μl plasma was extracted by 1 mL degassed cold (− 20 °C) acetonitrile: isopropanol: water (3:3:2; v/v/v) solution. 500 μl of the supernatant was evaporated to dryness. Triglycerides and lipids were removed by adding 500 μl of ACN: Water (1:1) and evaporating the supernatant. The dried extracts were derivitazed in two steps with methoxyamine hydrochloride followed by silylation with N-methyl-N-trimethylsiliyl trifluoroacetamide (MSTFA). A fatty acid methyl ester (FAME) mixture of C8 through C30 was added to the derivatized samples as retention indices markers. Derivatized samples were analyzed by Gas Chromatography–Mass Spectrometry (GC–MS) on an Agilent 6890 GC-LECO Pegasus III TOF equipped with a Cooled Injection System (CIS4), an Automated Linear Exchange system (ALEX), and a Multi Purpose Sampler (MPS, all Gerstel). The injector was run with an initial temperature of 50 °C and ramped to 275 °C at a rate of 12 °C/s. The injection volume was 0.5 μL and injector mode was splitless with purge time 25 s. GC conditions were set with a programmed oven temperature of 50 °C, held here for 1 min, then ramped to 330 °C at a rate of 20 °C/min, held at 330 °C for 5 min; with carrier gas flow rate at 1 ml/min. The GC column was RTX-5 MS, 30 m long, 0.25 mm i.d. × 0.25 μm film with 10 m integrated guard column. The transfer line and ion source temperature were set at 280 °C and 250 °C, respectively. Solvent delay was adjusted to 4.5 min and MS acquisition was optimized to 17 spectra per scan, using a mass range of 80 to 500 m/z. The Multichannel Plate (MCP) detector voltage was adjusted to 1850 V.
2.6. Data processing
Chromatogram acquisition, data handling, automated peak deconvolution and export of spectra were automatically performed by the Leco ChromaTOF software (v2.32). Peak picking was achieved in ChromaTOF (v2.32) at signal/noise levels of 5:1 throughout the chromatogram, with baseline subtraction just above the noise level, no smoothing, 3-s default peak widths, automatic mass-spectral deconvolution and peak detection and export of result spectra as *.csv files, in addition to export of raw data in open-access *.cdf formats. Data were further processed using the algorithms in the open-source BinBase metabolome database [14]. This algorithm used the settings: validity of chromatogram (< 10 peaks with intensity > 107 counts s− 1), unbiased retention index marker detection (MS similarity > 800 and exceeding thresholds for ion-ratio abundances for high m/z marker ions), and retention index calculation by 5th-order polynomial regression.
Spectra were cut to 5% base peak abundance, and matched to database entries from most- to least-abundant spectra, using the following matching filters: retention index window ± 2000 units (equivalent to about ± 2 s retention time), validation of unique ions and apex masses (unique ion must be included in apex masses and present at > 3% of base-peak abundance), mass spectrum similarity that must fit criteria dependent on peak purity and signal/noise ratios, optional ion-ratio settings to distinguish peaks with high similarity, and a final isomer filter (annotating the isomer spectrum with the closest RI fit). Signal intensities were reported as peak heights, using the unique ion as default, unless an alternative quantification ion was manually set in the BinBase administration software Bellerophon. All known artifact peaks—such as internal standards, column bleed, plasticizers or reagent peaks—were assigned by BinBase but not exported for further statistical calculations.
Metabolites were identified using the Fiehnlib libraries consisting of > 1000 authentic compounds and referenced using PubChem identifiers [15]. Daily quality controls were used comprising method blanks and five-point calibration curve samples of 31 pure reference compounds, which were repeatedly analyzed over the full analytical sequence, in addition to injection of one QC sample for every 10 biological samples. A quantification report table was produced for all database entries that were positively detected in > 50% of the samples of a study design class (as defined in the miniX database). This procedure results in 10–30% missing values, which could be caused by true negatives (compounds below the detection limit) or false negatives (compounds present but did not match quality criteria in the BinBase algorithm).
A subsequent post-processing module was employed to automatically replace missing values from the *.cdf files with the following parameters: for each positively detected metabolite, the average retention time was calculated for the day of analysis. Subsequently, for each chromatogram and each missing value, intensity of the quantification ion at this retention time was extracted by seeking its maximum value in a retention-time region of ± 1 s and subtracting the minimum (local background) intensity in a retention-time region of ± 5 s around the peak maximum. The resulting report table, therefore, did not contain any missing values. Replaced values were labeled as ‘lower confidence’ by color-coding.
2.7. Data normalization
Results were normalized by calculating the sum intensities of all identified compounds for each sample and dividing all data associated with a sample by the corresponding metabolite sum. The resulting data were multiplied by the average sum of all identified metabolites detected in the study (total average metabolome transformation)—disregarding unknown metabolites because these might potentially also represent artifacts. Intensities of identified metabolites with more than one peak (e.g. for the syn- and anti-forms of methoximated reducing sugars or amino acids with different derivatization status of amine groups) were summed to only one value in the transformed data set. The original non-transformed data set was retained for retrospective analysis. When comparing classes of samples with biologically different sum concentrations of identified metabolites (p < 0.05), these class averages were used for mean transformations. Result files were exported and processed by metabolomics BinBase database [14].
2.8. Statistical analysis
For the clinical data, the SAS software, version 9.1 (SAS Institute Inc., Cary, NC) was used. Descriptive statistics were calculated for each outcome. The data were log-transformed in order to improve the normality of residuals and homoscedasticity of errors where appropriate before analysis. Paired t-test was performed to determine the significance of within-group change. Inter-group comparisons were performed by analysis of covariance (ANCOVA), adjusted for the baseline values. When the overall difference among the groups was significant in ANCOVA, post-hoc pair wise group comparisons were performed using the Bonferroni multiple comparisons procedure to determine the significant differences. The longitudinal trajectories for changes in glucose and insulin during OGTT were estimated by repeated measures analysis of variance. Individual trajectories for changes in glucose and insulin were estimated from linear random-effects models. Each observed level was entered as the dependent variable. Treatment, time, and treatment x time interaction term were entered as independent variables. The coefficients for the interaction term were to estimate the additional changes in glucose and insulin level over time associated with treatment. To account for between-subject heterogeneity in the change of glucose or insulin, intercept and time were modeled as random effects. The 51 subjects included in the analysis had no missing endpoint values. A two-sided p-value < 0.05 was considered significant.
For the metabolomics data, to determine the baseline relationships among the metabolites and the clinical markers fasting plasma metabolites and several measures of insulin resistance and insulin secretion obtained from 79 PCOS patients were analyzed using multivariate regression splines (MARS). Model variables were automatically selected by the MARS algorithm from a pool of 22 nitrogenous compounds (mostly amino acids). In order to avoid over-fitting the algorithm pruned the number of variables to use only a maximum of 11 metabolites.
The metabolic pathways affected by fish, flaxseed and soybean oil treatments were determined using the PathwayTools software (version 17.0) from SRI International [16]. The HumanCyc, version 17.1 was used as reference [17]. The compounds which changed from the baseline (two tails student t-test p-value < 0.1), were used for pathway enrichment analysis. A minimum of 3 matches (hits) between the significant compound list and the reference pathway were required. Raw enrichment p-values were adjusted for multiple testing by Benjamini & Hochberg False Discovery Rate (FDR) [18]. A pathway with a FDR p-value < 0.01 was considered enriched. A network of significant compounds from the three oil treatments was overlaid on a KEGG reaction network [19], [20].
3. Results
3.1. Relationships between metabolites and clinical measures of insulin resistance and secretion
The multivariate regression splines (MARS) analysis indicated that 7 to 11 metabolites which provided predictive associations with insulin resistance and pancreatic function were BCAA, aromatic AA and other nitrogenous compounds (r2 = 0.70–0.86). A model built only on BCAA and aromatic AA alone was not as predictive (r2 for AIRg: 0.48 and fasting glucose: 0.24).
3.2. Changes in glucose homeostasis, plasma lipids and hs-CRP (Table 1 and Fig. 2)
Table 1.
Effects of fish, flaxseed and soybean oil treatments on anthropometric parameters, glucose homeostasis and cardiovascular risk markers (mean ± SD; Δ: change from the baseline).
| Fish oil (n = 17) |
Flaxseed oil (n = 17) |
Soybean oil (n = 17) |
||||
|---|---|---|---|---|---|---|
| Baseline | Δ | Baseline | Δ | Baseline | Δ | |
| Age (years) | 31.7 ± 7.8 | – | 29.4 ± 6.6 | – | 28.9 ± 4.1 | – |
| Weight (kg) | 100.0 ± 21.4 | 0.2 ± 0.8 | 94.1 ± 33.8 | 0.6 ± 1.6 | 90.0 ± 23.5 | 0.6 ± 1.6 |
| BMI (kg/m2) | 36.3 ± 7.8 | 0.3 ± 0.8 | 35.0 ± 10.3 | 0.2 ± 0.8 | 33.2 ± 7.4 | 0.1 ± 3.3 |
| Glucose (mmol/L) | 5.5 ± 0.7 | − 0.1 ± 0.9 | 5.2 ± 0.3 | 0.2 ± 0.5 | 4.9 ± 0.5 | 0.1 ± 0.5 |
| Insulin (pmol/L) | 153.5 ± 71.7 | 4.9 ± 63.1 | 172.9 ± 162.2 | 15.3 ± 160 | 122.2 ± 68.9 | 9.7 ± 34.2 |
| HgBA1 (%) | 5.5 ± 0.4 | − 0.1 ± 0.1⁎ | 5.5 ± 0.4 | − 0.1 ± 0.4 | 5.4 ± 0.4 | 0.1 ± 0.4 |
| Adiponectin (ng/ml) | 7.5 ± 4.1 | 1.0 ± 3.7 | 8.0 ± 4.9 | − 0.4 ± 2.1 | 6.5 ± 4.9 | − 0.3 ± 1.6 |
| Leptin (ng/ml) | 29.8 ± 14.4 | − 0.4 ± 10.3 | 27.1 ± 13.6 | 1.4 ± 4.2 | 28.1 ± 14.0 | 2.5 ± 12.0 |
| HOMA | 5.46 ± 2.8 | 0.10 ± 2.5 | 5.91 ± 6.1 | 0.9 ± 6.5 | 3.9 ± 2.4 | 0.4 ± 1.9 |
| ISIMatsuda | 2.4 ± 1.5 | − 0.5 ± 0.9⁎ | 2.5 ± 1.7 | − 0.1 ± 0.8 | 3.3 ± 1.7 | − 0.4 ± 1.0 |
| AUCGluc (mmol/L·2 h) | 15.7 ± 2.5 | 0.8 ± 2.3 | 15.9 ± 3.0 | 0.8 ± 2.8 | 14.8 ± 2.5 | 1.6 ± 2.3⁎ |
| AUCInsulin (pmol/L·2 h) | 1660 ± 944 | 326 ± 800b | 1924 ± 1373 | − 146 ± 717 | 1292 ± 800 | 167 ± 458 |
| AIRg (pmol/L) | 893 ± 705 | − 107 ± 231†,a | 627 ± 334 | 40 ± 210 | 669 ± 367 | − 119 ± 264† |
| DI | 1212 ± 779 | 229 ± 231 | 956 ± 713 | 11 ± 532 | 1784 ± 1798 | − 684 ± 1761 |
| SI | 1.5 ± 1.0 | 0.2 ± 1.2 | 1.6 ± 0.9 | 1.4 ± 1.2 | 3.1 ± 3.1 | − 0.7 ± 0.4 |
| Triglyceride (mmol/L) | 1.5 ± 0.6 | − 0.3 ± 0.4⁎ | 1.6 ± 1.0 | − 0.3 ± 0.5⁎ | 1.4 ± 0.7 | 0.7 ± 0.4 |
| Total-C (mmol/L) | 5.0 ± 0.9 | 0.2 ± 0.7 | 4.7 ± 1.0 | 0.3 ± 7 | 4.9 ± 0.7 | − 0.3 ± 1.1 |
| LDL-C (mmol/L) | 3.3 ± 0.7 | 0.2 ± 0.9 | 2.8 ± 0.7 | 0.2 ± 0.4 | 2.9 ± 0.8 | − 0.3 ± 0.9 |
| HDL-C (mmol/L) | 1.1 ± 0.2 | 0.1 ± 0.2 | 1.2 ± 0.33 | 0 ± 0.21 | 1.2 ± 0.3 | 0 ± 0 |
| ApoB (g/L) | 82.8 ± 20.9 | 3.4 ± 19.3 | 65.0 ± 51.5 | − 1.0 ± 45.9 | 76.8 ± 19.4 | 0.7 ± 14.0 |
| hs-CRP (mg/L) | 3.6 ± 3.2 | − 0.4 ± 2.4 | 3.8 ± 2.9 | − 0.1 ± 3.2 | 2.6 ± 2.5 | 0.1 ± 1.2 |
Inter-group comparisons were performed by analysis of covariance (ANCOVA), adjusted for the baseline values. When the overall difference among the groups was significant, post-hoc pair wise group comparisons were performed using the Bonferroni multiple comparisons procedure to determine the significant differences.
p < 0.05 for the change from the baseline, as analyzed by paired t-test.
p < 0.1 for the change from the baseline, as analyzed by paired t-test.
p < 0.05 as compared to the change with flaxseed oil.
p < 0.05 as compared to the change with soybean oil.
Fig. 2.
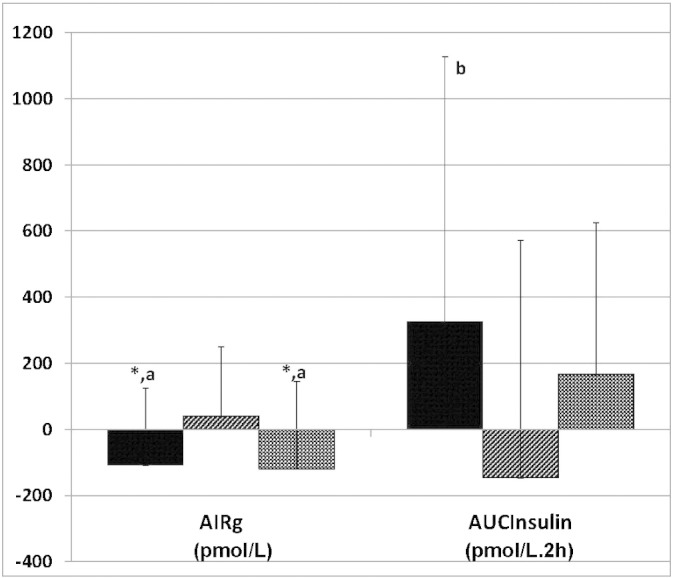
Effects of fish oil (FO), flaxseed oil (FLX) and soy bean oil (SBO) on early response (AIRg) measured by frequently sampled intravenous glucose tolerance test and on the cumulative insulin response measured by oral glucose tolerance test (AUCGlucose: pmolU/ml·2 h), shown as changes as compared to the pretreatment values (means ± SD; n = 17 in each treatment arm, b: p < 0.05 as compared to soybean oil). Paired t-test was performed to determine the significance of within-group change. Inter-group comparisons were performed by analysis of covariance (ANCOVA), adjusted for the baseline values. When the overall difference among the groups was significant in ANCOVA, post-hoc pair wise group comparisons were performed using the Bonferroni multiple comparisons procedure to determine the significant differences.
For glucose homeostasis, fish and soybean oils shared several effects which differed from those of flaxseed oil. Within-group, fish oil decreased HGBA1c (Δ: − 0.1 ± 0.1, p = 0.003) and the Matsuda's insulin sensitivity index (Δ: − 0.5 ± 0.9, p = 0.038); and tended to decrease early insulin secretion (AIRg; Δ: − 107 ± 231, p = 0.087) but overall insulin secretion did not decrease (AUCInsulin; Δ: 326 ± 800 pmol/L·2 h, p = 0.116). Between-group comparisons indicated that when compared to flaxseed oil, fish oil decreased early insulin secretion (AIRg: p = 0.024) but tended to increase cumulative insulin secretion (AUCInsulin: p = 0.062) (Fig. 2). The disposition index (DI) tended to be higher in fish oil treatment as compared to soybean oil (p = 0.059).
For plasma lipids, the effects of fish and flaxseed oils were similar and they differed from the effects of soybean oil. Plasma triglyceride decreased with both fish oil (Δ: − 0.3 ± 0.4 mmol/L, p = 0.015) and flaxseed oil (Δ: − 0.3 ± 0.5 mmol/L, p = 0.018). Between group comparisons showed that the effects of fish oil on triglyceride and LDL-cholesterol tended to differ from the effects of soybean oil (inter-group p = 0.055 for Δ triglyceride and p = 0.081 for Δ LDL-cholesterol).
3.3. Changes in fasting plasma amino acids (Table 2 and Fig. 3)
Table 2.
Effects of flaxseed, fish and soybean oils on serum metabolites (expressed as quantifier peak height means ± SD).
| Fish oil (n = 17) |
Flaxseed oil (n = 17) |
Soybean oil (n = 17) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Δ | Baseline | Δ | Baseline | Δ | ||
| BCAA & essential | Isoleucine | 26,678 ± 4346 | 5012 ± 5541⁎,a | 30,792 ± 5640 | 177 ± 6428 | 29,001 ± 3364 | 4757 ± 44,902⁎ |
| Leucine | 46,568 ± 6844 | 8250 ± 11,380⁎ | 54,082 ± 8522 | − 685 ± 13,363 | 51,347 ± 6444 | 7290 ± 7512 | |
| Valine | 83,560 ± 12,332 | 15,951 ± 19,284⁎,a | 97,055 ± 15,998 | 1758 ± 16,175 | 97,313 ± 9100 | 8854 ± 9124⁎ | |
| Lysine | 39,275 ± 5554 | 4651 ± 8419 | 47,970 ± 11,256 | − 1666 ± 13,342 | 42,997 ± 7995 | 1385 ± 1427 | |
| Aromatic AA | Phenylalanine | 13,450 ± 2016 | 1381 ± 3327 | 16,946 ± 3834 | − 1416 ± 4354 | 15,024 ± 2569 | 1250 ± 1291 |
| Tyrosine | 3678 ± 581 | 270 ± 606 | 4002 ± 891 | − 101 ± 808 | 3719 ± 763 | 81 ± 82 | |
| Tryptophan | 27,201 ± 2940 | 2146 ± 5686 | 32,698 ± 5822 | 193 ± 6259 | 32,418 ± 5529 | − 623 ± 643 | |
| Sulfur AA | Methionine | 1364 ± 808 | 205 ± 647 | 2230 ± 816 | 302 ± 1056 | 2065 ± 1113 | 570 ± 713⁎ |
| Meth. sulfoxide | 2916 ± 1105 | 712 ± 775⁎ | 2490 ± 936 | − 63 ± 1233 | 2596 ± 1006 | 329 ± 750 | |
| Cysteine | 1499 ± 570 | − 860 ± 548⁎,b | 1447 ± 515 | − 496 ± 569⁎ | 1504 ± 709 | − 448 ± 458⁎ | |
| Cystine | 2544 ± 437 | − 1567 ± 462⁎,b | 2313 ± 375 | − 680 ± 482⁎ | 2143 ± 750 | − 486 ± 503⁎ | |
| 1C | Glycine | 97,499 ± 12,398 | 25,763 ± 24,277a | 118,891 ± 25,440 | 5949 ± 21,923 | 105,798 ± 13,610 | 13,456 ± 13,870⁎ |
| Serine | 26,831 ± 7673 | 7833 ± 9553⁎,a | 33,660 ± 6329 | − 737 ± 9471 | 31,522 ± 4824 | 3443 ± 3550 | |
| Glutamic acid | 22,635 ± 6032 | 4028 ± 5203a | 24,052 ± 7426 | − 1978 ± 8630 | 17,068 ± 7982 | − 1284 ± 1324 | |
| Glutamine | 15,770 ± 9001 | 2321 ± 5884b | 19,576 ± 7855 | 3175 ± 8630 | 27,978 ± 14,942 | 769 ± 792 | |
| Non-Es | Alanine | 156,533 ± 32,486 | 13,995 ± 32,828 | 159,479 ± 27,885 | − 666 ± 28,425 | 148,432 ± 25,918 | 10,227 ± 10,543 |
| Proline | 47,965 ± 11,792 | 17,534 ± 17,771⁎ | 51,408 ± 11,339 | 7959 ± 18,550 | 51,626 ± 8353 | 8827 ± 9100⁎ | |
| Others | Urea | 234,794 ± 5489 | − 16,224 ± 72,117 | 242,284 ± 42,579 | − 13,799 ± 38,147 | 255,009 ± 55,765 | − 30,644 ± 31,587 |
| Uric acid | 11,992 ± 3200 | 754 ± 3740 | 14,303 ± 3146 | 1089 ± 3224 | 14,573 ± 3303 | 1155 ± 1192 | |
| Uridine | 112 ± 45 | 41 ± 82 | 138 ± 33 | 1 ± 58 | 195 ± 49 | 12 ± 12 | |
| 3-βOH butyrate | 8920 ± 9001 | − 4899 ± 9248 | 7640 ± 4441 | 54 ± 6123 | 5490 ± 2573 | 3101 ± 2989 | |
Inter-group comparisons were performed by analysis of covariance (ANCOVA), adjusted for the baseline values. When the overall difference among the groups was significant, post-hoc pair wise group comparisons were performed using the Bonferroni multiple comparisons procedure to determine the significant differences.
p < 0.05 for the change from the baseline, as analyzed by paired t-test.
p < 0.05 as compared to the change with flaxseed oil;
p < 0.05 as compared to the change with soybean oil.
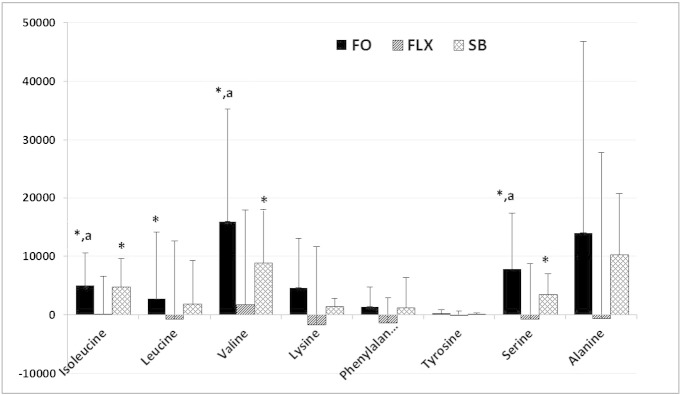
Fig. 3.
Effects of fish oil (FO), flaxseed oil (FLX) and soy bean oil (SBO) on fasting plasma amino acids, shown as changes as compared to the pretreatment values (mean ± SD; n = 17 in each treatment arm; *: p < 0.05 for the change from the baseline; a: p < 0.05 as compared to flaxseed oil). Paired t-test was performed to determine the significance of within-group change. Inter-group comparisons were performed by analysis of covariance (ANCOVA), adjusted for the baseline values. When the overall difference among the groups was significant in ANCOVA, post-hoc pair wise group comparisons were performed using the Bonferroni multiple comparisons procedure to determine the significant differences.
Fish and soybean oils increased several AA in the plasma. As compared to the baseline, fish oil increased isoleucine (p = 0.006), leucine (p = 0.035), valine (p = 0.019), methionine (p = 0.013), serine (p = 0.024) and proline (p = 0.007) Soybean oil also increased isoleucine (p = 0.001), valine (p = 0.016), glycine (p = 0.023) and proline (p = 0.045) They both decreased cysteine and cysteine. Increases in valine and isoleucine correlated with the increases in insulin response during OGTT (for valine, insulin at 60 min: r = 0.321, p = 0.022; at 90 min: r = 0.353, p = 0.011; AUCInsulin: r = 0.388, p = 0.005; for isoleucine, insulin at 90 min: r = 0.352, p = 0.011; AUCInsulin: 0.319, p = 0.022).
Flaxseed oil did not affect plasma AA levels, except decreasing cysteine (p = 0.016) and cystine (p = 0.001).
3.4. Metabolic pathways and networks affected by fish, flaxseed and soybean oil treatments
Fish oil exerted the biggest biochemical impact, affecting AA degradation (15 hits, p = 2 × 10− 7); AA biosynthesis (13 hits, p = 3 × 10− 6); C1 compound utilization (7 hits, p = 6 × 10− 4); amine and polyamine degradation (7 hits, p = 2 × 10− 3); purine nucleotide degradation (5 hits, p = 2 × 10− 3); TCA cycle (4 hits, p = 2 × 10− 3); and alanine, glycine, l-carnitine biosynthesis. Soybean oil also affected AA degradation (12 hits, p = 2 × 10− 5) or AA acid biosynthesis (11 hits, p = 4 × 10− 5). Flaxseed oil had minimal impact, affecting only alanine biosynthesis and l-cysteine degradation (each 3 hits; p = 0.002 for both).
Using enrichment analysis, it is difficult to distinguish whether the synthesis or the degradation pathway is affected because the same compounds are involved in both pathways. However, increases in specific degradation compounds and the higher number of hits on degradation pathways suggested that fish and soybean oil treatments affected primarily AA degradation.
4. Discussion
Polyunsaturated fatty acid supplements used in this study caused modest but significant changes in glucose homeostasis, plasma lipids and, unexpectedly, amino acid metabolism. Use of metabolomics provided a novel perspective for the understanding of the effects of PUFA on glucose homeostasis. Unexpectedly, long chain n-3 PUFA rich fish oil and n-6 PUFA rich soybean oil showed similarities in their effects on glucose homeostasis, plasma AA and nitrogenous compounds. On the other hand, flaxseed oil, which also belongs to n-3 class, behaved distinctly different than fish oil.
It is generally accepted that the metabolic effects of dietary PUFA are class-specific: Here we demonstrated that their effects on lipid metabolism may be class-specific because both fish and flaxseed oils decreased plasma triglyceride. However, changes seen in glucose homeostasis could not be explained on the basis of n-3 vs. n-6 classification.
We have found that fish oil decreased the Matsuda index, suggesting that insulin resistance increased. Although it is widely stated that fish oil increases insulin sensitivity, this is based on studies in experimental animals [21]. The studies in humans have shown either unchanged or increased insulin resistance [22], [23], [24]. Findings of experimental vs. clinical studies on pancreatic insulin secretion differ as well. The experimental studies indicate that both the essential and the long chain n-6 PUFA stimulate insulin secretion [25]. Thus n-3 PUFA are expected to decrease insulin secretion by competing with n-6 PUFA [26]. Insulin secretion occurs in two phases: early and late. As pancreas starts to fail, the early insulin secretion decreases, the late phase becomes more prominent. We found that both n-6 PUFA rich soybean oil and long chain n-3 rich fish oil tended to decrease the early insulin secretion (AIRg) but increased the late response and overall insulin secretion.
Several clinical studies investigated the effects of fish oil on blood glucose levels. Although individual studies showed variable changes in insulin secretion and insulin sensitivity, the overall glycemic control did not change [27]. We found that fish oil did not reduce glucose disposal (DI) possibly because of the compensatory increase in late insulin secretion; and DI was higher during fish oil treatment as compared to soybean oil. This may explain the decrease in HgBA1c during fish oil supplementation.
In the literature, the variability in the effects of n-3 PUFA on glucose homeostasis has been attributed to the differences in the dose of the supplements, subject populations and underlying disease states. However, the effects of n-3 PUFA on lipid metabolism have been quite consistent even though the study conditions have been as variable [1], [28]. This raises the possibility that the effects of PUFA on lipid metabolism may be direct and class-specific; whereas, their effects on glucose homeostasis may be much more complex.
The recent literature emphasized that serum BCAA, essential AA and aromatic AA related to various aspects of glucose homeostasis [12], [29], [30]. We reported similar results in a group of women with metabolic syndrome who did not have PCOS [13]. In this study a battery of AA and nitrogenous compounds showed predictive associations with fasting glucose, fasting insulin, HOMA, AUCInsulin and AIRg as well. Moreover addition, fish and soybean oils which affected glucose homeostasis also increased BCAA leucine, isoleucine and valine, while flaxseed oil, which did not affect glucose homeostasis, did not increase BCAA either. Regardless the treatment, the increases in plasma valine and leucine correlated directly with the increase in insulin response during OGTT. There may be two mechanisms: First, BCAA may have caused insulin resistance which then led to compensatory increase in insulin response. Second, BCAA may have acted as insulin secretagouge in the pancreas potentiating insulin response to glucose. We have previously demonstrated that whey protein, a rich source of essential and BCAA, stimulated insulin secretion in PCOS without changing plasma glucose levels [31].
We demonstrated that fish oil increased three out of four BCAA by 18% to 19% and soybean oil increased them by 9% to 16% while flaxseed oil did not affect plasma AA, AA metabolism or glucose homeostasis. Pathway analysis indicated that the increase in AA levels resulted primarily from decreased degradation. The rate limiting step in BCAA degradation is the branched chain α-ketoacid dehydrogenase (BCKD) [32]. Changes in β-oxidation of fatty acids have been associated with altered mitochondrial BCKD activity and insulin resistance; and it is known that fish oil alters mitochondrial β-oxidation of fatty acids [33]. Thus, a possible model of indirect regulation of glucose homeostasis may be that PUFA can modulate the plasma concentrations of AA by altering their degradation; and in turn AA can regulate insulin resistance and insulin secretion. It has been shown that fatty acids modify the effects of BCAA on insulin action [34] Lu et al. investigated the effects of fish oil on plasma metabolites and hepatic gene expression [35]. They found that fish oil activated PPARα in the live and altered gene expression of the enzymes involved BCAA degradation as well as plasma levels of several of the AA listed in Table 2 of our manuscript. A recent article in dyslipidemic and control human subjects also demonstrated that fish oil treatment regulated the genes involved in AA metabolism [36]. Thus emerging evidence supports the regulatory role of PUFA on AA metabolism in animal models and humans.
Finally, changes in sulfur containing AA are also noteworthy. Fish and soybean oils decreased cysteine by 57% and 30% respectively; fish oil also increased methionine sulfoxide by 24%. These findings are consistent with increased oxidative stress during increased PUFA intake. Increased oxidative stress is known to cause insulin resistance by increasing inflammation [37].
In summary, our findings suggest the novel possibility that dietary PUFA may alter glucose homeostasis indirectly, secondary to its effects on the AA metabolism. Alternatively, alterations in AA metabolism can be the consequence of the PUFA-induced changes in insulin sensitivity. The small sample size and wide range of BMI of the study subjects reduced the power for detection of several of the changes in glucose homeostasis parameters. Further research is necessary to investigate the potential mechanisms and to understand if these findings manifest outside the context of PCOS.
Transparency document
Transparency document.
Footnotes
Clinical trial #: NCT 022715060-1
The Transparency documents associated with this article can be found, in online version.
References
- 1.Kasim Karakas S. In: Omega-3 fish oils and lipoprotein metabolism. Wildman REC, editor. 2006. [Google Scholar]
- 2.Kasim Karakas S. In: Omega-3 fish oils and insulin resistance. Wildman REC, editor. 2006. [Google Scholar]
- 3.Arterburn L.M., Hall E.B., Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 4.Mourente G., Tocher D.R. In vivo metabolism of [1–14C]linolenic acid (18:3(n-3)) and [1–14C]eicosapentaenoic acid (20:5(n-3)) in a marine fish: time-course of the desaturation/elongation pathway. Biochim. Biophys. Acta. 1994;1212:109–118. doi: 10.1016/0005-2760(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 5.Vargas M.L., Almario R.U., Buchan W., Kim K., Karakas S.E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metab. Clin. Exper. 2011;60:1711–1718. doi: 10.1016/j.metabol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas M.L., Almario R.U., Buchan W., Kim K., Karakas S.E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism. 2011 doi: 10.1016/j.metabol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E., Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giesbertz P., Daniel H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:48–54. doi: 10.1097/MCO.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 9.Felig P., Marliss E., Cahill G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 10.Fiehn O., Garvey W.T., Newman J.W., Lok K.H., Hoppel C.L. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5:e15234. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccolo B.D., Comerford K.B., Karakas S.E., Knotts T.A., Fiehn O. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J. Nutr. 2015;145:691–700. doi: 10.3945/jn.114.203943. [DOI] [PubMed] [Google Scholar]
- 14.Scholz M., Fiehn O. Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing: 169–180. 2007. SetupX—a public study design database for metabolomic projects. [PubMed] [Google Scholar]
- 15.Kind T., Wohlgemuth G., Lee do Y., Lu Y., Palazoglu M. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karp P.D., Paley S.M., Krummenacker M., Latendresse M., Dale J.M. Pathway tools version 13.0: integrated software for pathway/genome informatics and systems biology. Brief. Bioinform. 2010;11:40–79. doi: 10.1093/bib/bbp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero P., Wagg J., Green M.L., Kaiser D., Krummenacker M. Computational prediction of human metabolic pathways from the complete human genome. Genome Biol. 2005;6:R2. doi: 10.1186/gb-2004-6-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 19.Karnovsky A., Weymouth T., Hull T., Tarcea V.G., Scardoni G. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics. 2011 doi: 10.1093/bioinformatics/btr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killcoyne S., Carter G.W., Smith J., Boyle J. Cytoscape: a community-based framework for network modeling. Methods Mol. Biol. 2009;563:219–239. doi: 10.1007/978-1-60761-175-2_12. [DOI] [PubMed] [Google Scholar]
- 21.Fedor D., Kelley D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 22.Kabir M., Skurnik G., Naour N., Pechtner V., Meugnier E. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am. J. Clin. Nutr. 2007;86:1670–1679. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 23.Karlstrom B.E., Jarvi A.E., Byberg L., Berglund L.G., Vessby B.O. Fatty fish in the diet of patients with type 2 diabetes: comparison of the metabolic effects of foods rich in n-3 and n-6 fatty acids. Am. J. Clin. Nutr. 2011;94:26–33. doi: 10.3945/ajcn.110.006221. [DOI] [PubMed] [Google Scholar]
- 24.Mostad I.L., Bjerve K.S., Lydersen S., Grill V. Effects of marine n-3 fatty acid supplementation on lipoprotein subclasses measured by nuclear magnetic resonance in subjects with type II diabetes. Eur. J. Clin. Nutr. 2008;62:419–429. doi: 10.1038/sj.ejcn.1602703. [DOI] [PubMed] [Google Scholar]
- 25.Band A.M., Jones P.M., Howell S.L. The mechanism of arachidonic acid-induced insulin secretion from rat islets of Langerhans. Biochim. Biophys. Acta. 1993;1176:64–68. doi: 10.1016/0167-4889(93)90178-r. [DOI] [PubMed] [Google Scholar]
- 26.Holness M.J., Smith N.D., Greenwood G.K., Sugden M.C. Acute omega-3 fatty acid enrichment selectively reverses high-saturated fat feeding-induced insulin hypersecretion but does not improve peripheral insulin resistance. Diabetes. 2004;53(Suppl. 1):S166–S171. doi: 10.2337/diabetes.53.2007.s166. [DOI] [PubMed] [Google Scholar]
- 27.Hendrich S. (n-3) Fatty acids: clinical trials in people with type 2 diabetes. Adv. Nutr. 2010;1:3–7. doi: 10.3945/an.110.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eslick G.D., Howe P.R., Smith C., Priest R., Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int. J. Cardiol. 2008 doi: 10.1016/j.ijcard.2008.03.092. [DOI] [PubMed] [Google Scholar]
- 29.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah S.H., Svetkey L.P., Newgard C.B. Branching out for detection of type 2 diabetes. Cell Metab. 2011;13:491–492. doi: 10.1016/j.cmet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Gurusinghe D., Gill S., Almario R.U., Lee J., Horn W.F. In polycystic ovary syndrome, adrenal steroids are regulated differently in the morning versus in response to nutrient intake. Fertil. Steril. 2010;93:1192–1199. doi: 10.1016/j.fertnstert.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011;2:445–456. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froyland L., Madsen L., Vaagenes H., Totland G.K., Auwerx J. Mitochondrion is the principal target for nutritional and pharmacological control of triglyceride metabolism. J. Lipid Res. 1997;38:1851–1858. [PubMed] [Google Scholar]
- 34.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y., Boekschoten M.V., Wopereis S., Muller M., Kersten S. Comparative transcriptomic and metabolomic analysis of fenofibrate and fish oil treatments in mice. Physiol. Genomics. 2011;43:1307–1318. doi: 10.1152/physiolgenomics.00100.2011. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt S., Stahl F., Mutz K.O., Scheper T., Hahn A. Different gene expression profiles in normo- and dyslipidemic men after fish oil supplementation: results from a randomized controlled trial. Lipids Health Dis. 2012;11:105. doi: 10.1186/1476-511X-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.