Abstract
The long non-coding RNA (lncRNA) SPRIGHTLY (formerly SPRY4-IT1), which lies within the intronic region of the SPRY4 gene is upregulated in human melanoma cells compared to melanocytes. SPRIGHTLY regulates a number of cancer hallmarks including proliferation, motility, and apoptosis. To better understand its oncogenic role, SPRIGHTLY was stably transfected into human melanocytes, which resulted in increased cellular proliferation, colony formation, invasion, and development of a multinucleated dendritic-like phenotype. RNA sequencing and mass spectrometric analysis of SPRIGHTLY-expressing cells revealed changes in the expression of genes involved in cell proliferation, apoptosis, chromosome organization, regulation of DNA damage responses, and cell cycle. The proliferation marker Ki67, minichromosome maintenance genes (MCM2-5), the anti-apoptotic gene X-linked inhibitor of apoptosis (XIAP) and the baculoviral IAP repeat-containing 7 (livin) were all upregulated in SPRIGHTLY-expressing melanocytes, while the pro-apoptotic tumor suppressor gene DPPIV/CD26 was downregulated, followed by an increase in ERK 1/2 phosphorylation, suggesting an increase in MAPK activity. Since downregulation of DPPIV is known to be associated with malignant transformation in melanocytes, SPRIGHTLY-mediated DPPIV downregulation may play an important role in melanoma pathobiology. Together, these findings provide important insights into how SPRIGHTLY regulates cell proliferation and anchorage-independent colony formation in primary human melanocytes.
INTRODUCTION
Melanoma is a skin cancer that arises from pigment-producing cells called melanocytes, and it is the leading cause of skin cancer-related death in the United States. Since melanoma is intrinsically resistant to many existing therapies, there is a pressing need to better understand the gene-regulatory pathways that contribute to melanomagenesis.
A class of regulatory RNAs greater than 200 nucleotides in length known as long non-coding RNAs (lncRNAs) have recently gained attention as oncogenes or tumor suppressor genes (Amaral and Mattick, 2008; Taft et al., 2010). LncRNAs were originally dismissed as non-functional transcriptional “noise” (Clark et al., 2011) since although some lncRNAs are translated into short polypeptides, the vast majority of lncRNAs are rarely or never translated (Banfai et al., 2012; Gascoigne et al., 2012). However, lncRNAs exhibit exquisite spatial and temporal context-dependent expression in different cell types, commensurate with their presumed regulatory role (Khaitan et al., 2011; Mercer et al., 2008; Sunwoo et al., 2009). At the molecular level, lncRNAs influence target gene expression at specific genomic loci either by directly interacting with chromatin regulatory proteins and/or by modulating the activity of their interacting partners (Dinger et al., 2008; Khalil et al., 2009; Pandey et al., 2008; Rinn and Chang, 2012; Tsai et al., 2010; Umlauf et al., 2008). LncRNAs can function as decoys for bound proteins and can alter protein structure and function (Rinn and Chang, 2012). LncRNAs play important physiological roles in normal cellular development and differentiation (Dinger et al., 2008), but changes in lncRNA expression are also associated with several diseases including cancer, heart disease, Alzheimer’s disease, psoriasis, and spinocerebellar ataxia type 8 (Esteller, 2011). For examples, in cancer, increased HOTAIR expression is associated with poor prognosis pancreatic cancer (Kim et al., 2013) and increased expression of PCGEM1 and PCA3/DD3 are associated with the development of prostate cancer (Ifere and Ananaba, 2009).
We previously identified a number of lncRNAs that are differentially expressed in melanoma cell lines relative to melanocytes and keratinocytes (Khaitan et al., 2011; Mazar et al., 2010). One of these, SPRIGHTLY (GenBank accession ID AK024556), was highly expressed and localized predominantly in the cytoplasm in melanoma cells but expressed at low levels in primary human melanocytes (Khaitan et al., 2011). SPRIGHTLY is derived from the intronic region of the SPRY4 gene and its predicted secondary structure contains several long hairpins (Khaitan et al., 2011). Loss of function of SPRIGHTLY in melanoma cells prevented cell growth and differentiation and induced apoptosis (Khaitan et al., 2011).
Here, we sought to examine how SPRIGHTLY contributes to melanocyte dedifferentiation and melanomagenesis by characterizing its molecular function. We hypothesized that the lncRNA SPRIGHTLY and its target genes dedifferentiate melanocytes and contribute to the development of human melanomas. To test the hypothesis, we ectopically expressed SPRIGHTLY in normal human melanocytes and knocked it down in melanoma cells.
SPRIGHTLY ectopically expressed in human melanocytes increased cellular proliferation, invasion, colony-formation, and induced a multinucleated dendritic-like phenotype. RNA sequencing and mass spectrometric (MS) analysis revealed changes in subsets of genes and proteins involved in cell proliferation, apoptosis, chromosome organization, regulation of the DNA damage response, and cell cycle progression. Accordingly, the cell proliferation marker Ki67, minichromosome maintenance genes (MCM2-5), and the anti-apoptotic genes X-linked inhibitor of apoptosis (XIAP) and baculoviral IAP repeat-containing 7 (livin) were all upregulated in SPRIGHTLY-expressing melanocytes. In contrast, expression of the pro-apoptotic tumor suppressor gene DPPIV was downregulated. Loss-of-function experiments in the melanoma cell line A375 confirmed the opposite effects. SPRIGHTLY contributes to the regulation of proliferation and apoptosis pathway genes in melanocytes and melanomas.
RESULTS
Ectopic expression of SPRIGHTLY in normal human melanocytes results in multi-nuclear and multi-dendrite cells
SPRIGHTLY is expressed at significantly lower levels in human melanocytes than melanoma cells (Khaitan et al., 2011). To establish the molecular and cellular functions of SPRIGHTLY in melanocytes, normal human melanocytes were engineered to ectopically express the SPRIGHTLY transcript using a lentiviral vector. The same vector without SPRIGHTLY was used as a control. RNA-FISH (Supplementary Figure S1a) and RNA-seq (Supplementary Figure S1b) confirmed ectopic expression of SPRIGHTLY in engineered cells. Interestingly, morphological examination of melanocytes that ectopically express SPRIGHTLY (SPRIGHTLY-EE) after one month of transfection revealed approximately 25% altered dendritic-like cell morphology with multiple enlarged nuclei compared to Vector Only controls (Figure 1a). Next, we stained SPRIGHTLY-EE cells with MELAN-A to observe probable pigmentation changes. Results (Supplementary Figure S2) reveal that, SPRIGHTLY did not interfere with the melanocyte pigmentation. It has previously been reported that oncogene-induced senescence results in multinucleated giant cells (Leikam et al., 2008). Oncogene-induced senescence is thought to be a natural anti-tumorigenic effect that occurs in response to extreme growth stimulatory signals from activated oncogenes.
Figure 1. Morphological and gene ontology changes in melanocytes that ectopically express SPRIGHTLY.
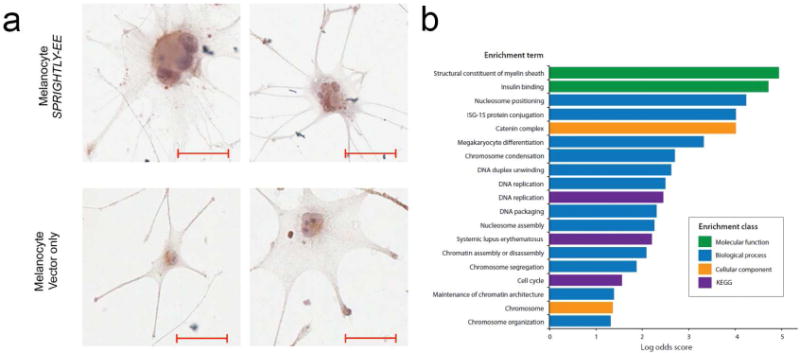
(a) Melanocytes ectopically expressing SPRIGHTLY (melanocyte SPRIGHTLY-EE) show a multi-nucleated and multi-dendritic-like phenotype. (b) Gene ontology (GO) and KEGG pathway enrichments determined from genes significantly differentially expressed between SPRIGHTLY-EE melanocytes compared to Vector Only control melanocytes by RNA-sequencing. P-values <0.005. Scale bar = 10 um
SPRIGHTLY regulates chromatin and cell cycle pathway genes in normal human melanocytes
To identify the molecular mechanisms by which ectopic expression of SPRIGHTLY modifies cell phenotype, SPRIGHTLY-EE and Vector Only cells were subjected to RNA-seq, MS, qPCR, and proto-array-based analyses. Comparison of the transcriptomic profiles revealed 740 significantly (p<0.05) differentially expressed genes (Supplementary Dataset S1). There was gene ontology (GO) enrichment for biological processes involved in DNA packaging, chromosome organization, DNA repair, cell cycle, and maintenance or establishment of chromatin architecture (mainly due to upregulation of core histone genes) in SPRIGHTLY-EE cells (Figure 1b and Supplementary Dataset S2). Pathway analysis demonstrated significant (p<0.005) enrichment for cell cycle, apoptosis, and DNA replication pathways, which was in keeping with the proliferative and multinuclear phenotype of SPRIGHTLY-expressing melanocytes. Notably, enrichment for chromatin-related gene expression is consistent with the multi-nucleated character of SPRIGHTLY-overexpressing cells and suggests that SPRIGHTLY may have a role in inducing DNA replication and nuclear division.
Further enrichment analysis of RNA-seq data for biological processes and cellular and molecular function revealed the presence of distinct gene clusters. These functional cluster categories are depicted in Figure 2 a, b, and c. There was significant enrichment for genes in the “chromosome organization, assembly, segregation, and condensation, DNA packaging, nucleosome positioning and assembly, DNA replication, and maintenance of chromatin architecture” biological process groups (Figure 2a). Genes in the “cellular function catenin complex and chromosome groups” were overrepresented (Figure 2b), and genes in the “molecular function structural constituent of myelin sheath and insulin-binding” groups were also enriched (Figure 2c).
Figure 2. Gene ontology (GO) enrichment.
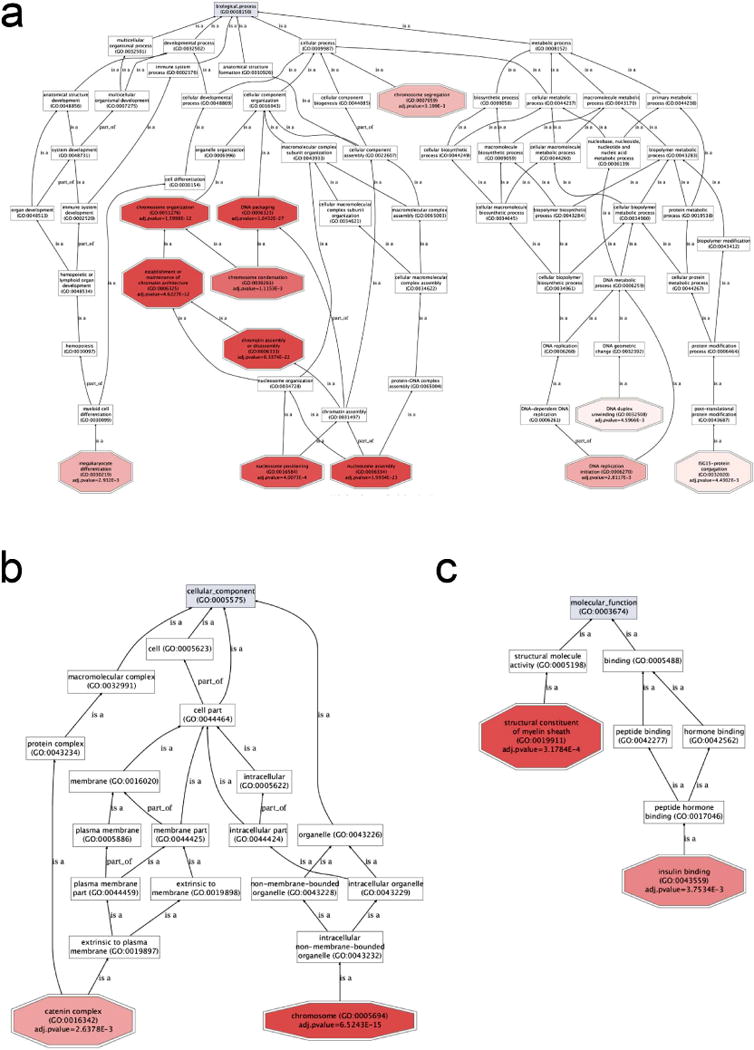
Statistically significant and differentially expressed genes were used for GO enrichment (SPRIGHTLY-EE vs. Melanocyte-VO). GoGraphViz visualizations showing: (a) biological process, (b) cellular component, and (c) molecular function GO terms. Adjusted P-values less than 0.005 for GO terms were considered significant. GO enrichments and visualizations were performed using Babelomics 4.3 (Medina et al., 2010).
Cell proliferation genes are perturbed when SPRIGHTLY is ectopically expressed in melanocytes and knocked down in melanoma cells
Since cell cycle and proliferative pathway genes appear to be perturbed in GO analyses, RNA samples were subjected to cell cycle and proliferation qPCR array analysis (Qiagen). Notably, mini-chromosome maintenance (MCM; MCM2, MCM3, MCM4, and MCM5), antigen-MKI67, cyclin-dependent kinase 1 (CDK1), and cell-division cycle protein 20 (CDC20) genes were significantly upregulated in SPRIGHTLY-EE cells, suggesting that SPRIGHTLY may play an important role in cell cycle progression and proliferation (Figure 3). We therefore performed cell proliferation, invasion and colony formation assays in SPRIGHTLY-EE cells and found that SPRIGHTLY expressing melanocytes are more proliferative, invasive and form anchorage-independent colonies (Supplementary Figures S3 a, b, c), further supporting the potential role of SPRIGHTLY in melanomagenesis. We additionally assayed SPRIGHTLY-EE cells for changes in cell senescence (β-gal assay), but could detect no differences with control cells (data not shown).
Figure 3. Differentially expressed proliferation, cell cycle, and apoptosis-related genes in SPRIGHTLY-EE cells compared to Melanocyte-VO.
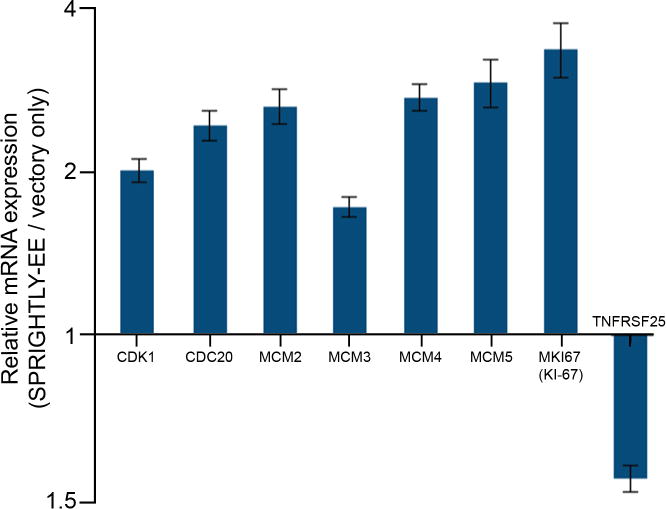
Relative expression of CDK1, CDC20, MCM2, MCM3, MCM4, MCM5, Ki-67 and TNFRSF25 in SPRIGHTLY-EE vs. Melanocyte-VO.
Since we observed SPRIGHTLY-EE cells are showing increased cell proliferation, invasion and colony forming ability, we hypothesize that these cells may be capable of forming tumors in SCID mice. To examine the oncogenic potential of SPRIGHTLY in vivo using mouse models we implanted SPRIGHTLY-EE cells into SCID mice. As a control, we injected vector only parental melanocytes, and included groups of five mice for each experiment. The results are depicted in Supplementary Figure S4. Following three months after cell implantation, we observed that SPRIGHTLY-EE cells, but not vector only parental cells, initiated subcutaneous cell migration.
MCM2 is the most widely studied MCM family member, and expression of MCM2 has been directly linked to cancer cell proliferation and invasion (Guzinska-Ustymowicz et al., 2008; Lian et al., 2013; Liu et al., 2013; Wojnar et al., 2011). MCM2 is upregulated in primary cutaneous melanomas and cutaneous melanoma metastases compared to benign nevi (Boyd et al., 2008; de Andrade et al., 2013). Since ectopic expression of SPRIGHTLY in melanocytes increased MCM2, we decided to investigate whether MCM2 knockdown by siRNA in the stage IV melanoma cell line A375 has opposite effects. qPCR confirmed excellent MCM2 knockdown efficiency (~90%; Figure 4a). Boyd et al. (2008) reported that MCM2 protein expression differs significantly in melanocytic neoplasms and can help distinguish benign tumors from their malignant counterparts. Therefore, we used an anti-MCM2 antibody to measure MCM2 protein expression in A375 parental and MCM2 siRNA knock-down to further confirm siRNA knockdown efficiency (Figure 4b).
Figure 4. MCM2 knockdown on cell viability, growth, and invasiveness.
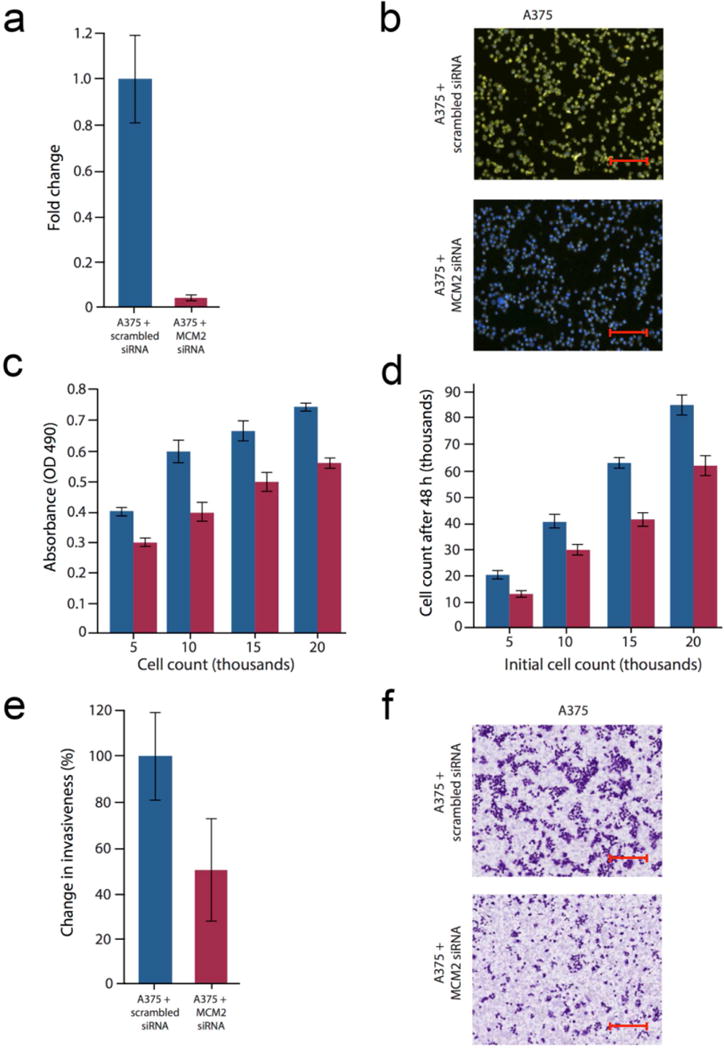
A375 cells transfected with an MCM2-specific siRNA and compared to scramble control siRNA. (a) qRT-PCR demonstrate the effect of MCM2-specific knockdown relative to scrambled control. (b) IHC staining of A375 cells using MCM2-specific antibodies. MCM2 staining shown in yellow (fluorescein isothiocyanate) and nuclei in blue (DAPI). Scale bar = 200 um. (c) Knockdown of MCM2 affects A375 cell viability (MTS assay). (d) Knockdown of MCM2 affects A375 cell number. (e) Knockdown of MCM2 suppresses A375 invasion. (f) Staining of A375 cells (crystal violet) 48 h after transfection with MCM2 siRNA reveals a decrease in cell invasion. All results are expressed as a mean±SD of three experiments. Scale bar = 200 um. Data for (a) and (e) were normalized to the scramble-transfected control.
Since MCM2 is a known cell proliferation gene/marker, we next measured cell proliferation in MCM2 knockdown A375 cells by direct cell counting. MCM2 knockdown reduced A375 cell numbers by ~30% over a 48 h period compared to scramble siRNA control cells (Figure 4c). This was corroborated in cell viability assays (MTS), which also revealed ~30% decrease in viability in MCM2 knockdown cells over the same period of time (Figure 4d). Cell invasion was reduced by ~50% in MCM2 knockdown A375 cells compared to controls (Figure 4e and f), an effect replicated by knockdown of the SPRIGHTLY transcript (Khaitan et al., 2011). Next, we examined whether MCM2 knockdown had any effect on the activity of effectors of apoptosis, but did not find any changes either in caspase 3 or 7 activity. Of note, SPRIGHTLY expression did not change when MCM2 was knocked down in A375 cells. However, SPRIGHTLY knockdown in A375 melanoma cells did result in downregulation of both MCM2 and Ki67, suggesting that SPRIGHTLY does regulate cell proliferation and likely to be located upstream of MCM2 and Ki67 gene regulatory pathways.
Next, to further confirm the cell proliferation, Ki67 immunostaining was performed in normal human melanocytes, SPRIGHTLY-EE cells, and A375 cells (Figure 5a). Ki67 was poorly expressed in melanocytes, showed increased expression in SPRIGHTLY-EE cells, and was highly expressed in A375 melanoma cells, supporting the notion that SPRIGHTLY contributes to increased cell proliferation in SPRIGHTLY-EE cells. This observation was further confirmed by knockdown of SPRIGHTLY in A375 cells followed by measuring Ki67 expression using RNA-seq. As expected, Ki-67 expression was profoundly downregulated in SPRIGHTLY knockdown A375 cells (Supplementary Figure S5). Moreover RNA-seq data were further analyzed for proliferation pathway genes using Ingenuity pathway analysis software, and several proliferative pathway genes were perturbed by SPRIGHTLY knockdowns (Supplementary Figure S6). Together, these data indicate that SPRIGHTLY is important for cell proliferation in melanocytes and melanoma cells.
Figure 5. Ki-67 and DPPIV expression in melanocytes ectopically expressing SPRIGHTLY.
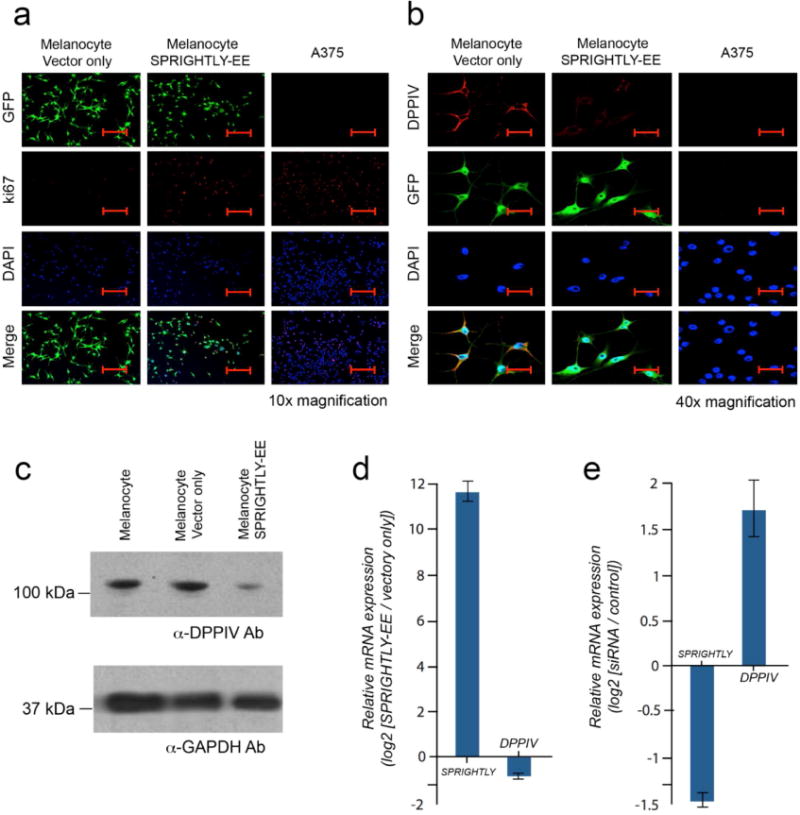
(a) Immunofluorescence staining of Ki-67 in SPRIGHTLY-EE, Vector-only melanocytes, and A375 cells. Ki-67 nuclear staining is highest in A375 cells. Ki-67 staining is elevated in SPRIGHTLY-EE cells compared to Vector-only controls. Scale bar = 80 um. (b) Immunofluorescence staining of DPPIV in SPRIGHTLY-EE cells, Vector-only melanocytes, and A375 cells. Control melanocytes display abundant cell-surface DPPIV, A375 cells express little or no DPPIV, and SPRIGHTLY-EE cells show intermediate expression levels, i.e., an inverse pattern to Ki-67. Scale bar = 20 um. (c) DPPIV protein levels (western blot) in SPRIGHTLY-EE cells relative to melanocytes alone and melanocytes transfected with empty vector. (d) Ectopic expression of SPRIGHTLY downregulates DPPIV mRNA levels in melanocytes. (e) siRNA-mediated knockdown of SPRIGHTLY upregulates DPPIV mRNA levels in A375 melanoma cells.
Anti-apoptotic XIAP and livin are upregulated, and pro-apoptotic DPPIV is downregulated, in SPRIGHTLY-EE cells
Further analysis of protein array data revealed that a group of anti-apoptotic proteins, including livin and the X-linked inhibitor of apoptosis (XIAP) proteins, were induced in SPRIGHTLY-EE cells compared to controls. Conversely, pro-apoptotic proteins such as TRAIL-R2 (DR5) and dipeptidyl peptidase-IV (DPPIV) were clearly decreased (Supplementary Figure S7). These results suggest that SPRIGHTLY expression is associated with changes in anti-apoptotic, pro-proliferative, and DNA-packaging genes, although whether these are indirect or direct effects remains to be determined.
Of the identified gene products modulated by SPRIGHTLY, DPPIV, is known to be directly associated with the molecular etiology of human melanomas (Nielsen et al., 2011; Pethiyagoda et al., 2000). DPPIV is a known pro-apoptotic tumor suppressor that is usually highly expressed in normal melanocytes but shows reduced expression in SPRIGHTLY-EE cells and has undetectable expression in A375 cells (Figure 5b). We therefore analyzed protein expression of DPPIV in SPRIGHTLY-EE and Vector Only cells by western blotting, which confirmed that DPPIV protein expression is significantly lower in SPRIGHTLY-EE cells compared to controls (Figure 5c). Since it has been previously reported that DPPIV expression is known to inhibit MAPK-extracellular signal-regulated kinase (ERK) 1/2 activation (Wesley et al., 2005), we decided to check ERK 1/2 phosphorylation by western blot analysis. The results are depicted in the Supplementary Figure S8. An examination of SPRIGHTLY-EE cells revealed an increase in phosphorylation of ERK 1/2 compared to that seen in Melanocyte Vector only control cells, suggesting that the loss of DPPIV may allow for an increase in activation of MAPK. Given that DPPIV expression can be silenced by promoter hypermethylation in melanomas (McGuinness and Wesley, 2008), DPPIV promoter CpG islands were examined for possible promoter hypermethylation. However, no aberrant CpG island methylation was identified (Supplementary Figure S9), indicating that DPPIV downregulation may occur via an alternate (i.e., other than epigenetic) mechanism.
To further examine the relationship between SPRIGHTLY and DPPIV, SPRIGHTLY and DPPIV mRNA levels were measured by qPCR under two opposing conditions (increased and decreased levels of SPRIGHTLY). Our results revealed a reciprocal relationship between DPPIV and SPRIGHTLY expression levels: decreased DPPIV mRNA levels were associated with increased SPRIGHTLY, while increased DPPIV mRNA levels were associated with decreased SPRIGHTLY expression (Figure 5e and e). Since DPPIV is a known pro-apoptotic tumor suppressor gene that is highly expressed in normal human melanocytes but its expression is lost in malignant melanoma, our results imply that a higher SPRIGHTLY:DPPIV ratio is most likely contribute to the malignant phenotype. Together, our study provides evidence that SPRIGHTLY may contribute to malignant transformation of melanocytes by regulating a number of cancer hallmarks.
DISCUSSION
Long non-coding RNAs (lncRNAs) are involved in numerous aspects of human physiology and pathology. Until recently, these transcripts had been dismissed as non-functional noise and were regarded as junk. Although our understanding of the function of lncRNAs has increased rapidly, there remain large knowledge gaps about how lncRNAs are regulated and how they regulate other genes and proteins.
In this study, we provide evidence that stable lentiviral expression of the lncRNA SPRIGHTLY in normal human melanocytes contributes to the regulation of cell proliferation, apoptosis, chromosome organization, regulation of the DNA damage response, and cell cycle in melanocytes. Previously, we reported that SPRIGHTLY which is transcribed from the first intron of the Sprouty 4 gene (SPRY4), is expressed at low levels in normal human melanocytes but is highly upregulated in human melanoma cells and patient samples (Khaitan et al., 2011). Importantly, siRNA-mediated knockdown of SPRIGHTLY in melanoma cells decreased cell proliferation and invasion and increased apoptosis. To investigate this further, we affinity purified SPRIGHTLY from melanoma cells and used mass spectrometry (MS) to identify protein interactions; of these, the protein phosphatidate phosphatase (lipin 2) was found to be a major binding partner involved in lipid metabolism (Mazar et al., 2014). These findings support a role for SPRIGHTLY in multiple regulatory pathways in melanomas and suggest that SPRIGHTLY and its target genes may be important in melanocyte dedifferentiation and their transformation into melanomas.
Here, we report that melanocytes engineered to stably express SPRIGHTLY display increased cell proliferation and a multinucleated dendritic-like morphology. The melanocyte dendrite is a specialized structure that is formed in response to hormones (e.g., α-melanocyte stimulating hormone) and UV light and functions to transport melanosomes to neighboring keratinocytes. Scott and Cassidy (Scott and Cassidy, 1998) reported that transfection of an expression vector encoding constitutively active rac1 protein induces the formation of branching actin-based structures in B16F1 murine melanoma cells and melanoma cell lines. However, we did not observe an increase in rac1 expression in SPRIGHTLY-EE cells compared to parental controls. Therefore, we believe that SPRIGHTLY induced multi-dendrite formation is rac1 independent.
RNA sequencing and MS analysis of SPRIGHTLY-EE cells revealed changes in subsets of genes involved in cell proliferation, apoptosis, chromosome organization, regulation of the DNA damage response, and cell cycle. Interestingly, the cell proliferation marker MKI67 and minichromosome maintenance genes (MCM2-5), as well as the anti-apoptotic genes X-linked inhibitor of apoptosis (XIAP) and baculoviral IAP repeat-containing 7 (livin), were all upregulated in SPRIGHTLY-expressing melanocytes. In contrast, expression of the pro-apoptotic and tumor suppressor gene DPPIV was downregulated.
The protein encoded by the DPPIV gene is a trans-membrane glycoprotein with a serine exopeptidase activity. It is constitutively expressed on the surface of numerous cell types (including melanocytes) and plays an important role in immune regulation, signal transduction, and apoptosis (Pro and Dang, 2004; Yu et al., 2010). In melanocytes, downregulation of DPPIV/CD26 is associated with malignant transformation (Morrison et al., 1993; Van den Oord, 1998; Wesley et al., 1999). Conversely, inducible transduction of DPPIV into melanoma cells reverses the malignant phenotype to that of normal melanocytes (Wesley et al., 1999), suggesting that DPPIV/CD26 downregulation represents an important event in the pathogenesis of melanoma. Supporting this, DPPIV/CD26 has successfully been used as a diagnostic biomarker to discriminate malignant melanomas from deep penetrating nevi (Roesch et al., 2006).
Morrison et al. (1993) showed that DPPIV/CD26 loss occurs late during malignant transformation and is associated with the emergence of growth factor independence and the development of specific chromosomal abnormalities. In addition, melanoma cells transfected with DPPIV have a longer lag period before entering the logarithmic growth phase, with growth inhibited when cells reach confluence. DPPIV reduces melanoma tumor growth proportional to expression (Wesley et al., 1999), and DPPIV has been shown to promote apoptosis and inhibit cell migration and angiogenesis (Arscott et al., 2009). DPPIV expression increases the percentage of cells in G0–G1 phase, indicating that DPPIV/CD26 may promote cell cycle arrest (Wesley et al., 2004). DPPIV/CD26 expression decreases the ability of melanoma cells to grow in soft agar, indicating that DPPIV/CD26 is important for the inhibition of anchorage-independent growth, and DPPIV/CD26 inhibits the invasion of malignant melanoma cell lines (Pethiyagoda et al., 2000). Interestingly, ectopic expression of DPPIV/CD26 has been shown to induce marked phenotypic changes, with cells expressing DPPIV/CD26 exhibiting a more organized growth pattern and sheet-like appearance compared to parental melanoma cells and cells transfected with control vector (Pro and Dang, 2004).
In our study, ectopic expression of SPRIGHTLY was associated with downregulation of DPPIV/CD26 in transfected melanocytes but not in parental melanocytes or control cells; this was validated using multiple assays including protein arrays, western blotting, and immunofluorescent staining. Conversely, knockdown of SPRIGHTLY in melanoma cells upregulated DPPIV/CD26. Consistent with these findings, SPRIGHTLY-transfected melanocytes showed an increase in growth rate, invasiveness, and anchorage-independent colony formation (Supplementary Figure S3). Since over-expression of SPRIGHTLY promoted anchorage-independent growth of melanocytes, we conducted in vivo tumor formation studies (utilizing SCID mice). As depicted in Supplementary Figure S4, three months following cell implantation we observed SPRIGHTLY-EE cells, but not vector only parental cells, produced subcutaneous cell migration. However, we believe that should we have been able to maintain the mice for a longer period (more than three months), even greater transformational phenotypes might have become evident. Likewise, combining SPRIGHTLY with mutations in genes such as PTEN, CDKN2A, BRAF, NRAS, GNQ11, TP53 etc., may have revealed additional oncogenic potential.
Although the exact mechanism by which SPRIGHTLY regulates DPPIV/CD26 remains to be determined, DPPIV/CD26 has been reported to be regulated at the transcriptional level by promoter methylation, interferons, and retinoic acid (Bauvois et al., 2000) and at the protein level by post-translational events (Pereira et al., 2003; Swenson, 2007). Here, DPPIV promoter CpG islands were examined for possible promoter hypermethylation; however, no aberrant CpG island methylation was identified. Since SPRIGHTLY is predominantly present in the cytoplasm in melanoma cells (Khaitan et al., 2011) and localized to the polysome fraction (the site of protein synthesis), it is possible that SPRIGHTLY regulates DPPIV post-transcriptionally or translationally by an unknown mechanism.
Interestingly, we found that cellular proliferation marker Ki-67, cyclin-dependent kinase 1 (CDK1), and mini-chromosome maintenance protein family genes (MCM2-5) were significantly upregulated in SPRIGHTLY-EE cells. MCM proteins are central players in both the initiation and the elongation of eukaryotic DNA replication (Forsburg, 2004) and are regulated by cyclin-dependent kinases (CDKs) (Nguyen et al., 2001) including CDK1 (synonym: CDC2), which was upregulated in melanocytes that ectopically expressed SPRIGHTLY. The activation of CDK1/CDC2 is dependent on mitogen-activated protein kinase (MAPK) (Guadagno and Ferrell, 1998; Palmer et al., 1998). It is of note that DPPIV expression is known to inhibit MAPK-extracellular signal-regulated kinase (ERK) 1/2 activation (Wesley et al., 2005). It serves as no surprise that in our study, the loss of DPPIV may have led to increased phosphorylation of ERK 1/2, suggesting increased activation of MAPK. Therefore, it is possible that the downregulation of DPPIV/CD26 may be responsible for the upregulation of MCMs and CDK1/CDC2 in melanocytes ectopically expressing SPRIGHTLY via reduction of the inhibitory activity on MAPK. Interestingly, since SPRIGHTLY forced-expression led to increased phosphorylation of ERK 1/2, and increased cell proliferation and anchorage-independent colony formation, it will be important to investigate the activation and the mutation status of other melanoma related MAPK pathway genes (ex: BRAF, NRAS). This continues as ongoing research in our laboratory.
The downregulation of DPPIV by SPRIGHTLY has significant implications with respect to the biological behavior and metastatic potential of melanoma. As a serine protease and membrane glycoprotein, DPPIV inactivates glucagon-like peptide 1, an incretin and gut-derived peptide important for post-prandial glucose homeostasis. However, DPPIV has many other substrates including CXCL12/SDF1, the unique CXCR4 ligand. Wesley et al. (1999) first highlighted that DPPIV expression suppresses the malignant phenotype of melanocytes, and others have recently demonstrated that CXCL12/CXCR4 signaling antagonists suppress CXCR4+ pulmonary metastases in preclinical models of melanoma (O’Boyle et al., 2013). Since stromal CXCL12 production drives the recruitment and migration of melanoma, it is tempting to speculate that SPRIGHTLY participates in malignant transformation by downregulating melanocyte DPPIV activity and dissipating the metastastic milieu created by the stroma, thereby promoting metastasis.
Collectively, our study demonstrates that ectopic expression of SPRIGHTLY in melanocytes results in downregulation of DPPIV/CD26 and upregulation of cell proliferation genes, consequently altering the melanocytic phenotype toward malignant melanoma. The findings provide both direct evidence for the melanomagenic role of SPRIGHTLY and how it regulates cell proliferation in melanocytes and melanomas. We also postulate that SPRIGHTLY and its interacting molecular partners may be involved in the development and malignant transformation of congenital melanocytic nevi. Further clinicopathological studies to determine the association between SPRIGHTLY and its target genes in malignant transformation of melanocytic nevi and clinical outcomes are warranted. Finally, elevated expression of SPRIGHTLY in melanoma cells compared to melanocytes, its accumulation in the cytoplasm, and its effects on cell dynamics suggest that this lncRNA and its target genes may play an important role in primary human melanocyte development and the molecular etiology of human melanomas.
MATERIALS AND METHODS
Further details are available in Supplementary Materials online.
Cell lines
Human melanocytes (ScienCell, Carlsbad, CA, HEM-l, Cat No 2200) were grown in MelM media containing MelGS growth supplements, 0.5% fetal bovine serum (FBS), and penicillin and streptomycin. A375 melanoma cells (ATCC CRL-1619) were grown in Complete Tu Medium containing a 4:1 mixture of MCDB-153 medium with 1.5 g/L sodium bicarbonate and Leibovitz’s L-15 medium with 2 mM L-glutamine, 2% FBS, and 1.68 mM CaCl2. The cells were grown in a humidified tissue culture incubator at 37°C, 5% CO2 atmosphere.
Lentiviral vector constructs
A third-generation replication-defective HIV-1-based lentiviral vector was obtained from the Sanford-Burnham virus core facility. This vector system includes the 5′ and 3′ lentiviral LTRs and all necessary elements for effective transduction. Green fluorescent protein (GFP) served as a transduction marker, and woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), which is believed to promote RNA processing and nuclear export, was used to boost expression of the gene of interest in target cells by facilitating the production of mature mRNA from transcripts initiated by the vector’s internal promoter (pCMV). To construct the SPRIGHTLY expression lentiviral vector, DNA encoding full length SPRIGHTLY was cloned into the lenti-transfer vector MCS region NheI site and then packaged into viral particles in 293T cells by co-transfection with packaging-defective helper plasmids using FuGENE 6 Transfection Reagent (Roche, Mannheim, Germany). Empty vector was also packaged in 293T cells as Vector Control. Virus-containing supernatants were collected 48 h later, and the lentiviral titers were determined by high-content screening (HCS).
Supplementary Material
Acknowledgments
We thank the Sanford-Burnham Analytical Genomics core facility for deep-sequencing, Bioinformatics core for data analysis support, Histology and Microscope facility for IHC studies, and Ms. Debbie McFadden (SBMRI) for formatting the manuscript. This work was supported by National Institutes of Health grants CA165184, NCI 5P30CA030199 and FL Biomed BHC 5BC08 to RJP and 2R01CA125255, Bankhead Coley 4BB17, and NSF CBET-1403535 to MK. MED is supported by a Career Development Award and an Australia Fellowship, respectively, from the National Health and Medical Research Council of Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome. 2008;19:454–92. doi: 10.1007/s00335-008-9136-7. [DOI] [PubMed] [Google Scholar]
- Arscott WT, LaBauve AE, May V, Wesley UV. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: relevance of chemokine regulation and caspase activation. Oncogene. 2009;28:479–91. doi: 10.1038/onc.2008.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfai B, Jia H, Khatun J, Wood E, Risk B, Gundling WE, Jr, et al. Long noncoding RNAs are rarely translated in two human cell lines. Genome Res. 2012;22:1646–57. doi: 10.1101/gr.134767.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene. 2000;19:265–72. doi: 10.1038/sj.onc.1203292. [DOI] [PubMed] [Google Scholar]
- Boyd AS, Shakhtour B, Shyr Y. Minichromosome maintenance protein expression in benign nevi, dysplastic nevi, melanoma, and cutaneous melanoma metastases. J Am Acad Dermatol. 2008;58:750–4. doi: 10.1016/j.jaad.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Clark MB, Amaral PP, Schlesinger FJ, Dinger ME, Taft RJ, Rinn JL, et al. The reality of pervasive transcription. PLoS biology. 2011;9:e1000625. doi: 10.1371/journal.pbio.1000625. discussion e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade BA, Leon JE, Carlos R, Delgado-Azanero W, Mosqueda-Taylor A, de Almeida OP. Expression of minichromosome maintenance 2, Ki-67, and geminin in oral nevi and melanoma. Ann Diagn Pathol. 2013;17:32–6. doi: 10.1016/j.anndiagpath.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–45. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev. 2004;68:109–31. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne DK, Cheetham SW, Cattenoz PB, Clark MB, Amaral PP, Taft RJ, et al. Pinstripe: a suite of programs for integrating transcriptomic and proteomic datasets identifies novel proteins and improves differentiation of protein-coding and non-coding genes. Bioinformatics. 2012;28:3042–50. doi: 10.1093/bioinformatics/bts582. [DOI] [PubMed] [Google Scholar]
- Guadagno TM, Ferrell JE., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–5. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Guzinska-Ustymowicz K, Stepien E, Kemona A. MCM-2, Ki-67 and PCNA protein expressions in pT3G2 colorectal cancer indicated lymph node involvement. Anticancer Res. 2008;28:451–7. [PubMed] [Google Scholar]
- Ifere GO, Ananaba GA. Prostate cancer gene expression marker 1 (PCGEM1): a patented prostate- specific non-coding gene and regulator of prostate cancer progression. Recent patents on DNA & gene sequences. 2009;3:151–63. doi: 10.2174/187221509789318360. [DOI] [PubMed] [Google Scholar]
- Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, et al. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res. 2011;71:3852–62. doi: 10.1158/0008-5472.CAN-10-4460. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–25. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikam C, Hufnagel A, Schartl M, Meierjohann S. Oncogene activation in melanocytes links reactive oxygen to multinucleated phenotype and senescence. Oncogene. 2008;27:7070–82. doi: 10.1038/onc.2008.323. [DOI] [PubMed] [Google Scholar]
- Lian M, Fang J, Han D, Ma H, Feng L, Wang R, et al. Microarray gene expression analysis of tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma. PLoS One. 2013;8:e84854. doi: 10.1371/journal.pone.0084854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li JS, Tian DP, Huang B, Rosqvist S, Su M. MCM2 expression levels predict diagnosis and prognosis in gastric cardiac cancer. Histol Histopathol. 2013;28:481–92. doi: 10.14670/HH-28.481. [DOI] [PubMed] [Google Scholar]
- Mazar J, Sinha S, Dinger ME, Mattick JS, Perera RJ. Protein-coding and non-coding gene expression analysis in differentiating human keratinocytes using a three-dimensional epidermal equivalent. Mol Genet Genomics. 2010;284:1–9. doi: 10.1007/s00438-010-0543-6. [DOI] [PubMed] [Google Scholar]
- Mazar J, Zhao W, Khalil AM, Lee B, Shelley J, Govindarajan SS, et al. The functional characterization of long noncoding RNA SPRY4-IT1 in human melanoma cells. Oncotarget. 2014;5:8959–69. doi: 10.18632/oncotarget.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness C, Wesley UV. Dipeptidyl peptidase IV (DPPIV), a candidate tumor suppressor gene in melanomas is silenced by promoter methylation. Front Biosci. 2008;13:2435–43. doi: 10.2741/2856. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–21. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison ME, Vijayasaradhi S, Engelstein D, Albino AP, Houghton AN. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med. 1993;177:1135–43. doi: 10.1084/jem.177.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VQ, Co C, Li JJ. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–73. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- Nielsen PS, Riber-Hansen R, Steiniche T. Immunohistochemical double stains against Ki67/MART1 and HMB45/MITF: promising diagnostic tools in melanocytic lesions. Am J Dermatopathol. 2011;33:361–70. doi: 10.1097/DAD.0b013e3182120173. [DOI] [PubMed] [Google Scholar]
- O’Boyle G, Swidenbank I, Marshall H, Barker CE, Armstrong J, White SA, et al. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br J Cancer. 2013;108:1634–40. doi: 10.1038/bjc.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–47. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Molecular cell. 2008;32:232–46. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Pereira DA, Gomes L, El-Cheikh MC, Borojevic R. Dipeptidyl peptidase IV (CD26) activity in the hematopoietic system: differences between the membrane-anchored and the released enzyme activity. Braz J Med Biol Res. 2003;36:567–78. doi: 10.1590/s0100-879x2003000500003. [DOI] [PubMed] [Google Scholar]
- Pethiyagoda CL, Welch DR, Fleming TP. Dipeptidyl peptidase IV (DPPIV) inhibits cellular invasion of melanoma cells. Clinical and Experimental Metastasis. 2000;18:391–400. doi: 10.1023/a:1010930918055. [DOI] [PubMed] [Google Scholar]
- Pro B, Dang NH. CD26/dipeptidyl peptidase IV and its role in cancer. Histol Histopathol. 2004;19:1345–51. doi: 10.14670/HH-19.1345. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annual Review of Biochemistry. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Wittschier S, Becker B, Landthaler M, Vogt T. Loss of dipeptidyl peptidase IV immunostaining discriminates malignant melanomas from deep penetrating nevi. Mod Pathol. 2006;19:1378–85. doi: 10.1038/modpathol.3800663. [DOI] [PubMed] [Google Scholar]
- Scott GA, Cassidy L. Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J Invest Dermatol. 1998;111:243–50. doi: 10.1046/j.1523-1747.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–59. doi: 10.1101/gr.087775.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson LI. Progress in tumor marker research. New York: Nova Biomedical Books; 2007. [Google Scholar]
- Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220:126–39. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umlauf D, Fraser P, Nagano T. The role of long non-coding RNAs in chromatin structure and gene regulation: variations on a theme. Biol Chem. 2008;389:323–31. doi: 10.1515/BC.2008.047. [DOI] [PubMed] [Google Scholar]
- Van den Oord JJ. Expression of CD26/dipeptidyl-peptidase IV in benign and malignant pigment-cell lesions of the skin. Br J Dermatol. 1998;138:615–21. doi: 10.1046/j.1365-2133.1998.02171.x. [DOI] [PubMed] [Google Scholar]
- Wesley UV, Albino AP, Tiwari S, Houghton AN. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J Exp Med. 1999;190:311–22. doi: 10.1084/jem.190.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65:1325–34. doi: 10.1158/0008-5472.CAN-04-1852. [DOI] [PubMed] [Google Scholar]
- Wesley UV, Tiwari S, Houghton AN. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004;109:855–66. doi: 10.1002/ijc.20091. [DOI] [PubMed] [Google Scholar]
- Wojnar A, Pula B, Piotrowska A, Jethon A, Kujawa K, Kobierzycki C, et al. Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2 proteins in invasive ductal breast carcinoma. Anticancer Res. 2011;31:3027–33. [PubMed] [Google Scholar]
- Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, et al. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010;277:1126–44. doi: 10.1111/j.1742-4658.2009.07526.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


