Abstract
Purpose
Patients with high-grade gliomas (HGG) are frequently excluded from first-in-human solid tumor trials because of perceived poor prognosis, excessive toxicities, concomitant drug interactions, and poor efficacy. We conducted an analysis of outcomes from select, single-agent phase I studies in patients with HGG. We compared outcomes to pooled analysis of published studies in solid tumors with various molecular and cytotoxic drugs evaluated as single agents or as combinations.
Patient and Methods
Individual records of patients with recurrent HGG enrolled onto Adult Brain Tumor Consortium trials of single-agent, cytotoxic or molecular agents from 2000 to 2008 were analyzed for baseline characteristics, toxicities, responses, and survival.
Results
Our analysis included 327 patients with advanced, refractory HGG who were enrolled onto eight trials involving targeted molecular (n = 5) and cytotoxic (n = 3) therapies. At enrollment, patients had a median Karnofsky performance score of 90 and median age of 52 years; 62% were men, 63% had glioblastoma, and the median number of prior systemic chemotherapies was one. Baseline laboratory values were in an acceptable range to meet eligibility criteria. Patients were on the study for a median of two cycles (range, < one to 56 cycles), and 96% were evaluable for primary end points. During cycle 1, grade ≥ 3 nonhematologic and grade ≥ 4 hematologic toxicities were 5% (28 of 565 adverse events) and 0.9% (five of 565 adverse events), respectively, and 66% of these occurred at the highest dose level. There was one death attributed to drug. Overall response rate (complete and partial response) was 5.5%. Median progression-free and overall survival times were 1.8 and 6 months, respectively.
Conclusion
Patients with HGG who meet standard eligibility criteria may be good candidates for solid tumor phase I studies with single-agent molecular or cytotoxic drugs with favorable preclinical rationale and pharmacokinetic properties in this population.
INTRODUCTION
The primary objectives of phase I oncology trials are to determine the safety, toxicity, maximum-tolerated dose (MTD), preliminary efficacy, and recommended dose of novel agents for phase II and III studies.1 In the era of targeted therapies, phase I trials are increasingly enriched for patients with predictive biomarkers to obtain an efficacy signal to make go or no-go decisions in drug development.2
To maximize patient safety and minimize the chance of nonreproducible results, phase I studies use strict eligibility criteria that have historically excluded patients with primary CNS tumors such as glioblastoma.3,4 The reasons given to exclude such patients include poor prognosis, excessive toxicities, neurologic complications, drug-drug interactions (DDIs) with antiepileptics, and limited efficacy as a result of poor penetration across the blood-brain barrier.3,4 A recent analysis of the National Cancer Institute's Cancer Therapy Evaluation Program (NCI-CTEP) phase I clinical trial database of more than 3,000 patients with solid tumors enrolled between 2000 and 2010 found only eight patients who had primary or metastatic CNS tumors (< 0.2%).5 To date, there are no studies that have systematically evaluated the historical barriers that prevent access to phase I clinical trials for patients with CNS tumors. We conducted a multi-institutional pooled analysis of phase I oncology studies of single-agent molecular or cytotoxic drugs in patients with high-grade glioma (HGG) enrolled onto Adult Brain Tumor Consortium trials and analyzed baseline patient characteristics and organ function, toxicities, MTD, responses, and survival. We compared our findings to patients with solid tumors enrolled onto phase I oncology studies sponsored by NCI-CTEP and the European Drug Development Network where molecular and/or cytotoxic drugs were evaluated as either single agents or in combinations.5–8
PATIENTS AND METHODS
We queried a prospectively database maintained by the Adult Brain Tumor Consortium, a multi-institutional consortium, and included phase I trials of single-agent cytotoxic or molecular agents activated between the years 2000 and 2008 for which complete data were available. Molecular agents are defined as drugs that target an extra- or intracellular mechanism different from those associated with conventional chemotherapy such as DNA, tubulin, or cell division machinery. Eligible patients were ≥ 18 years of age with histologically proven HGG and received at least one dose of study drug. All patients had recurrent HGG and met standard phase I eligibility criteria.
The following patient and laboratory characteristics were reviewed: age at diagnosis, sex, race, performance status, prior radiation therapy, prior chemotherapies, and baseline laboratory evaluations including WBC count, hemoglobin, platelet count, coagulation, AST/ALT, total protein, and renal function. In addition, the type of concurrent antiepileptic drug was documented. For cross comparisons between trials, Karnofsky performance score was converted to Eastern Cooperative Oncology Group performance status.9 These baseline characteristics were compared (Table 1) to a recently published study summarizing the outcomes of 3,104 patients with solid tumors (all carcinomas, bone and soft tissue sarcomas, melanoma, and skin and neuroendocrine tumors) enrolled onto 127 trials sponsored by NCI-CTEP during 2000 to 2010 evaluating molecular and cytotoxic drugs as single agents or in combination.5 Toxicities in all protocols were graded using Common Terminology Criteria for Adverse Events (version 4.0), and older protocols using older versions (version 2.0 or 3.0) were converted to version 4.0 using conversion tables.10 We evaluated all drug-related serious adverse events (SAEs) and dose-limiting toxicities (DLTs) during cycle 1. For each trial, cycle length was defined as per individual protocol (21 or 28 days). Radiographic response to treatment was measured by New Approach to Brain Tumor Therapy or MacDonald criteria.11,12
Table 1.
Baseline Patient Demographic and Clinical Characteristics in HGG and Solid Tumor Studies
| Characteristic | HGG |
Solid Tumors5 |
P | ||
|---|---|---|---|---|---|
| No. of Patients (N = 327) | % | No. of Patients (N = 3,104) | % | ||
| Age, years | — | ||||
| Median | 52 | 58 | |||
| Range | 18-86 | 18-87 | |||
| Sex | < .001 | ||||
| Men | 204 | 62 | 1,539 | 50 | |
| Women | 123 | 38 | 1,565 | 50 | |
| ECOG performance status | < .001 | ||||
| Median | 1 | 1 | |||
| 0 | 40 | 12 | 892 | 29 | |
| 1 | 213 | 65 | 2,056 | 66 | |
| ≥ 2 | 74 | 23 | 156 | 5 | |
| Median No. of prior chemotherapies | < .001 | ||||
| 0-2 | 1 | 95 | 860 | 28 | |
| ≥ 3 | 311 | 5 | 1,539 | 72 | |
| Missing | 16 | 5 | |||
| Resection | 73 | 22 | — | — | — |
| Biopsy | 254 | 78 | |||
| Prior radiation therapy | < .001 | ||||
| Yes | 318 | 97 | 1,446 | 47 | |
| No | 0 | 0 | 1,653 | 53 | |
| Missing | 9 | 3 | 5 | 0 | |
| Concurrent AEDs | 259 | 100 | NA | NA | — |
| EIAED | 168 | 65 | |||
| Non-EIAED | 91 | 35 | |||
| WBC, × 109/L | — | ||||
| Median | 6.6 | 6.7 | |||
| Range | 2.3-17.9 | 2.1-38.2 | |||
| Hemoglobin, g/dL | — | ||||
| Median | 14 | 12.2 | |||
| Range | 13-17 | 8-17.3 | |||
| Platelets, × 109/L | — | ||||
| Median | 218 | 255 | |||
| Range | 90-636 | 78-1,114 | |||
| AST, U/L | — | ||||
| Median | 21 | 27 | |||
| Range | 18-75 | 7-176 | |||
| ALT, U/L | — | ||||
| Median | 29 | 23 | |||
| Range | 7-157 | 2-170 | |||
| Alkaline phosphatase | — | ||||
| Median | 80 | 102 | |||
| Range | 3-225 | 25-10,405 | |||
| Total bilirubin, mg/dL | — | ||||
| Median | 0.5 | 0.5 | |||
| Range | 0.1-2 | 0.1-1.9 | |||
| Creatinine | |||||
| Median | 0.8 | — | |||
| Range | 0.4-1.5 | ||||
| PT, seconds | — | ||||
| Median | 11.7 | ||||
| Range | 1.1-34.7 | ||||
| PTT, seconds | — | ||||
| Median | 26 | ||||
| Range | 12.7-42.4 | ||||
| Brain | 327 | 100 | 8 | < 1 | < .001 |
| Glioblastoma | 207 | 63 | — | ||
| Nonglioblastoma | 120 | 37 | — | ||
| Drug class | < .001 | ||||
| Molecular | 222 | 67 | 1,345 | 43 | |
| Cytotoxic | 105 | 32 | 575 | 19 | |
| Molecular and cytotoxic | 0 | 0 | 1,184 | 38 | |
| No. of study drugs | 3,270 | < .001 | |||
| 1 | 100 | 1,137 | 37 | ||
| ≥ 2 | 0 | 1,967 | 63 | ||
NOTE. The χ2 test was used for all categorical variables. Standard deviations were not published for solid tumor studies, and statistical comparisons between data sets were not performed.
Abbreviations: AED, antiepileptic drug; EIAED, enzyme-inducing antiepileptic drug; ECOG, Eastern Cooperative Oncology Group; HGG, high-grade glioblastoma; NA, not applicable; PT, prothrombin time; PTT, partial thromboplastin time.
Statistics
We used descriptive statistics for continuous variables and frequency counts for categorical variables. The χ2 test was used for the comparison of clinical and demographic factors in HGG and solid tumor studies (STSs). The Kaplan-Meier method was used for the calculation of progression-free survival (PFS) and overall survival (OS) estimates. SAS software (version 9.4; SAS Institute, Cary, NC) was used for all study analyses.
RESULTS
Patient and Trial Characteristics
A total of 327 patients were accrued to eight phase I trials that were conducted from 2000 to 2008 (Table 1). Trial drugs are listed in Table 2.13–29 The median age was 52 years (range, 18 to 86 years) with a median Eastern Cooperative Oncology Group performance status of 0 (range, 0 to 2). All patients had a diagnosis of HGG at the time of trial entry, and 63% had glioblastoma. The median number of prior chemotherapies was one (range, zero to six prior chemotherapies), and temozolomide was first-line treatment in 128 (64%) of 198 patients for whom data were available. Ninety-seven percent of patients received prior radiation therapy as part of initial management of HGG; these data were missing for nine patients (3%). Seventy-nine percent of all patients were on treatment with at least one antiepileptic drug (AED). Of these, 65% were enzyme-inducing AEDs (EIAEDs), and 12% of patients were on two AEDs. Baseline median laboratory characteristics for HGG and STS cohorts are listed in Table 1. Because standard deviations were not published, statistical comparisons between laboratory values were not performed. The findings were comparable for median age and baseline laboratory values between these two groups of patients. As expected, there were significant differences (P < .001, χ2; Table 1) between the two cohorts in terms of performance status, sex, number of prior systemic therapies, number of study drugs, prior radiation therapy, and metastatic sites.
Table 2.
Trial Characteristics and MTD of Drugs in Glioma Trials Compared With Those Conducted in Solid Tumors
| Agent and Class | Neuro-Oncology Trials |
Solid Tumor Studies |
% Difference in MTD Between Non-EIAED and Standard | ||||
|---|---|---|---|---|---|---|---|
| Study | Trial Start Date | MTD | Study | Trial Start Date | MTD | ||
| BAY 43-9006 (sorafenib), molecular kinase inhibitor | Nabors et al13 | December 2004 | Non-EIAED: 800 mg twice a day; EIAED: 600 mg BID | Strumberg et al14 | July 2002 | 400 mg twice a day | +100 |
| EM-1421 (terameprocol), cytotoxic transcription inhibitor | Grossman et al15 | January 2007 | 1,700 mg per day (pegylated formulation) | Goel et al16, Zonnenberg et al17 | December 2005 | 2,200 mg per day (nonpegylated formulation) | NA |
| Karenitecan, cytotoxic topoisomerase inhibitor | Grossman et al18 | January 2002 | Non-EIAED: 1.5 mg/m2; EIAED: 2.0 mg/m2 | Schilsky et al19 | Before 2000 | 1.0 mg/m2 | +50 |
| Atrasentan, small-molecule endothelin inhibitor | Phuphanich et al20 | June 2002 | 70-95 mg per day | Ryan et al21 | July 1997 | 60 mg per day | +16 |
| BMS-247550 (ixabepilone), cytotoxic microtubule inhibitor | Peereboom et al22 | October 2002 | Non-EIAED: 6.8 mg/m2; EIAED: 9.6 mg/m2 | Abraham et al23 | September 2000 | 6 mg/m2 per day | +13 |
| COL-3, small-molecule metalloproteinase inhibitor | Rudek et al24 | July 2000 | Non-EIAED: 75 mg/m2 per day; EIAED: 100 mg/m2 per day | Syed et al25 | October 1997 | 50 mg/m2 per day | +50 |
| PS341 (bortezomib), small-molecule proteasome inhibitor | Phuphanich et al26 | May 2001 | Non-EIAED: 1.70 mg/m2; EIAED: 2.5 mg/m2 | Aghajanian et al27 | July 1999 | 1.56 mg/m2 per dose | +9 |
| EMD 121974 (cilengitide), small-molecule integrin inhibitor | Nabors et al28 | September 2000 | Not reached | Eskens et al29 | December 1999 | Not reached | NA |
Abbreviations: EIAED, enzyme-inducing antiepileptic drug; MTD, maximum-tolerated dose; NA, not applicable.
Toxicities
A total of 7,075 all-grade adverse events (AEs) were recorded in 327 patients across all cycles. Because the definition of DLTs can vary between studies, we broadened AEs to include protocol-defined DLTs and other unanticipated, all-grade, SAEs that in the opinion of the investigator were possibly, probably, or definitely related to drug during cycle 1 (drug-related SAEs [rSAE]; Table 3). AEs within each category are listed on a per-patient basis, and therefore, a patient may have experienced more than one AE in a category, but only the highest grade AE is reported. A total of 565 all-grade AEs were recorded during cycle 1 with the following distribution: grade 1 to 2, 90.6%; grade 3 to 4 nonhematologic, 3.9%; grade 3 to 4 hematologic, 5.3%; and grade 5, 0.2%. There were 33 rSAEs (5.8%) during cycle 1 that occurred in 31 patients, which is a patient event rate of 9.4% (31 of 327 patients). As expected, more rSAEs occurred at higher doses, with rSAEs occurring at the highest dose level and one dose level below the highest in 22 (66%) of 33 events, at the intermediate dose level in seven (21%) of 33 events, and at the lower dose level in 27% of events. Of 565 related toxicities, the most common SAEs were thrombocytopenia (2.1%), leukopenia (2.1%), constitutional (1.4%), dermatologic (1%), and neurologic (< 1%). Neurologic toxicities were mostly grade 1 and 2 events. In addition, there were four intracranial hemorrhages, of which two were grade 2 events during cycle 1, one was a grade 4 event during cycle 4, and one was a grade 5 event during cycle 1; all were attributed to disease progression. In the 327 patients, there were seven disease-related deaths (2.4%) during cycle 1 and one death (0.3%) related to study drug (ixabepilone) as a result of grade 5 infection. The seven disease-related deaths were unrelated to study drug (CNS hemorrhage in one patient, disease progression in five patients, and unclassified in one patient). The toxicities of the HGG and STS cohort are listed in Appendix Table A1 (online only).
Table 3.
Cycle 1 Drug-Related Toxicities (possible, probable, or definite) in Patients With HGG by Category
| Category | No. of Events (%) |
|||
|---|---|---|---|---|
| Toxicity Grade |
Related SAE | |||
| 1-2 | 3-4 | 5 | ||
| All toxicities (n = 565) | 512 (90.6) | 52 (9.2) | 1 (< 1) | 33 (5.8) |
| Constitutional | 89 (15.7) | 8 (1.4) | 8 (1.4) | |
| Cardiovascular | 22 (3.8) | 1 (< 0.5) | 1 (< 0.5) | |
| GI | 84 (14.8) | 2 (< 0.5) | 2 (< 0.5) | |
| Respiratory | 4 (< 1) | 2 (< 0.5) | 2 (< 0.5) | |
| Metabolic | 34 (6) | 3 (< 0.5) | 3 (< 0.5) | |
| Dermatologic | 35 (6) | 6 (1) | 6 (1) | |
| Neurologic | 35 (6) | 5 (< 1) | 5 (< 1) | |
| Hematologic | ||||
| WBC | 42 (7) | 12 (2) | 5 (< 1) | |
| Hemoglobin | 73 (13) | 1 (0.8) | ||
| Platelets | 61 (11) | 12 (2) | ||
| Others | 29 (5) | 1 (< 1) | 1 | |
| Subcategories of all related neurologic toxicities during cycle 1 | ||||
| Encephalopathy | 4 | 0 | 0 | |
| Headache | 18 | 1 | 0 | |
| Neuropathy | 18 | 2 | 0 | |
| Other | 35 | 3 | 0 | |
| Total | 75 | 6 | 0 | |
NOTE. Toxicities within each category are listed on a per-patient basis. The sum of neurologic subcategories is greater than listed under neurology category.
Abbreviations: HGG, high-grade glioma; SAE, serious adverse event.
Impact on the Final Determination of MTD
The MTD was successfully declared in all HGG phase I studies (Table 2). The final dose was in a range similar to the previously determined dose in STS. Using the MTD from STS as the first established dose, patients with glioma on non-EIAEDs had marginally higher doses declared as the MTD, with the exception of one study (terameprocol) where the MTD was lower. In the cilengitide trial, the MTD was not reached in both cohorts, and in the terameprocol study, the drug formulation was different. Patients with HGG on non-EIAEDs had MTDs that were identical to 100% higher than that declared in STS. The start date of phase I studies of identical study drugs lagged by approximately 2 years in patients with HGG compared with STS.
Efficacy and Survival
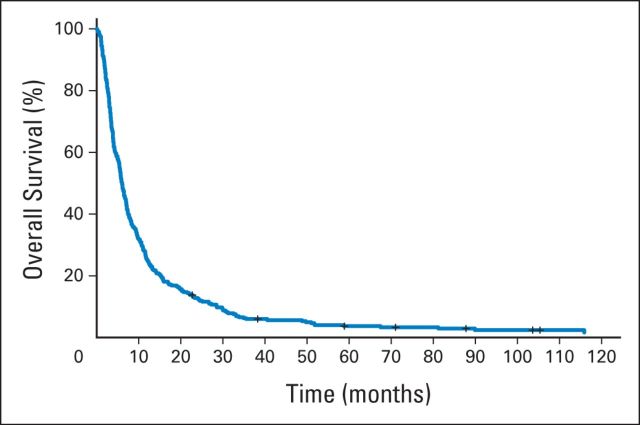
Of 327 patients, 96% completed at least one cycle of an investigational agent, and the median number of cycles received was two (range, < one to 56 cycles). Median time on study was 1.7 months (range, 0.03 to 51.8 months). Reasons for removal from study included disease progression (78%), drug toxicity (7%), refusal of further treatment (7%), treatment delay of more than 14 days (0.6%), death (0.9%), and other causes (6%). In this cohort, 14 patients (4%) were unable to complete cycle 1 for the previously mentioned reasons. As noted earlier, radiographic responses were reported using modified MacDonald or New Approach to Brain Tumor Therapy criteria and were centrally confirmed in most cases. Best response to treatment was complete response (CR), partial response (PR), stable disease (SD), and progressive disease in five (1.5%), 13 (4%), 72 (22%), and 202 patients (61%), respectively, with 35 patients not evaluable for response. There were 47 (65.2%) of 72 patients with SD who remained on study for 3 or more months, which we arbitrarily defined as clinically beneficial. Median times on study for patients with best response as CR, PR, SD, and progressive disease were 14.6 months (range, 3.6 to 51 months), 7 months (range, 1.6 to 21.3 months), 3.6 months (range, 1 to 21.8 months), and 1.4 months (range, 0 to 38 months), respectively. The overall clinical benefit rate (CR + PR + SD ≥ 3 months) was 19.8%. All patients were observed for survival, and the median follow-up time was 6 months (range, 0.1 to 131 months). Median PFS and OS time were 1.8 and 6 months, respectively (Fig 1). PFS rates at 3 and 6 months were 28% and 13%, respectively.
Fig 1.
Kaplan-Meier curve of overall survival of 327 patients with primary glioma enrolled onto phase I oncology trials between 2000 and 2008.
DISCUSSION
The annual incidence rate of primary CNS tumors in adults is 27.86 per 100,000, and gliomas, including glioblastoma, account for 65% of malignant tumors.30 There are few active treatments approved, and patients with refractory disease face limited treatment options. The Cancer Genome Atlas (TCGA) data have demonstrated that there are a wide range of mutations involved in glioblastoma that potentially can be targeted by novel drugs.31 Patients with HGG are unable to gain access to most phase I clinical trials under current eligibility criteria.3,4,32 Historical reasons that continue to be used for the exclusion of patients with HGG include poor life expectancy, poor performance status, fear of excessive CNS toxicities, potential drug exclusion by the blood-brain barrier, and DDIs.3,4 Our data demonstrate that patients with HGG who meet standard phase I eligibility criteria and are enrolled onto trials of appropriately chosen single-agent drugs successfully met phase I end points, namely safety, toxicity, and efficacy.
To our knowledge, this is the first report that retrospectively examines these variables from a prospectively maintained database of patients with HGG enrolled onto phase I oncology trials across multiple institutions in North America. We compared our findings with the published outcomes of patients with solid tumors enrolled onto phase I oncology trials.5–8 These cohorts are ideal to evaluate baseline patient and laboratory characteristics because they all include patients with advanced, refractory disease using similar eligibility criteria during a similar time frame. These large cohorts represent multi-institutional studies that were conducted in either North America or Europe and were sponsored by cooperative groups with rigorously maintained databases. We acknowledge several limitations in our study. An important difference between the HGG and STS cohorts is that they were sometimes treated with different phase I drugs, and therefore, any direct comparison of toxicities or safety is not possible. Our HGG cohort only included studies with single agents, whereas STSs included both single-agent and combination studies. We expect combination studies to have higher rates of toxicities and responses, and again, any direct comparison between cohorts is not appropriate. Our initial efforts to directly compare HGG and STS trials that used identical drugs was unsuccessful because published trials reported toxicities, efficacy, and other variables as summary data with little uniformity in reporting between studies.
The primary objectives of a phase I study are to evaluate the safety and tolerability of the study drug during cycle 1.1 Patients who discontinue treatment before the completion of cycle 1 for reasons other than DLT are not evaluable for the primary toxicity end point and must be replaced. This negatively affects drug development by increasing time and costs.33,34 The early discontinuation rate during cycle 1 in phase I STSs is estimated to be 14% to 16.5%.8,34 In our study, only 14 (4%) of 327 patients with HGG were unable to complete cycle 1 as a result of disease progression or other causes. Early discontinuation is a reflection of disease progression, patient fitness, and organ function and not a reflection of number or type of study drug(s). Our results demonstrate that select patients with HGG who meet standard phase I eligibility criteria have comparable fitness and organ function as their solid tumor counterparts. Our data disprove the old belief that the performance status of patients with HGG is too poor for participation in phase I studies.
The patients with HGG in our cohort continued on study for a median of two cycles and came off study for disease progression, which is typical for most phase I STSs. In patients with HGG, safety and toxicity assessments were successfully completed in 96% of all patients. In our cohort, 35 (10.7%) of 327 patients were not evaluable for efficacy because of disease progression, a rate similar to that seen in STSs (12.8%).7
Only a handful of phase I studies in solid tumors have reported on the risk of grade 3 or higher toxicities. In single-agent and combination studies, the rates of grade 3 and 4 toxicities are estimated to range from 10.3% to 36%.35,36 Horstmann et al7 reported a grade 4 toxicity rate of 13% and 15% for trials evaluating single-agent molecular and cytotoxic agents, respectively, for studies conducted during 1991 to 2002. In the modern era, Molife et al37 reported grade 3 and 4 toxicity rates during cycle 1 of 14.1% and 1.9%, respectively, with single-agent molecular drugs. In patients with HGG on single-agent studies evaluating molecular or cytotoxic drugs, grade 3 or higher nonhematologic toxicities during cycle 1 constituted 5% (28 of 565 events) of all toxicities, and the rate of grade 4 or higher hematologic toxicities was 0.9% (five of 565 events). In patients with HGG, drug-related neurologic complications during cycle 1 were mostly grade 1 to 2 events; there were few grade 3 events (six of 565 events, 1%) and no grade 4 or 5 events. The feared complication of intracranial hemorrhage occurred in 1.2% of patients. Serious hemorrhagic or thrombotic toxicity in phase I STSs is approximated at 0.8%.5 The wide range in toxicities observed among the cohorts is likely a reflection of differences in the type and number of agents tested, trial design, dose levels, and improvements in supportive care. The serious toxicities observed in this cohort of patients with HGG enrolled onto single-agent trials seem to be well within the acceptable toxicity rates seen in STSs.
A common criticism is that AEDs will result in DDIs and impact MTD. Our data confirm this for patients on EIAEDs. However, in patients with HGG who were not on EIAEDs, the MTD is identical or marginally higher than the previously established MTD in STS. It is reassuring to note that the MTD of identical compounds determined in HGG studies is similar to what was observed in STS. It is not clear whether the slight differences we observed are statistically or biologically significant. Nevertheless, this suggests that repeating dose-escalation studies starting from the lowest dose level is unnecessary in patients with HGG on non-EIAEDs. We suspect that the slight differences may be a consequence of the 1- to 5-year lag in initiating clinical trials in HGG, which provided improved understanding of toxicity profile and supportive care. In multiple phase I studies, the most important predictor of DLT (and MTD) was performance status, albumin level, and dose level. Importantly, the number of prior chemotherapies and age have not been shown to impact DLT and MTD. However, we were unable to discern whether patients with HGG and solid tumors had any significant differences in baseline characteristics because standard deviations were not reported in published STSs. This slight difference in MTD may also be explained by concurrent corticosteroid use in patients with HGG. Corticosteroids such as dexamethasone are relatively weak inducers of CYP450 enzymes, and available data suggest that they have little effect on the exposure or safety profile of drugs like irinotecan in HGG and bortezomib in patients with myeloma or lymphoma.38,39 To further mitigate concerns of DDIs, there has been a major shift in neuro-oncology toward the use of second- and third-generation non-EIAEDs that are cleared through the kidney and do not interact with CYP450 enzymes. Prophylactic AEDs are currently not recommended in patients with CNS tumors with no history of seizures.40 When indicated, non-EIAEDs are used, and thus, the potential for DDIs has been largely removed. We recommend that phase I clinical researchers determine the eligibility criteria based on biologic rationale and pharmacokinetic properties of the drug instead of excluding all patients on AEDs or corticosteroids.
Finally, the greatest barriers to enrollment of patients with HGG to phase I studies are the perceived poor prognosis and lack of efficacy. In our study, we show that the overall response rate to these select single agents is 5.5%, which is in the range of overall responses seen in STSs with a single agents (3.2% to 7%).6,7 We recommend that patients with HGG be included in single-agent phase I STSs when there is sound biologic rationale and favorable pharmacokinetic properties. Most phase I eligibility criteria expect patients to have a life expectancy of 90 days. In patients with glioma, the 3-month PFS rate, median PFS time, and median OS time are 76%, 1.8 months, and 6 months, respectively, which are similar to those seen in STSs.8,33
Not all patients with HGG will be appropriate to participate in phase I studies. Our study suggests that a subset of patients with HGG who meet eligibility can be included in single-agent phase I STS studies when study drugs demonstrate good biologic rationale and the predicted pharmacokinetics is favorable in this population. Patients with HGG are fit, have a low early discontinuation rate (< 4%), and can prevent delays arising from patient replacement. Dedicated phase I studies in HGG may only be necessary when biologic rationale and/or pharmacokinetic properties mandates it in this population. Because actionable mutations are rapidly being discovered in HGG and solid tumors, there is little justification to deny or delay access to some early-phase clinical trials of potentially life-saving drugs for patients with HGG.
Appendix
Table A1.
Cycle 1 Toxicities of Cytotoxic and Molecular Agents in Patients With HGG and STS Enrolled Onto Phase I Oncology Studies
| Toxicity | Toxicity Grade (No. [%]) |
No. of DLTs | Cytotoxic Agents |
Molecular Agents |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1-2 | 3-4 | 5 | No. of Patients (glioma) | Rate per 1,000 Patients (glioma) | Rate per 1,000 Patients (solid tumors) | No. of Patients (glioma) | Rate per 1,000 Patients (glioma) | Rate per 1,000 Patients (solid tumors) | ||
| No. of patients | 105 | 575 | 222 | 1,345 | ||||||
| Constitutional | 89 (90.6) | 8 (1.4) | 8 | 3 | 28.6 | 45.2 | 5 | 22.5 | 53.5 | |
| Cardiovascular | 22 (3.8) | 1 (< 0.5) | 1 | 1 | 9.5 | 17.4 | 0 | 0 | 44.6 | |
| GI | 84 (14.8) | 2 (< 0.5) | 2 | 2 | 19.0 | 33 | 0 | 0 | 57.2 | |
| Respiratory | 4 (< 1) | 2 (< 0.5) | 2 | 2 | 19.0 | 5.2 | 0 | 0 | 7.4 | |
| Metabolic | 34 (6) | 3 (< 0.5) | 3 | 1 | 9.5 | 31.3 | 2 | 9.0 | 51.3 | |
| Dermatologic | 35 (6) | 6 (1) | 6 | 0 | 0 | 0 | 6 | 27.02 | 26 | |
| Neurologic | 35 (6) | 5 (< 1) | 5 | 0 | 0 | 1.7 | 5 | 22.5 | 5.9 | |
| Hematologic | ||||||||||
| WBC | 42 (7) | 12 (2) | 5 | 1 | 9.5 | 104.3 | 4 | 18.0 | 32.7 | |
| Hemoglobin | 73 (13) | 1 (0.8) | 0 | 0 | 0.6 | 0 | 0 | 0.7 | ||
| Platelets | 61 (11) | 12 (2) | 0 | 0 | 2.6 | 0 | 0 | 1.5 | ||
| Others | 29 (5) | 1 (< 1) | 1 | 1 | 9.5 | 26.1 | 0 | 0 | 17.8 | |
NOTE. Patients in HGG cohort only received single agents, whereas STS patients received single agents or a combination of drugs. Grade 3 or higher nonhematologic and grade 4 hematologic toxicities are calculated as a rate per 1,000 patients.
Abbreviations: DLT, dose-limiting toxicity; HGG, high-grade glioma; STS, solid tumor studies.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Mrinal M. Gounder, Lakshmi Nayak, Solmaz Sahebjam, Alona Muzikansky, Stuart Grossman, Lisa M. DeAngelis, Patrick Y. Wen
Provision of study materials or patients: Serena Desideri, Xiaobu Ye
Collection and assembly of data: Mrinal M. Gounder, Lakshmi Nayak, Solmaz Sahebjam, Alona Muzikansky, Armando J. Sanchez, Serena Desideri, Patrick Y. Wen
Data analysis and interpretation: Mrinal M. Gounder, Lakshmi Nayak, Solmaz Sahebjam, Alona Muzikansky, Armando J. Sanchez, Xiaobu Ye, Stuart Grossman, S. Percy Ivy, L. Burt Nabors, Micheal Prados, Lisa M. DeAngelis, Patrick Y. Wen
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of the Safety and Benefit of Phase I Oncology Trials for Patients With Primary CNS Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Mrinal M. Gounder
Consulting or Advisory Role: Amgen, Daiichi Sankyo, Karyopharm Therapeutics
Speakers' Bureau: Amgen
Lakshmi Nayak
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Bristol-Myers Squibb (I)
Other Relationship: Peer reviewer for UpToDate
Solmaz Sahebjam
Research Funding: Cortice Biosciences (Inst), Merck (Inst)
Alona Muzikansky
No relationship to disclose
Armando J. Sanchez
No relationship to disclose
Serena Desideri
No relationship to disclose
Xiaobu Ye
No relationship to disclose
S. Percy Ivy
No relationship to disclose
L. Burt Nabors
Consulting or Advisory Role: Cavion
Micheal Prados
Research Funding: Genentech/Roche (Inst)
Stuart Grossman
Stock or Other Ownership: Axxia Pharmaceuticals
Honoraria: Roche/Genentech, MedImmune
Consulting or Advisory Role: Midatech
Research Funding: Erimos Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent related to a subcutaneous polymeric opioid delivery system has been awarded.
Lisa M. DeAngelis
Consulting or Advisory Role: Juno Therapeutics, CarThera, Pharmaco-Kinesis
Patrick Y. Wen
Employment: Harvard Medical School
Leadership: Dana-Farber Cancer Center
Honoraria: Merck
Consulting or Advisory Role: Abbvie, Roche/Genentech, Novartis, Novocure, Midatech, Momenta Pharmaceuticals, Vascular biogenics, Regeneron, Cavion, Foundation Medicine, Cubist
Speakers' Bureau: Merck
Research Funding: AstraZeneca, Agios, Angiochem, Exelixis, Genentech/Roche, GlaxoSmithKline, Karyopharm Therapeutics, Merck, Novartis, Sanofi, Vascular Biogenics
Travel, Accommodations, Expenses: Roche
REFERENCES
- 1.Eisenhauer EA, Twelves C, Buyse ME. Phase I Cancer Clinical Trials: A Practical Guide (ed 1) New York, NY: Oxford University Press; 2006. [Google Scholar]
- 2.Carden CP, Sarker D, Postel-Vinay S, et al. Can molecular biomarker-based patient selection in phase I trials accelerate anticancer drug development? Drug Discov Today. 2010;15:88–97. doi: 10.1016/j.drudis.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Gounder MM, Spriggs DR. Inclusion of patients with brain metastases in phase I trials: An unmet need. Clin Cancer Res. 2011;17:3855–3857. doi: 10.1158/1078-0432.CCR-11-0759. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Schiff D, Cloughesy TF, et al. It is time to include patients with brain tumors in phase I trials in oncology. J Clin Oncol. 2011;29:3211–3213. doi: 10.1200/JCO.2011.36.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman DM, Eaton AA, Gounder MM, et al. Nomogram to predict cycle-one serious drug-related toxicity in phase I oncology trials. J Clin Oncol. 2014;32:519–526. doi: 10.1200/JCO.2013.49.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Hunsberger S, Boerner SA, et al. Meta-analysis of the relationship between dose and benefit in phase I targeted agent trials. J Natl Cancer Inst. 2012;104:1860–1866. doi: 10.1093/jnci/djs439. [DOI] [PubMed] [Google Scholar]
- 7.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 8.Olmos D, A'Hern RP, Marsoni S, et al. Patient selection for oncology phase I trials: A multi-institutional study of prognostic factors. J Clin Oncol. 2012;30:996–1004. doi: 10.1200/JCO.2010.34.5074. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Cancer Therapy Evaluation Program: Instructions and Guidelines Clinical Data Update System, 3, 2011. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/cdus.htm.
- 10.Bramwell V, Rouesse J, Steward W, et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma: Reduced local recurrence but no improvement in survival—A study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1994;12:1137–1149. doi: 10.1200/JCO.1994.12.6.1137. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 13.Nabors LB, Supko JG, Rosenfeld M, et al. Phase I trial of sorafenib in patients with recurrent or progressive malignant glioma. Neuro Oncol. 2011;13:1324–1330. doi: 10.1093/neuonc/nor145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 15.Grossman SA, Ye X, Peereboom D, et al. Phase I study of terameprocol in patients with recurrent high-grade glioma. Neuro Oncol. 2012;14:511–517. doi: 10.1093/neuonc/nor230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goel S, Burris H, Mendelson D, et al. A phase I study of intravenous tetra-O-methyl nordihydroguaiaretic acid in patients with refractory malignancy. J Clin Oncol. 2007;(suppl 18S):25. abstr 3584. [Google Scholar]
- 17.Zonnenberg BA, Groenewegen G, Janus TJ, et al. Phase I dose-escalation study of the safety and pharmacokinetics of atrasentan: An endothelin receptor antagonist for refractory prostate cancer. Clin Cancer Res. 2003;9:2965–2972. [PubMed] [Google Scholar]
- 18.Grossman SA, Carson KA, Phuphanich S, et al. Phase I and pharmacokinetic study of karenitecin in patients with recurrent malignant gliomas. Neuro Oncol. 2008;10:608–616. doi: 10.1215/15228517-2008-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilsky R, Hausheer F, Bertucci D, et al. Phase I trial of karenitecin (KT) administered intravenously daily for five consecutive days in patients with advanced solid tumor using accelerated dose titration. Proc Am Soc Clin Oncol. 2000;19:195a. (abstr 758) [Google Scholar]
- 20.Phuphanich S, Carson KA, Grossman SA, et al. Phase I safety study of escalating doses of atrasentan in adults with recurrent malignant glioma. Neuro Oncol. 2008;10:617–623. doi: 10.1215/15228517-2008-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan CW, Vogelzang NJ, Vokes EE, et al. Dose-ranging study of the safety and pharmacokinetics of atrasentan in patients with refractory malignancies. Clin Cancer Res. 2004;10:4406–4411. doi: 10.1158/1078-0432.CCR-04-0083. [DOI] [PubMed] [Google Scholar]
- 22.Peereboom DM, Supko JG, Carson KA, et al. A phase I/II trial and pharmacokinetic study of ixabepilone in adult patients with recurrent high-grade gliomas. J Neurooncol. 2010;100:261–268. doi: 10.1007/s11060-010-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham J, Agrawal M, Bakke S, et al. Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five days. J Clin Oncol. 2003;21:1866–1873. doi: 10.1200/JCO.2003.03.063. [DOI] [PubMed] [Google Scholar]
- 24.Rudek MA, New P, Mikkelsen T, et al. Phase I and pharmacokinetic study of COL-3 in patients with recurrent high-grade gliomas. J Neurooncol. 2011;105:375–381. doi: 10.1007/s11060-011-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syed S, Takimoto C, Hidalgo M, et al. A phase I and pharmacokinetic study of Col-3 (Metastat), an oral tetracycline derivative with potent matrix metalloproteinase and antitumor properties. Clin Cancer Res. 2004;10:6512–6521. doi: 10.1158/1078-0432.CCR-04-0804. [DOI] [PubMed] [Google Scholar]
- 26.Phuphanich S, Supko JG, Carson KA, et al. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol. 2010;100:95–103. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 28.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskens FA, Dumez H, Hoekstra R, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39:917–926. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 30.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadatos-Pastos D, Banerji U. Revisiting the role of molecular targeted therapies in patients with brain metastases. J Neurooncol. 2011;105:467–474. doi: 10.1007/s11060-011-0661-y. [DOI] [PubMed] [Google Scholar]
- 33.Arkenau HT, Olmos D, Ang JE, et al. 90-Days mortality rate in patients treated within the context of a phase-I trial: How should we identify patients who should not go on trial? Eur J Cancer. 2008;44:1536–1540. doi: 10.1016/j.ejca.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Hyman DM, Eaton AA, Gounder MM, et al. Predictors of early treatment discontinuation in patients enrolled on Phase I oncology trials. Oncotarget. doi: 10.18632/oncotarget.2909. [epub ahead of print on January 21, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachelot T, Ray-Coquard I, Catimel G, et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol. 2000;11:151–156. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]
- 36.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 37.Molife LR, Alam S, Olmos D, et al. Defining the risk of toxicity in phase I oncology trials of novel molecularly targeted agents: A single centre experience. Ann Oncol. 2012;23:1968–1973. doi: 10.1093/annonc/mds030. [DOI] [PubMed] [Google Scholar]
- 38.Prados MD, Yung WK, Jaeckle KA, et al. Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol. 2004;6:44–54. doi: 10.1215/S1152851703000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellmann A, Rule S, Walewski J, et al. Effect of cytochrome P450 3A4 inducers on the pharmacokinetic, pharmacodynamic and safety profiles of bortezomib in patients with multiple myeloma or non-Hodgkin's lymphoma. Clin Pharmacokinet. 2011;50:781–791. doi: 10.2165/11594410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors—Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]