Abstract
Persistent organic pollutants (POPs) and polycyclic aromatic hydrocarbons (PAHs) used in agricultural, industrial, and domestic applications are widely distributed and bioaccumulate in food webs, causing adverse effects to the biosphere. A review of published data for 1977–2015 for a wide range of vegetation around the globe indicates an extensive load of pollutants in vegetation. On a global perspective, the accumulation of POPs and PAHs in vegetation depends on the industrialization history across continents and distance to emission sources, beyond organism type and climatic variables. International regulations initially reduced the concentrations of POPs in vegetation in rural areas, but concentrations of HCB, HCHs, and DDTs at remote sites did not decrease or even increased over time, pointing to a remobilization of POPs from source areas to remote sites. The concentrations of compounds currently in use, PBDEs and PAHs, are still increasing in vegetation. Differential congener specific accumulation is mostly determined by continent—in accordance to the different regulations of HCHs, PCBs and PBDEs in different countries—and by plant type (PAHs). These results support a concerning general accumulation of toxic pollutants in most ecosystems of the globe that for some compounds is still far from being mitigated in the near future.
Hundreds of thousands of organic compounds produced synthetically for use in agricultural, industrial, and domestic applications have been annually spread widely around the globe since the beginning of the industrial revolution1. Some of these compounds known as persistent organic pollutants (POPs) are intentionally produced for use as pesticides (e.g. hexachlorobenzene (HCB), also a byproduct in the manufacture of chlorinated solvents; hexachlorocyclohexanes (HCHs); and dichlorodiphenyltrichloroethanes (DDTs)), industrial fluids (polychlorinated biphenyls (PCBs)), and flame retardants (polybromodiphenyl ethers (PBDEs)) (Table S1). Others, such as polycyclic aromatic hydrocarbons (PAHs) are unintentional byproducts of the incomplete combustion of organic matter. POPs and PAHs are toxic and semivolatile and have the capacity to bioaccumulate and biomagnify in food webs. These chemicals enter food webs predominantly through the equilibrium partition of primary producers with the atmosphere2. In terrestrial ecosystems, the accumulation of POPs in vegetation is thus the major vector of POPs and, consequently, a crucial process in determining the extent of environment (and human) exposure to POPs. High concentrations of POPs and PAHs can indeed cause endocrine disruption, altered neurological development, immune system modulation, and cancer3.
The atmosphere is the principal source of POPs and PAHs in vegetation4,5,6. POPs and PAHs enter vegetation by gaseous equilibrium partitioning between the vegetation and surrounding air and by dry/wet particle-bound deposition; partitioning from soil to plant roots is a secondary entrance pathway. In contrast to hydrophilic compounds7, lipophilic pollutants such as POPs and PAHs are generally not translocated within a plant through the phloem, because they are retained in lipophilic tissues, and thus the metabolism of the compounds is not significant8. All these mechanisms of pollutant uptake and bioaccumulation in plants are mostly dependent on the physicochemical properties of the compounds but also on environmental (e.g. temperature, soil composition, and wind velocity) and especially on plant (e.g. surface area, lipid and VOCs content—, hydration state and growth rate) parameters9,10,11. However, some researchers hold that the equilibrium between plants and air is slow and thus plant tissues accumulate POPs throughout their life span, which reinforces the role of plant physiological adaptations and life cycle in the bioaccumulation of POPs10,11.Anyway, the highest theoretical maximum reservoir capacity for POPs is found in areas with low temperatures and coniferous forests (e.g. Siberia, Canada, and Scandinavia) where both the gas-to-leaf partition and the plant characteristics such as surface area are maximized, and the lowest concentrations are typically in warm and desert areas (e.g. Sahara) where most semivolatile compounds are in the gas phase and organic matrices able to retain POPs are rare12. Compounds retained in leaves by this filtering effect ultimately accumulate in soils under forest canopies after leaf senescence because they have reduced mobility across the phloem. Soils under forest canopies accumulate 3–5 times more POPs than bare soils13, and this retention substantially increases their overall global residence times14.
The widespread distribution and environmental and health concerns of POPs and PAHs led to international environmental agreements to eliminate or restrict their production, use, and release. The first global regulation to outlaw, limit the use or curtail inadvertent production of POPs was agreed in the Stockholm Convention on Persistent Organic Pollutants in May 2001 that entered into force on May 2004. Twelve groups of substances, informally known as the dirty dozen, were initially included. Little information is available for the temporal trends of these compounds in ecosystems, but a decrease in POP dispersion has been detected since the onset of the regulations15,16. POP concentrations, however, are still high in most ecosystems, causing health risks to humans and wildlife17,18, and substantial gaps in information remain about the temporal trends of POPs in ecosystems and the levels of toxicity and environmental stress incurred at the level of the ecosystem. A wide range of POPs has been remobilized into the atmosphere of the remote Arctic over the past two decades as a result of climate change, confirming that Arctic warming could undermine global efforts to reduce environmental and human exposure to these toxic chemicals19 and underscoring the need for an assessment of current temporal and spatial trends of POPs.
We examined the trends of temporal and spatial accumulation of airborne organic contaminants in vegetation at a global scale from an ecosystem perspective (i) to assess the level of contamination by POPs and PAHs in a maximized number of plant species—as the first entrance of POPs in food webs and thus crucial in determining the extent of wildlife and human exposure to POPs; (ii) to assess the main factors affecting the concentrations of POPS and PAHs in the different ecosystems; (iii) to assess the impacts of international POP regulations on these concentrations; and verify the limited available data and models indicating that atmospheric levels of most POPs are declining slowly in some regions of the northern hemisphere16,20. The target analytes included current-use (DDTs – only for malaria and dengue control – PBDEs and PAHs) and historic-use (HCB, HCHs, and PCBs) pollutants in vegetation from industrial, urban, rural and remote areas.
Results
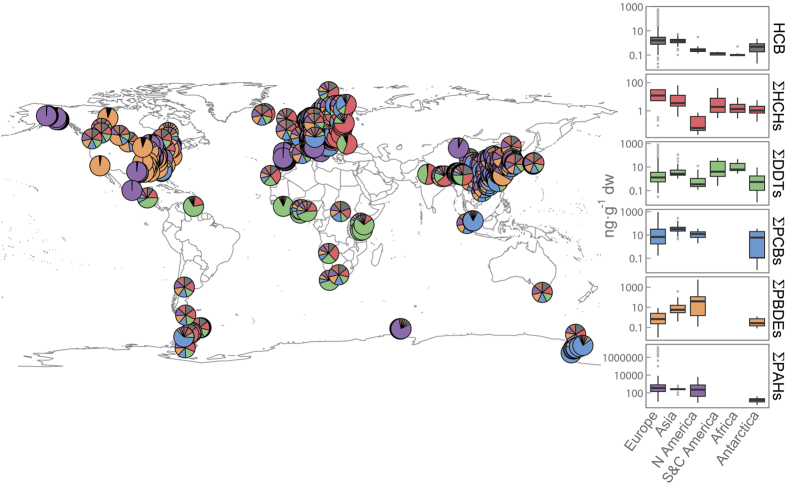
We found 79 publications providing concentrations of HCB, HCHs, DDTs, PCBs, PBDEs, and PAHs in vascular plants, mosses and lichens from around the globe (mean values and references in Table S2). The publications spanned 1977 to 2015 and 54% of the studies included rural areas (Fig. S1). The Northern Hemisphere, particularly Europe, was best represented (Fig. 1), but data from South and Central America, Africa, and Australia were limited: 1650 observations in Europe versus 204 in North America, 15 in South and Central America, 28 in Africa, 427 in Asia, and 235 in Antarctica. Most of the studies focused on gymnosperms, particularly Pinus sylvestris (388 of 727 observations), and information on bryophytes, lichens, and angiosperms were lacking (Table S2).
Figure 1. Continental distribution of POPs in vegetation around the globe.
The map shows the proportion of each family of compounds reviewed for all sampling locations with published data. The boxplots compare the concentrations of each family of POPs across continents. Interquartile ranges (25th and 75th percentile) are shown by the height of the boxes, and the horizontal lines represent medians (50th percentile). Whiskers range from the 10th to 90th percentiles. Concentrations are in ng·g−1 dry weight. The map was generated using the R packages rgdal60, maptools61, and mapplots62. Permission is granted to Nature Publishing Group to publish the image in all formats under an Open Access license.
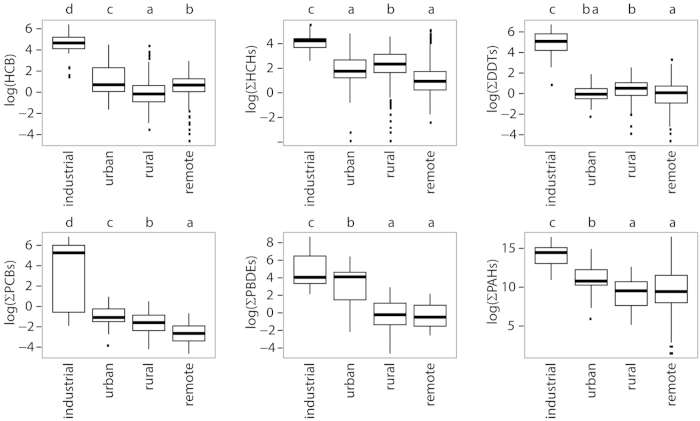
Concentrations of POPs and PAHs were high on most continents (Fig. 1), particularly Asia, Europe, and North America for most compounds; and Antarctica usually had the lowest concentrations. Central and South America, Africa, and Asia had the highest p,p′-DDT/p,p′-DDE ratios (3.3, 2.5, and 5, respectively) and concentrations of DDTs. In addition to the continental differences, land use was another significant driver of POP and PAH concentrations: vegetation from urban and industrial areas concentrated up to five orders of magnitude more POPs and PAHs than rural and remote areas (Fig. 2). Concentrations were similar or sometimes higher in rural than in remote areas. In contrast, differences among large plant types (e.g. lichens—plant-like composite organism, bryophytes, gymnosperms, and angiosperms) were less variable than concentrations among continents or land use areas at global scale (Fig. S2).
Figure 2. Variability of POPs and PAHs in vegetation from remote, rural, urban, and industrial environments.
Box plots of the logarithm of the concentration of each family of compounds. Small letters refer to significant differences among land uses (Tukey’s HSD, P < 0.05). Different letters indicate significant differences. Interquartile ranges (25th and 75th percentile) are shown by the height of the boxes, and horizontal lines represent medians (50th percentile). Whiskers range from the 10th to 90th percentiles, and values outside this range are indicated by small squares. Concentrations are in ng·g−1 dry weight.
We fit multilevel models (MLMs) to resolve the influences of location (continent or land use), plant type, climate, and temporal trends on the global distributions of POPs and PAHs in vegetation. The MLMs make it possible to estimate simultaneously the responses of total concentration of each family of compounds to predictor (independent) variables, in addition to also giving a summary of the predictor determinants of congener composition21. Totals for each family were always calculated based on the same, most usually measured compounds (see Methods for details). Year, latitude, altitude, mean annual temperature (MAT), mean annual precipitation (MAP), plant type, continent, and land use (urban and industrial study areas were excluded from the MLM to avoid potential point-source contamination) were included in the best-fitting multilevel models, selected using Akaike’s information criterion (AIC), as the main drivers determining the global distributions of POPs and PAHs in vegetation (Fig. 3, Table S3). Spatial autocorrelation was significant (P < 0.001) and included in all models.
Figure 3. Multilevel model of the selected effects of abiotic and biotic variables on the concentrations of POPs in plants.
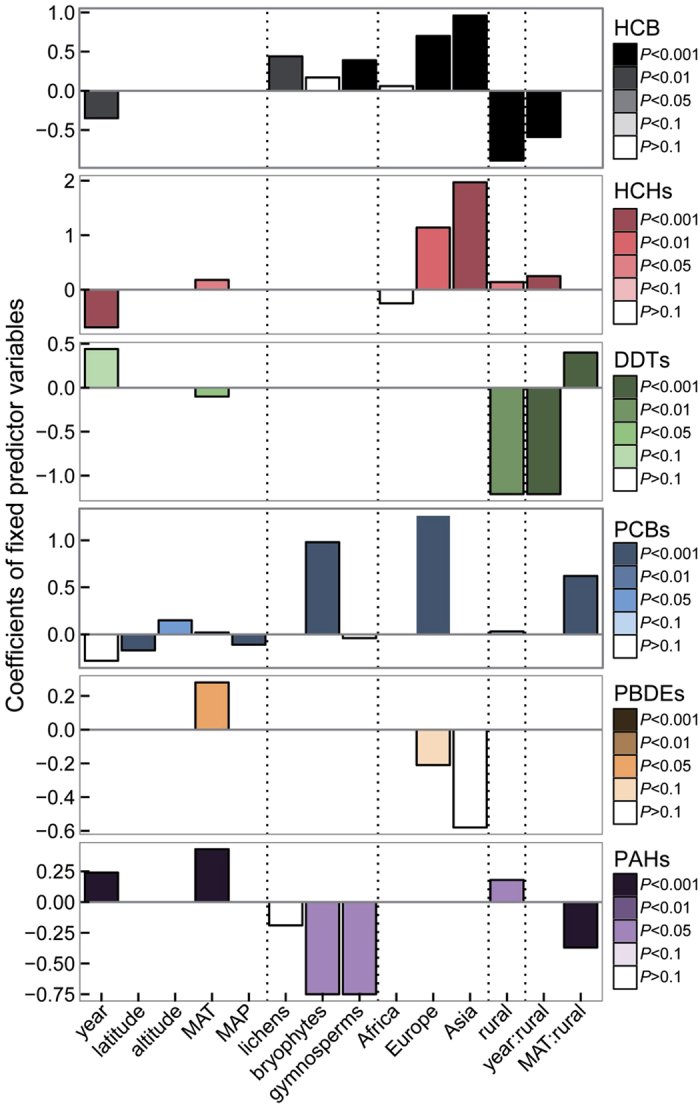
Constant and varying effects by congeners are included for the predictor variables included in the lowest-AIC models. All models accounted for spatial correlation structure and nugget (which were significant in all cases, P < 0.001) and were run using the restricted maximum likelihood criterion for optimizing parameter estimates. MAT, mean annual temperature; MAP, mean annual precipitation.
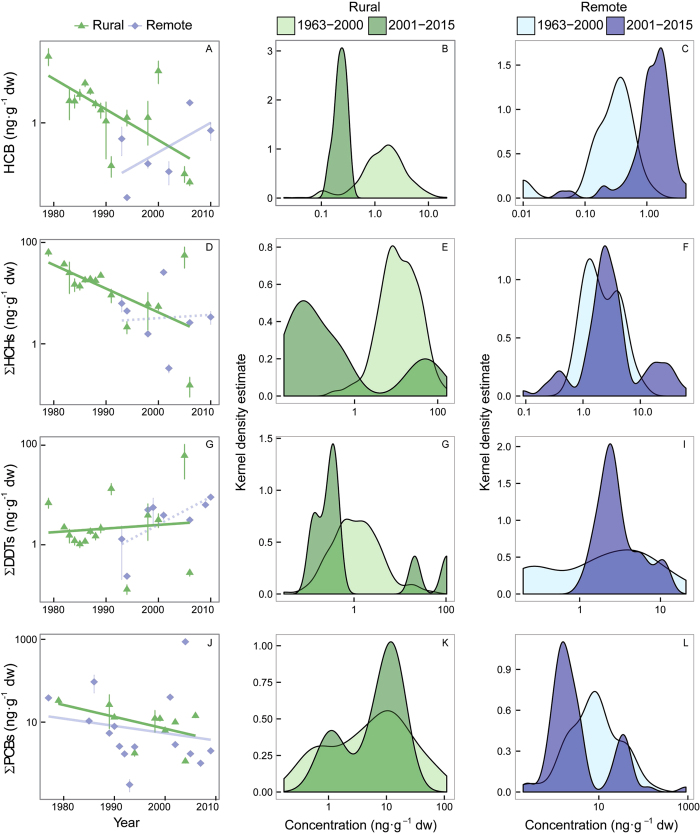
Continent and land use (remote and rural areas) were the strongest factors in most models accounting for the accumulation of POPs and PAHs in vegetation (Fig. 3). Year was also a significant variable explaining the accumulation of most POPs and PAHs in vegetation (Fig. 3). Year was associated negatively with the historically used HCB, HCHs, and PCBs, positively with the currently emitted PAHs, and not clearly associated with DDTs and PBDEs. Interestingly, this decreasing trend of the historically used compounds was not consistent among land-use areas, indicated by the positive interaction between year and land use (Fig. 3). Indeed, HCB, HCH, and DDT concentrations decreased only in rural areas but increased at remote sites (e.g. HCB, Fig. 4A) or showed no trend (e.g. HCHs and DDTs, Fig. 4D,G). In contrast, PBDE and PAH concentrations increased with time in rural areas and had no significant relationship with time in remote areas (Fig. 5, Fig. S3 for gymnosperms).
Figure 4. Individual relationships among HCB, ΣHCHs, and ΣDDTs (ng·g−1 dry weight) and year.
The equations for rural and remote areas are: (A) HCB rural: y = −0.04× + 91, r2 = 0.19, df = 1354, P < 0.0001, and HCB remote: y = 0.07× − 140, r2 = 0.43, df = 1,95, P < 0.0001; (D) ΣHCHs rural: y = −0.07× + 148, r2 = 0.31, df = 1,356, P < 0.0001, and ΣHCHs remote: y = 0.08× − 16, r2 = 0.01, df = 1,143, P = 0.2; (G) ΣDDTs rural: y = −0.01× + 23, r2 = 0.01, df = 1,356, P = 0.09, and ΣDDTs remote: y = 0.01× − 13, r2 = 0.00, df = 1,155, P = 0.4; and (J) ΣPCBs rural: y = −0.02× + 50, r2 = 0.11, df = 1,102, P < 0.001, and ΣPCBs remote: y = −0.03× + 71, r2 = 0.24, df = 1,124, P < 0.0001. Panels (B), (E), (H) and (K) and (C), (F), (I) and (L) show the estimated curve for kernel density for both rural and remote regions, respectively, before and after 2000.
Figure 5. Individual relationships among ΣPBDEs and ΣPAHs (ng·g−1 dry weight) and year.
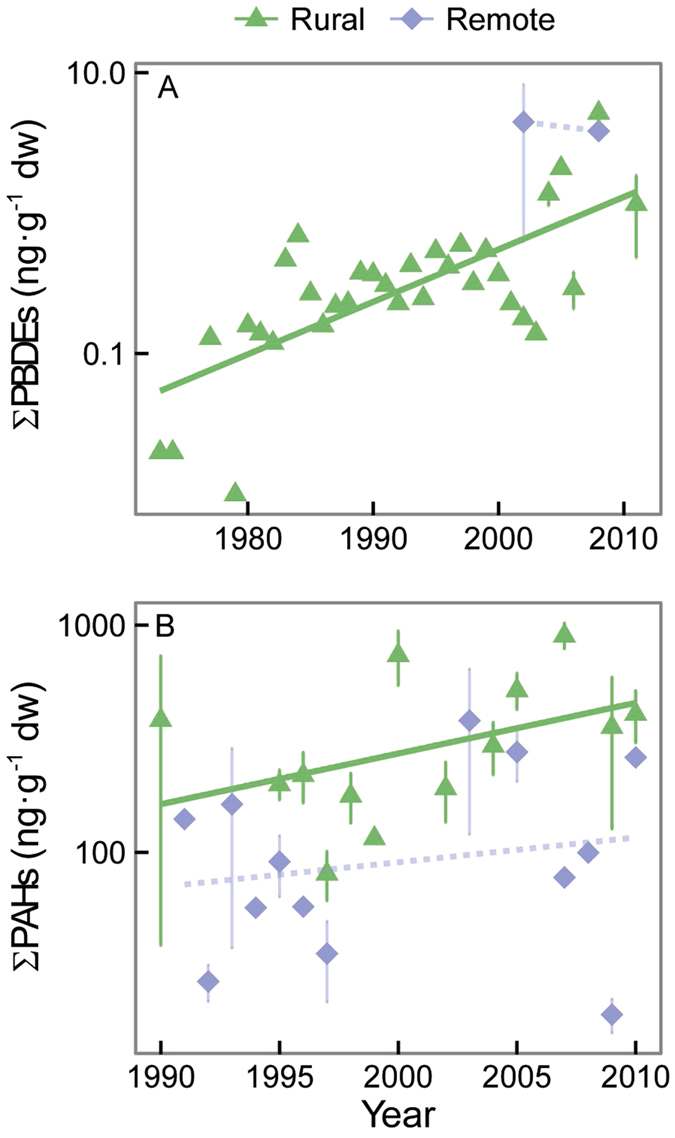
The equations for rural and remote areas are: (A) ΣPBDEs rural: y = 0.05× − 98, r2 = 0.48, df = 1,91, P < 0.0001, and ΣPBDEs remote: y = 0.03× − 64, r2 = 0.05, df = 1,18, P = 0.3; and (B) ΣPAHs rural: y = 0.06× − 125, r2 = 0.32, df = 1,192, P < 0.0001, and ΣPAHs remote: y = −0.01× + 28, r2 = 0.02, df = 1,47, P = 0.3.
The strength of the climatic variables accounting for the variation of POPs and PAHs was smaller than all other variables, but still significant as expected at this scale. HCHs, PBDEs, and PAHs were positively associated with MAT, and PCBs were negatively associated with latitude and MAP (Fig. 3), indicating a dependence on fresh release from diffuse or point sources in warmer temperate areas. DDTs, however, were negatively associated with MAT, and PCBs were positively associated with altitude (Fig. 3), suggesting global fractionation22,23.
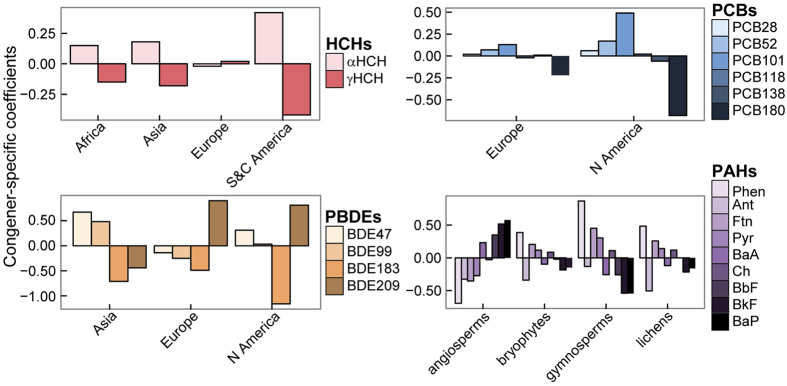
In the same MLMs, the different congeners of HCHs, PCBs, PBDEs, and PAHs had different concentration pattern depending on the continent or the plant type (different slope per congener in the MLM, Fig. 6). Concentrations were usually higher for α-HCH than γ-HCH on all continents except Europe. PCB101 was the PCB congener that bioaccumulated the most in vegetation, followed by lighter (PCB28 and 52) and heavier (PCB118, 138, and 180) PCBs. These differences were slightly more accentuated in North America. Concentrations were higher for BDE209 than all other PBDEs in North America and Europe, but were lower in Asia. The most important differences in PAH concentrations were in angiosperms versus gymnosperms, mosses and lichens, with higher proportions of PAHs of high molecular weight.
Figure 6. Coefficients from the multilevel model giving the significant congener-specific responses to continent and plant type*.
The values are the species-specific changes of slope plus the estimates for fixed effects. *Lichens as plant-like composite organisms.
Discussion
The global distribution of POPs and PAHs in vegetation is remarkable. The concentrations presented in this review generally did not exceed the thresholds established in toxicological assays24,25,26 but were nevertheless high considering the dates most of these compounds were restricted or banned. These concentrations may be of concern, because these hydrophobic and persistent compounds biomagnify across food webs, sometimes achieving toxic concentrations in top predators, including humans27.
Despite the high variability in POP and PAH sources and concentrations and in the number and location of studies that have analyzed these compounds in vegetation (Table S2), the industrialization history and distance to emission sources were key parameters determining the distribution of POPs and PAHs total concentration and congener composition around the globe, beyond plant type (with the different plant physiologies and life cycles compared) and climatic variables like temperature (Fig. 3). Europe was best represented for the number of studies and, together with Asia and North America, their vegetation accumulated the highest concentrations of most compounds, with the exception of HCHs and DDTs that dominated in remote areas of Africa, Asia, and Central America. These results concurred for example with soil global PCB budgets28 and PCB historical global production and consumption29. The proportion of the different congeners of HCHs, PCBs and PBDEs also differed among continents (Fig. 6). Concentrations were usually higher for α-HCH than γ-HCH everywhere except Europe, indicating a more direct and/or recent release of lindane (γ-) in Europe versus the historic HCH mixture known as technical HCH, which is less pure and has higher proportions of α-HCH30. Moderately hydrophobic PCB congeners (e.g. PCB101) dominated in most plant types, consistent with the McLachlan equilibrium model partitioning air and vegetation and considering the life span of the leaves and the effects of growth dilution and particle deposition31. PBDEs congener composition illustrated the stricter regulations in Europe and North America against the use and release of the less brominated PBDEs, while, Asia still presented higher proportion of these tetra- and penta-BDEs.
The concentrations of most compounds tended to decrease over time in rural areas, and those of other compounds have remained stable or have even increased at remote sites (Figs 3 and 4). The legislation established in most regions to reduce and eliminate the production, use, and release of most POPs thus appear to have been effective but insufficient, because POPs are still found and are accumulating in remote, usually cold, regions of the planet. Some studies at rural sites show a similar decreasing trend16,32, but less information is available for remote sites. These results seem thus to point to a problematic redistribution of POPs from point sources of contamination to remote regions. The travel of these semi-volatile compounds to remote regions where they have never been emitted depends on the physicochemical properties of the compounds and usually involves a cold condensation effect that traps these semivolatile compounds in colder areas22,23.
In rural areas, the concentrations of PAHs, PBDEs and DDTs did not experience the current decreasing trend observed for all other compounds reviewed here. In the case of PAHs, the trend of increasing PAH concentrations in vegetation over time is striking (Figs 3 and 5B). PAHs are emitted to the atmosphere from the burning of residential/commercial biomass (60.5%), open-field biomass (from agricultural waste, deforestation, and wildfires, 13.6%), and fossil fuels by on-road motor vehicles (12.8%)33. Jones et al.34 and Salamova et al.35 found evidence of a decline in PAH concentrations in rural vegetation in the UK and in the air at some sites around the Great Lakes in North America, but PAH concentrations in other locations show little evidence of decline (e.g. in air35,36 and mussels37) or even an increase (e.g. in air36 and mussels38). These variable temporal trends of PAHs concentrations, together with the positive relationship between PAHs concentrations and MAT in rural areas (Fig. 3), reinforce the strong influence of local/regional emission sources to PAH concentrations in the environment and underscore the concerns about the high PAH concentrations in the environment given the toxicity and environmental consequences of this group of compounds39. Unfortunately, with the dispersion of data we have, we could not find a strong enough signal of the different PAHs congeners in the MLM analysis to elucidate the sources (e.g. biogenic, industrial or degradative processes) of these compounds over time and/or across the different regions. However, we did find different congener patterns of PAHs among plant types (Fig. 6). Angiosperms accumulated higher proportions of high molecular weight PAHs in relation to other plant types. This pattern could be originated by a potential lower biotransformation capabilities of PAHs in angiosperms in relation to all other vegetation groups40. Similar results were reported for some arthropod lineages, which were more or less able to debrominate PBDEs depending on the evolutionary history of each organism41.
The concentrations of PBDEs similarly increased over time (Fig. 5A), but year was not selected as a predictor variable in the best MLM (Fig. 3), potentially due to the low sample sizes in past studies. Defining the global status of PBDEs is even more challenging than with other compounds due to the variation in PBDE regulations among countries. Finally, the global temporal increasing trend of DDT concentrations is mostly affected by the allowance of DDT use in regions with malaria and dengue fever to control populations of Anopheles mosquitoes42. Also, since dicofol (widely used as a pesticide in agriculture applications) is mainly synthesized from DDT, it contains DDT as an impurity (0.1–20%) and at present can therefore still be emitted to the atmosphere43. Indeed, Central America, Africa, and Asia had the highest DDT concentrations and very high p,p′-DDT/p,p′-DDE ratios, indicating recent use of DDT. Technical DDT generally contains 75% p,p′-DDT, 15% o,p′-DDT, 5% p,p′-DDE, and <5% other congeners, but p,p′-DDT is easily degraded in the environment to p,p′-DDE. Tanabe44 has identified the tropical belt as the major source of emission of organochlorine insecticides such as DDTs and HCHs, for the same reasons.
As expected at this global scale, the direct climatic influence on the distribution of POPs in vegetation was masked by the strong influence of development-derived factors such as continent, land use, or even year. The expected negative relationships of POPs concentrations along gradients of temperature (and latitude or altitude), consistent with the global distillation theory22,23, were only and slightly found for DDTs (MAT) and PCBs (altitude) (Fig. 3). In contrast, the positive effect of temperature on the distribution of POPs and PAHs around the globe, mostly in rural areas (significant interaction, Fig. 3), is likely underlining the different proximities to the sources of the sampling sites, which usually have higher temperatures45. These results reinforce the importance of not only the climatic and biogeochemical variables but also, and more importantly, other factors (such as proximity to sources, legislation, etc.) of each particular ecosystem on the overall multimedia partitioning of these persistent, hydrophobic, semi-volatile compounds at a global scale.
Strong efforts have been made to measure and assess the risk of particular POPs and PAHs in the global environment29,46,47, and similar efforts are starting to be developed for new chemicals such as current-use pesticides48. The constant release of new chemicals into the atmosphere every year, however, is large relative to the small fraction of chemicals measured1. Little is known in quantitative terms about the global sources of POP and PAH emissions. The implications for this progressive toxification of nature are still unresolved but certainly are one of the challenges that humanity will face in the near future. Our study demonstrates that (i) contamination levels in vegetation at rural and remote sites far from point sources of contamination are generally non-toxic but significant, and could cause adverse effects in the top trophic levels after being biomagnified, (ii) the accumulation of POPs and PAHs (and each particular congener) in vegetation depends on the industrialization history across continents and distance to emission sources, beyond plant type and climatic variables, such as temperature, and iii) global regulations initially reduced POP concentrations in vegetation in rural areas, but HCB, HCH, and DDT concentrations in remote sites are not decreasing and are even increasing (HCB) over time, suggesting a redistribution of these pollutants from their sources, while PBDE and PAH concentrations still show a hazardous increasing trend even in vegetation from rural areas. These concentrations found in vegetation consequently point to a concerning pollutant background in the majority of ecosystems of the planet that seems still far from being mitigated with the current international regulations. Changes in rates of transport and pools of POPs throughout the planet are additionally expected as the climate warms49, favoring re-emission of POPs from cold remote areas50,51 and increasing even more human and environmental exposure to POPs. Understanding the global load of these toxic persistent and semivolatile substances and the feedbacks between their dynamics and a changing environment is therefore crucial to enable the assessment and mitigation of this progressive toxification of nature.
Methods
Literature search and development of the database
We searched the Thomson Reuters Web of Knowledge database (http://thomsonreuters.com/) and Google Scholar (http://scholar.google.com/) for publications containing the name of each compound, congener or group of compounds and vegetation, or plant/moss/lichen, or particular species of plant/moss/lichen. We searched for additional studies by scanning the bibliographies of the publications identified by our initial search. Data were collected for 1977–2015 and 1432 locations around the globe (data summarized and references in Table S2). We extracted POP and PAH concentrations, plant/moss/lichen species names, locations, geographic coordinates, altitudes, and climatic variables for each location. All chemical concentrations were expressed in a dry weight basis (ng chemical per g dry weight). We did not express the concentrations in a lipid weight basis due to i) the low proportion of publications providing this information, ii) the low precision of the lipid gravimetric determination compared to total dry weight measurements, and iii) the presence of many other hydrophobic compounds such as membrane lipids and cuticular wax, cutin or terpenoids in addition to the lipids extracted by gravimetric methods11. Similarly, the possible influence of seasonal variation of starch content on the total dry weight basis11 was cushioned by the high number of samples considered and the assumption that the starch distribution was not skewed among sampling locations. Data from major urban or industrial centers were avoided and not used in the spatial analyses of this study.
Climatic or altitudinal information not available in the original articles were obtained from the WorldClim database 30 arc-second resolution grid52, and HadCRUT3 with 5° resolution grid53. The variables MAT, MAP, and altitude were selected from the WorldClim database, and cloud cover, potential evapotranspiration, and vapor pressure were selected from the HadCRUT3database. Antarctica was not well represented in these climatic databases, but most studies with data available from these polar locations fortunately also contained climatic data.
Statistical analysis
All concentrations were logarithmically transformed to control for heteroscedasticity. Only needle and foliar concentrations were used for gymnosperms and angiosperms in the analyses. We used analysis of variance (ANOVA), Tukey’s honestly significant difference (HSD) test, and multivariate analysis of variance (MANOVA) to determine the variance of the concentrations of all congeners in each family of compounds among plant types and land uses. The ANOVA tested the differences among the sums of the concentrations for each family of compounds, and the MANOVA determined the variance of the concentrations of all congeners in each family of compounds.
We used MLMs54 to both simultaneously quantify the distributional patterns of POPs and PAHs in addition to the abundances of the constituent congeners21. The models can be interpreted as several regressions in which differences in slopes and intercepts among congeners are random variables. Using predictor (independent) variables enables this approach to assess the effects of abiotic and biotic drivers on POP and PAH concentrations and congener compositions while taking into account spatial correlation in the residuals. Without using predictor variables, this approach tests for spatial correlations in POP and PAH concentrations and congener compositions55. To do so, we only used the congeners better represented in the bibliography, which are for HCHs: α-HCH and γ-HCH, DDTs: DDT and DDE, PCBs: 28, 52, 101, 118, 138 and 180, PBDEs: 47, 99, 183 and 209, and PAHs: Phen, Ant, Ftn, Pyr, BaA, Ch, BbF, BkF and BaP.
The variables we tested were year of vegetation sampling, latitude, longitude, altitude, temperature, MAT, MAP, cloud cover, potential evapotranspiration, vapor pressure, and plant type (we grouped lichens (plant-like composite organism), bryophytes, gymnosperms, and angiosperms to simplify the analysis). All variables were transformed if needed to minimize skews. We excluded variables that were closely correlated to one another, so all abiotic and biotic variables listed above had pairwise correlations <0.7 (Cohen’s scale56). We checked for normality and homogeneity of residuals with Shapiro-Wilks and Levene’s tests. This analysis simultaneously provided information about abiotic and biotic drivers of the POP and PAH concentrations and the responses of individual congeners to these drivers21. Finally, spatial autocorrelation structure was significant in all analyses (P < 0.01) and was introduced in the model to correct for the spatial structure of the data.
Data were analyzed using R, v. 3.2.357. Data from WorldClim and CRU were extracted using the R package raster, and the values of the four nearest raster cells were interpolated58. Data were analyzed using the R package lme459. The maps were generated using the R packages rgdal (to project the latitude/longitude coordinates60), maptools (to read in data from a shapefile and plotted the maps61), and mapplots (to add the pie charts to the maps62).
Additional Information
How to cite this article: Bartrons, M. et al. Spatial And Temporal Trends Of Organic Pollutants In Vegetation From Remote And Rural Areas. Sci. Rep. 6, 25446; doi: 10.1038/srep25446 (2016).
Supplementary Material
Acknowledgments
We thank H. Kylin whose comments improved the manuscript. This research was supported by the European Research Council Synergy grant ERC-SyG-610028 IMBALANCE-P, the Spanish Government grant CGL2013-48074-P and the Catalan Government projects SGR 2014-274 and SGR 2014-1249.
Footnotes
Author Contributions M.B. and J.P. designed the study and wrote the paper. M.B. collected data, performed analytical work, and prepared the figures. M.B., J.C. and J.P. discussed the results and commented on the manuscript.
References
- Muir D. C. G. & Howard P. H. Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol. 40, 7157–7166, doi: 10.1021/es061677a (2006). [DOI] [PubMed] [Google Scholar]
- Armitage J. M. & Gobas F. A. P. C. A Terrestrial Food-Chain Bioaccumulation Model for POPs. Environ. Sci. Technol. 41, 4019–4025, doi: 10.1021/es0700597 (2007). [DOI] [PubMed] [Google Scholar]
- WHO. Health risks of persistent organic pollutants from long-range transboundary air pollution. World Health Organization/IPCS, 1-274 (2003).
- Paterson S., Mackay D. & McFarlane C. A Model of Organic Chemical Uptake by Plants from Soil and the Atmosphere. Environ. Sci. Technol. 28, 2259–2266, doi: 10.1021/es00062a009 (1994). [DOI] [PubMed] [Google Scholar]
- Simonich S. L. & Hites R. A. Organic pollutant accumulation in vegetation. Environ. Sci. Technol. 29, 2905–2914, doi: 10.1021/es00012a004 (1995). [DOI] [PubMed] [Google Scholar]
- Collins C. D., Martin I. & Doucett W. Plant Uptake of Xenobiotics. (Springer, 2010). [Google Scholar]
- Cousins I. T. & Mackay D. Strategies for including vegetation compartments in multimedia models. Chemosphere 44, 643–654, doi: 10.1016/s0045-6535(00)00514-2 (2001). [DOI] [PubMed] [Google Scholar]
- Trapp S., Matthies M., Scheunert I. & Topp E. M. Modeling the bioconcentration of organic chemicals in plants. Environ. Sci. Technol. 24, 1246–1252, doi: 10.1021/es00078a013 (1990). [DOI] [Google Scholar]
- Paterson S., Mackay D., Tam D. & Shiu W. Y. Uptake of organic-chemicals by plants–a review of processes, correlations and models. Chemosphere 21, 297–331, doi: 10.1016/0045-6535(90)90002-b (1990). [DOI] [Google Scholar]
- Kylin H. & Bouwman H. Hydration State of the Moss Hylocomium splendens and the Lichen Cladina stellaris Governs Uptake and Revolatilization of Airborne alpha- and gamma-Hexachlorocyclohexane. Environ. Sci. Technol. 46, 10982–10989, doi: 10.1021/es302363g (2012). [DOI] [PubMed] [Google Scholar]
- Kylin H. & Sjodin A. Accumulation of airborne hexachlorocyclohexanes and DDT in pine needles. Environ. Sci. Technol. 37, 2350–2355, doi: 10.1021/es0201395 (2003). [DOI] [PubMed] [Google Scholar]
- Dalla Valle M., Dachs J., Sweetman A. J. & Jones K. C. Maximum reservoir capacity of vegetation for persistent organic pollutants: Implications for global cycling. Glob. Biogeochem. Cycle 18, GB4032 (2004). [DOI] [PubMed] [Google Scholar]
- Horstmann M. & McLachlan M. S. Atmospheric deposition of semivolatile organic compounds to two forest canopies. Atmos. Environ. 32, 1799–1809, doi: 10.1016/s1352-2310(97)00477-9 (1998). [DOI] [Google Scholar]
- Su Y. S. & Wania F. Does the forest filter effect prevent semivolatile organic compounds from reaching the Arctic? Environ. Sci. Technol. 39, 7185–7193, doi: 10.1021/es0481979 (2005). [DOI] [PubMed] [Google Scholar]
- Hassanin A., Johnston A. E., Thomas G. O. & Jones K. C. Time Trends of Atmospheric PBDEs Inferred from Archived U.K. Herbage. Environ. Sci. Technol. 39, 2436–2441, doi: 10.1021/es0486162 (2005). [DOI] [PubMed] [Google Scholar]
- Kong D., MacLeod M., Hung H. & Cousins I. T. Statistical Analysis of Long-Term Monitoring Data for Persistent Organic Pollutants in the Atmosphere at 20 Monitoring Stations Broadly Indicates Declining Concentrations. Environ. Sci. Technol. 48, 12492–12499, doi: 10.1021/es502909n (2014). [DOI] [PubMed] [Google Scholar]
- Martí-Cid R. et al. Dietary Exposure to Organochlorine Compounds in Tarragona Province (Catalonia, Spain): Health Risks. Human and Ecological Risk Assessment: An International Journal 16, 588–602, doi: 10.1080/10807031003788832 (2010). [DOI] [Google Scholar]
- Bonefeld-Jorgensen E. C. et al. Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin. Pharmacol. Toxicol. 115, doi: 10.1111/bcpt.12263 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. M., Hung H. L., Tian C. & Kallenborn R. Revolatilization of persistent organic pollutants in the Arctic induced by climate change. Nat. Clim. Chang. 1, 255–260, doi: 10.1038/nclimate1167 (2011). [DOI] [Google Scholar]
- Nizzetto L. et al. Past, Present, and Future Controls on Levels of Persistent Organic Pollutants in the Global Environment. Environ. Sci. Technol. 44, 6526–6531, doi: 10.1021/es100178f (2010). [DOI] [PubMed] [Google Scholar]
- Jackson M. M., Turner M. G., Pearson S. M. & Ives A. R. Seeing the forest and the trees: multilevel models reveal both species and community patterns. Ecosphere 3, art79, doi: 10.1890/es12-00116.1 (2012). [DOI] [Google Scholar]
- Wania F. & Mackay D. Global fractionation and cold condensation of low volatility organo-chlorine compounds in Polar Regions. Ambio 22, 10–18 (1993). [Google Scholar]
- Wania F. & Mackay D. Tracking the distribution of Persistent Organic Pollutants. Environ. Sci. Technol. 30, A390–A396 (1996). [DOI] [PubMed] [Google Scholar]
- Pakova V., Hilscherova K., Feldmannova M. & Blaha L. Toxic effects and oxidative stress in higher plants exposed to polycyclic aromatic hydrocarbons and their N-heterocyclic derivatives. Environ. Toxicol. Chem. 25, 3238–3245 (2006). [DOI] [PubMed] [Google Scholar]
- Calvelo Pereira R., Monterroso C. & Macias F. Phytotoxicity of hexachlorocyclohexane: Effect on germination and early growth of different plant species. Chemosphere 79, 326–333, doi: 10.1016/j.chemosphere.2010.01.035 (2010). [DOI] [PubMed] [Google Scholar]
- Chung M. K., Hu R., Wong M. H. & Cheung K. C. Comparative toxicity of hydrophobic contaminants to microalgae and higher plants. Ecotoxicology 16, 393–402, doi: 10.1007/s10646-007-0134-4 (2007). [DOI] [PubMed] [Google Scholar]
- Shunthirasingham C. et al. Mountain Cold-Trapping Increases Transfer of Persistent Organic Pollutants from Atmosphere to Cows’ Milk. Environ. Sci. Technol. 47, 9175–9181, doi: 10.1021/es400851d (2013). [DOI] [PubMed] [Google Scholar]
- Meijer S. N. et al. Global distribution and budget of PCBs and HCB in background surface soils: Implications or sources and environmental processes. Environ. Sci. Technol. 37, 667–672 (2003). [DOI] [PubMed] [Google Scholar]
- Breivik K., Sweetman A., Pacyna J. M. & Jones K. C. Towards a global historical emission inventory for selected PCB congeners–a mass balance approach 1. Global production and consumption. Sci. Total Environ. 290, 181–198 (2002). [DOI] [PubMed] [Google Scholar]
- Vijgen J. et al. Hexachlorocyclohexane (HCH) as new Stockholm Convention POPs—a global perspective on the management of Lindane and its waste isomers. Environ. Sci. Pollut. Res. 18, 152–162, doi: 10.1007/s11356-010-0417-9 (2011). [DOI] [PubMed] [Google Scholar]
- McLachlan M. S. Framework for the interpretation of measurements of SOCs in plants. Environ. Sci. Technol. 33, 1799–1804, doi: 10.1021/es980831t (1999). [DOI] [Google Scholar]
- Harrad S. J. et al. Polychlorinated biphenyls (PCBs) in the British environment: sinks, sources and temporal trends. Environ. Pollut. 85, 131–146 (1994). [DOI] [PubMed] [Google Scholar]
- Shen H. et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ. Sci. Technol. 47, 6415–6424, doi: 10.1021/es400857z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. C., Sanders G., Wild S. R., Burnett V. & Johnston A. E. Evidence for a decline of PCBs and PAHs in rural vegetation and air in the United Kingdom. Nature 356, 137–140, doi: 10.1038/356137a0 (1992). [DOI] [Google Scholar]
- Salamova A., Venier M. & Hites R. A. Revised Temporal Trends of Persistent Organic Pollutant Concentrations in Air around the Great Lakes. Environ. Sci. Technol. 2, 20–25, doi: 10.1021/acs.estlett.5b00003 (2015). [DOI] [PubMed] [Google Scholar]
- Prevedouros K., Jones K. C. & Sweetman A. J. Modelling the atmospheric fate and seasonality of polycyclic aromatic hydrocarbons in the UK. Chemosphere 56, 195–208, doi: 10.1016/j.chemosphere.2004.02.032 (2004). [DOI] [PubMed] [Google Scholar]
- Melwani A. R. et al. Mussel Watch update: long-term trends in selected contaminants from coastal California, 1977-2010. Mar. Pollut. Bull. 81, 291–302, doi: 10.1016/j.marpolbul.2013.04.025 (2014). [DOI] [PubMed] [Google Scholar]
- Parolini M. & Binelli A. Temporal trends of polycyclic aromatic hydrocarbons (PAHs) In Dreissena polymorpha specimens from Lake Maggiore (Northern Italy). Environ. Sci. Pollut. Res. Int. 21, 7006–7023, doi: 10.1007/s11356-014-2623-3 (2014). [DOI] [PubMed] [Google Scholar]
- Samanta S. K., Singh O. V. & Jain R. K. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 20, 243–248, doi: 10.1016/S0167-7799(02)01943-1 (2002). [DOI] [PubMed] [Google Scholar]
- McCutcheon S. C. & Schnoor J. L. Phytoremediation: Transformation and Control of Contaminants. (Wiley, 2004). [Google Scholar]
- Bartrons M., Grimalt J. O., de Mendoza G. & Catalan J. Pollutant Dehalogenation Capability May Depend on the Trophic Evolutionary History of the Organism: PBDEs in Freshwater Food Webs. Plos ONE 7, e41829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivaiah S., Tozan Y. & Breman J. G. Dichlorodiphenyltrichloroethane (DDT) for Indoor Residual Spraying in Africa: How Can It Be Used for Malaria Control? Am. J. Trop. Med. Hyg. 77, 249–263 (2007). [PubMed] [Google Scholar]
- Qiu X., Zhu T., Yao B., Hu J. & Hu S. Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol 39, 4385–4390 (2005). [DOI] [PubMed] [Google Scholar]
- Tanabe S. Global environmental contamination by bioaccumulative endocrine disrupters. Jap. J. Limnol. 64, 225–237 (2003). [Google Scholar]
- Hellström A., Kylin H., Strachan W. M. J. & Jensen S. Distribution of some organochlorine compounds in pine needles from Central and Northern Europe. Environ. Pollut. 128, 29–48, doi: 10.1016/j.envpol.2003.08.033 (2004). [DOI] [PubMed] [Google Scholar]
- Lohmann R., Breivik K., Dachs J. & Muir D. Global fate of POPs: Current and future research directions. Environ. Pollut. 150, 150–165, doi: 10.1016/j.envpol.2007.06.051 (2007). [DOI] [PubMed] [Google Scholar]
- Dalla Valle M., Codato E. & Marcomini A. Climate change influence on POPs distribution and fate: A case study. Chemosphere 67, 1287–1295 (2007). [DOI] [PubMed] [Google Scholar]
- Shunthirasingham C. et al. Current-use pesticide transport to Costa Rica’s high-altitude tropical cloud forest. Environ. Toxicol. Chem. 30, 2709–2717, doi: 10.1002/etc.671 (2011). [DOI] [PubMed] [Google Scholar]
- IPCC. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 1535 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013).
- Nizzetto L. & Perlinger J. A. Climatic, Biological, and Land Cover Controls on the Exchange of Gas-Phase Semivolatile Chemical Pollutants between Forest Canopies and the Atmosphere. Environ. Sci. Technol. 46, 2699–2707, doi: 10.1021/es2036527 (2012). [DOI] [PubMed] [Google Scholar]
- Cabrerizo A., Dachs J., Barcelo D. & Jones K. C. Climatic and Biogeochemical Controls on the Remobilization and Reservoirs of Persistent Organic Pollutants in Antarctica. Environ. Sci. Technol. 47, 4299–4306, doi: 10.1021/es400471c (2013). [DOI] [PubMed] [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G. & Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978, doi: 10.1002/joc.1276 (2005). [DOI] [Google Scholar]
- Brohan P., Kennedy J. J., Harris I., Tett S. F. B. & Jones P. D. Uncertainty estimates in regional and global observed temperature changes: A new data set from 1850. J. Geophys. Res.: Atm. 111, D12106, doi: 10.1029/2005JD006548 (2006). [DOI] [Google Scholar]
- Gelman A. & Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. (Cambridge University Press, 2007). [Google Scholar]
- Bartrons M. et al. Spatial patterns reveal strong abiotic and biotic drivers of zooplankton community composition in Lake Mývatn, Iceland. Ecosphere 6, art105, doi:10.1890/ES14-00392.1 (2015). [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. (L. Erlbaum Associates, 1988). [Google Scholar]
- Core Team. R. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. (2013). [Google Scholar]
- Hijmans R. J. raster: Geographic data analysis and modeling. R package version 2.4–15. http://CRAN.R-project.org/package=raster. (2015). [Google Scholar]
- Bates D., Maechler M., Bolker B. & Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. http://CRAN.R-project.org/package=lme4. doi: citeulike-article-id:7112638 (2014). [Google Scholar]
- Bivand R., Keitt T. & Rowlingson B. rgdal: Bindings for the Geospatial Data Abstraction Library. R package version 0.9-03. http://CRAN.R-project.org/package=rgdal (2015). [Google Scholar]
- Bivand R. & Lewin-Koh N. maptools: Tools for reading and handling spatial objects. R package version 0.8-36. http://CRAN.R-project.org/package=maptools (2015). [Google Scholar]
- Gerritsen H. mapplots: Data visualisation on maps. R package version 1.5. http://CRAN.R-project.org/package=mapplots (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.