Abstract
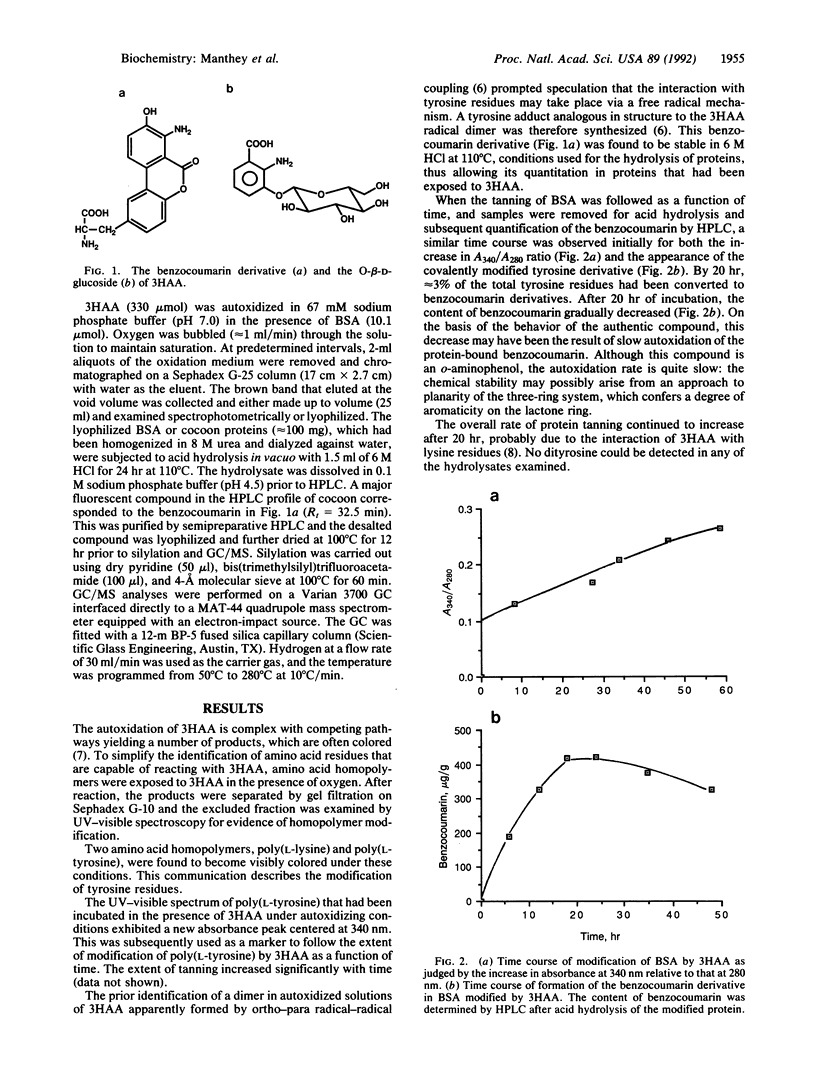
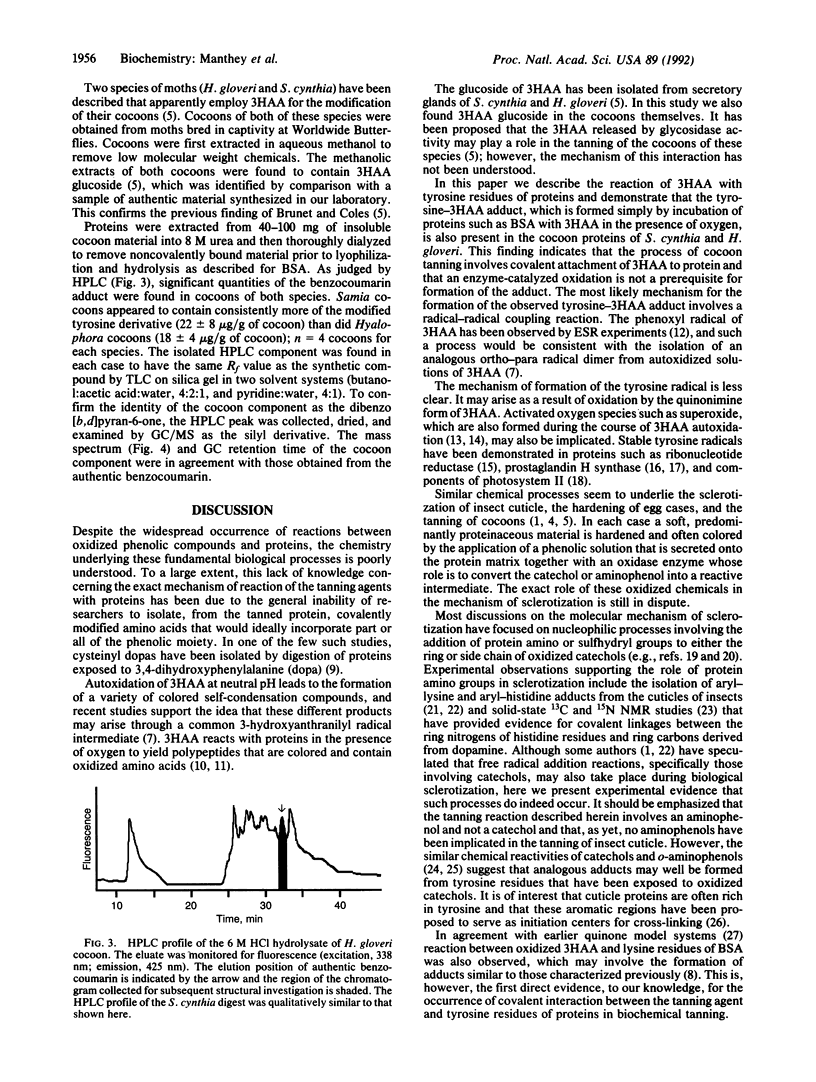
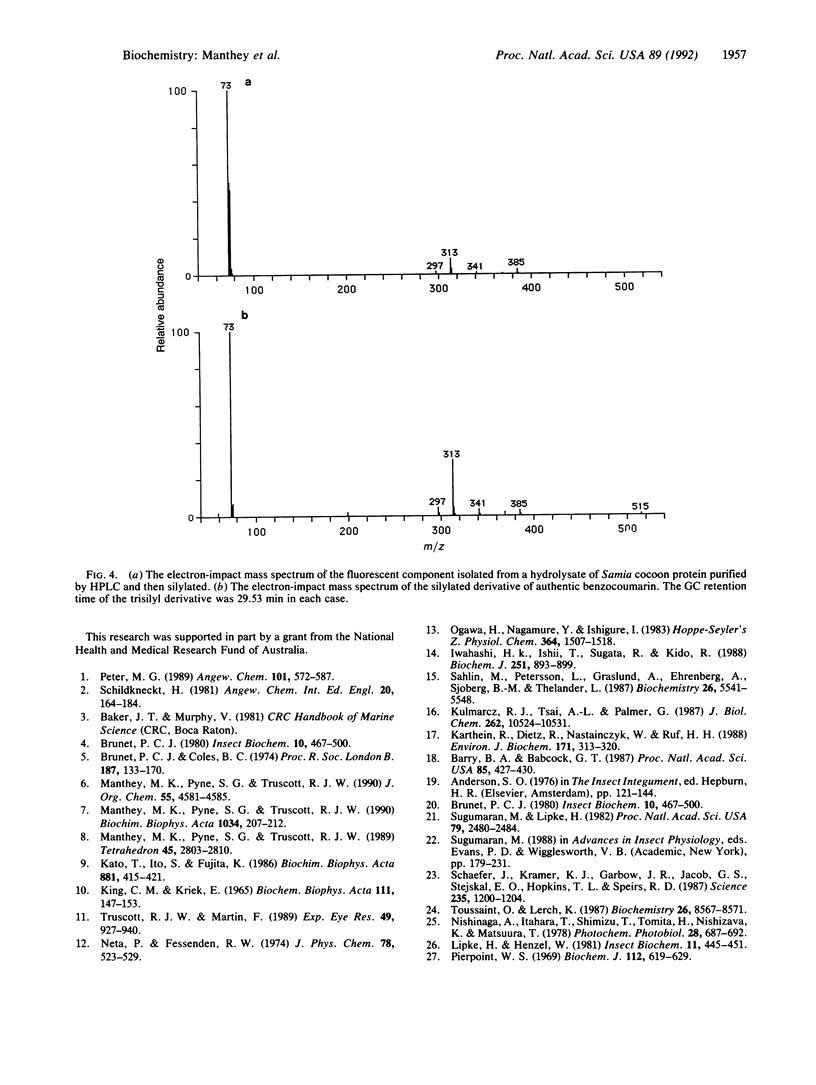
The binding of oxidized phenolic compounds to proteins is of importance in a number of biological systems, including the sclerotization of insect cuticle and the tanning of cocoons. 3-Hydroxyanthranilic acid (3HAA), an aminophenol, is a tryptophan metabolite that undergoes autoxidation readily, and proteins incubated in the presence of 3HAA and oxygen become colored and oxidized. Some moth species are thought to employ this reactivity of 3HAA with proteins for the tanning of cocoons, but the detailed mechanism of this process has not been studied previously. We show that one reaction pathway involves the covalent coupling of 3HAA with tyrosine to form a benzocoumarin derivative, a dibenzo[b,d]pyran-6-one. The stability of the benzocoumarin to conditions of acid hydrolysis normally used for protein digestion has enabled the isolation of the tyrosine adduct from bovine serum albumin that had been incubated with 3HAA. The adduct was also isolated from cocoons of Samia cynthia and Hyalophora gloveri, two species of moths reported to utilize 3HAA for cocoon tanning. These findings indicate that one mechanism of interaction of 3HAA with proteins involves a radical-radical coupling with tyrosine residues.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunet P. C., Coles B. C. Tanned silks. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):133–170. doi: 10.1098/rspb.1974.0067. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi H., Ishii T., Sugata R., Kido R. Superoxide dismutase enhances the formation of hydroxyl radicals in the reaction of 3-hydroxyanthranilic acid with molecular oxygen. Biochem J. 1988 May 1;251(3):893–899. doi: 10.1042/bj2510893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthein R., Dietz R., Nastainczyk W., Ruf H. H. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur J Biochem. 1988 Jan 15;171(1-2):313–320. doi: 10.1111/j.1432-1033.1988.tb13792.x. [DOI] [PubMed] [Google Scholar]
- Kato T., Ito S., Fujita K. Tyrosinase-catalyzed binding of 3,4-dihydroxyphenylalanine with proteins through the sulfhydryl group. Biochim Biophys Acta. 1986 May 2;881(3):415–421. doi: 10.1016/0304-4165(86)90034-6. [DOI] [PubMed] [Google Scholar]
- King C. M., Kriek E. The differential reactivity of the oxidation products of o-aminophenols towards protein and nucleic acid. Biochim Biophys Acta. 1965 Nov 15;111(1):147–153. doi: 10.1016/0304-4165(65)90480-0. [DOI] [PubMed] [Google Scholar]
- Kulmacz R. J., Tsai A. L., Palmer G. Heme spin states and peroxide-induced radical species in prostaglandin H synthase. J Biol Chem. 1987 Aug 5;262(22):10524–10531. [PubMed] [Google Scholar]
- Manthey M. K., Pyne S. G., Truscott R. J. Mechanism of reaction of 3-hydroxyanthranilic acid with molecular oxygen. Biochim Biophys Acta. 1990 May 16;1034(2):207–212. doi: 10.1016/0304-4165(90)90078-b. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Nagamura Y., Ishiguro I. Cinnabarinate formation in malpighian tubules of the silkworm. Bombyx mori: reaction mechanism of cinnabarinate formation in the presence of catalase and manganese ions. Hoppe Seylers Z Physiol Chem. 1983 Nov;364(11):1507–1518. doi: 10.1515/bchm2.1983.364.2.1507. [DOI] [PubMed] [Google Scholar]
- Pierpoint W. S. o-Quinones formed in plant extracts. Their reaction with bovine serum albumin. Biochem J. 1969 May;112(5):619–629. doi: 10.1042/bj1120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin M., Petersson L., Gräslund A., Ehrenberg A., Sjöberg B. M., Thelander L. Magnetic interaction between the tyrosyl free radical and the antiferromagnetically coupled iron center in ribonucleotide reductase. Biochemistry. 1987 Aug 25;26(17):5541–5548. doi: 10.1021/bi00391a049. [DOI] [PubMed] [Google Scholar]
- Schaefer J., Kramer K. J., Garbow J. R., Jacob G. S., Stejskal E. O., Hopkins T. L., Speirs R. D. Aromatic cross-links in insect cuticle: detection by solid-state 13C and 15N NMR. Science. 1987 Mar 6;235(4793):1200–1204. doi: 10.1126/science.3823880. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Lipke H. Crosslink precursors for the dipteran puparium. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2480–2484. doi: 10.1073/pnas.79.8.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint O., Lerch K. Catalytic oxidation of 2-aminophenols and ortho hydroxylation of aromatic amines by tyrosinase. Biochemistry. 1987 Dec 29;26(26):8567–8571. doi: 10.1021/bi00400a011. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Martin F. The reaction of proteins with 3-hydroxyanthranilic acid as a possible model for senile nuclear cataract in man. Exp Eye Res. 1989 Dec;49(6):927–940. doi: 10.1016/s0014-4835(89)80017-x. [DOI] [PubMed] [Google Scholar]


