SUMMARY
Anthropogenic stressors may influence hosts and their pathogens directly or may alter host–pathogen dynamics indirectly through interactions with other species. For example, in aquatic ecosystems, eutrophication may be associated with increased or decreased disease risk. Conversely, pathogens can influence community structure and function and are increasingly recognised as important members of the ecological communities in which they exist.
In outdoor mesocosms, we experimentally manipulated nutrients (nitrogen and phosphorus) and the presence of a fungal pathogen, Batrachochytrium dendrobatidis (Bd), and examined the effects on Bd abundance on larval amphibian hosts (Pseudacris regilla: Hylidae), amphibian traits and community dynamics. We predicted that resource supplementation would mitigate negative effects of Bd on tadpole growth and development and that indirect effects of treatments would propagate through the community.
Nutrient additions caused changes in algal growth, which benefitted tadpoles through increased mass, development and survival. Bd-exposed tadpoles metamorphosed sooner than unexposed individuals, but their mass at metamorphosis was not affected by Bd exposure. We detected additive rather than interactive effects of nutrient supplementation and Bd in this experiment.
Nutrient supplementation was not a significant predictor of infection load of larval amphibians. However, a structural equation model revealed that resource supplementation and exposure of amphibians to Bd altered the structure of the aquatic community. This is the first demonstration that sublethal effects of Bd on amphibians can alter aquatic community dynamics.
Keywords: Batrachochytrium dendrobatidis, eutrophication, food web, pathogen, trophic cascade
Introduction
The emergence of infectious diseases in wildlife threatens global biodiversity (Daszak, Cunningham & Hyatt, 2000). Environmental stressors such as habitat loss, climate change and contamination may interact with infectious diseases by directly affecting host and pathogen physiology, or through indirect mechanisms such as interactions with other species (Smith, Acevedo-Whitehouse & Pedersen, 2009). The effects of emerging infectious pathogens on hosts are typically investigated in laboratory-based studies, and while these studies are useful for determining susceptibility of hosts, they typically ignore direct and indirect influences of other biotic and abiotic factors. Furthermore, through their effects on hosts, infectious agents can have important effects on the structure and function of ecosystems (reviewed in Hatcher, Dick & Dunn, 2012), but as most laboratory-based studies exclude members of the community other than the host and the pathogen, they cannot detect these effects.
Human-induced modifications of the environment, such as eutrophication due to increased nutrient loading, can have profound effects on community dynamics including interactions between hosts and their pathogens (Johnson et al., 2010). At low to moderate levels, nutrient loading is generally positively associated with disease risk (Johnson & Carpenter, 2008). Nutrient supplementation may promote disease by directly influencing abundance, virulence and survival of disease agents (Smith & Schindler, 2009). Eutrophication may also promote infection through effects on host abundance and susceptibility. By definition, hosts and parasites share common resources, and parasites must compete effectively to obtain the resources needed to sustain themselves, complete their life cycle and avoid host immune responses (Hall et al., 2007, 2010; Smith, 2007). The availability of nutrients can have considerable effects on the host’s ability to mount these costly responses (Smith, Jones & Smith, 2005). In addition to direct effects on pathogens and hosts, eutrophication may also affect disease risk through indirect pathways, such as intermediate hosts or vectors, or trophic interactions (Lafferty & Holt, 2003; McKenzie & Townsend, 2007). For example, eutrophication can provide increased resources for growth and reproduction of snails, the first intermediate host of a trematode parasite, leading to increased density of infected snails and increased per capita rate of production of free-swimming stages which infect amphibians (Johnson et al., 2007; Rohr et al., 2008).
The emerging infectious disease chytridiomycosis, caused by the fungal pathogen Batrachochytrium dendrobatidis (hereafter Bd), has propelled amphibians to the forefront of the biodiversity crisis (Stuart et al., 2004; Skerratt et al., 2007; Wake & Vredenburg, 2008). The infective stage of the fungus, an aquatic flagellate zoospore, infects keratinised tissues of amphibian hosts, including mouthparts of larval amphibians. This hinders the efficiency with which infected larvae feed on periphyton (including attached algal particles, detritus and other organic matter) (Venesky, Parris & Storfer, 2009; Venesky, Wassersug & Parris, 2010), which can lead to reduced growth and slower developmental rates (e.g. Parris & Baud, 2004; Parris & Cornelius, 2004). Buck et al. (2012, 2015) suggested that larval amphibians also compete with zooplankton for suspended algal particles (phytoplankton), and this pattern may be augmented by Bd-induced mouthpart damage. Sublethal effects of Bd on larval amphibians suggest the potential for direct and indirect interactions between Bd and other members of the aquatic community.
We investigated the effects of nutrient supplementation on host–pathogen dynamics of Bd within a community context. In outdoor mesocosms containing tadpoles and a complex aquatic community, we manipulated nutrients (nitrogen and phosphorus) and the presence of the pathogen. Nutrient supplementation could exacerbate the effects of Bd on amphibians if (i) direct or indirect benefits to the pathogen or an increase in host survival increased the duration of infectivity and/or transmission rate of the pathogen (Johnson et al., 2010) or (ii) a nutrient-induced phytoplankton bloom shaded periphyton (Rohr et al., 2008; Staley, Rohr & Harwood, 2011; Halstead et al., 2014), a primary resource of scraping tadpoles. Alternatively, nutrient supplementation could mitigate pathological effects on amphibians if (iii) a nutrient-induced phytoplankton bloom tempered the effects of Bd by benefitting cladocerans, which have been shown to consume Bd zoospores (Buck, Truong & Blaustein, 2011; Searle et al., 2013), or 4) nutrient supplementation caused a phytoplankton bloom, minimising competitive effects between tadpoles and zooplankton for this shared resource (Wassersug, 1972; Altig, Whiles & Taylor, 2007; Whiles et al., 2010; Buck et al., 2011, 2015). We also investigated the effects of host–pathogen dynamics on other members of the aquatic community in the mesocosms.
Methods
In a completely randomised 2 × 2 factorial design in artificial ponds (mesocosms), we experimentally manipulated nitrogen and phosphorus concentrations and Bd. We crossed the presence or absence of added nutrients with the presence or absence of the pathogen. Each of the four treatments was replicated eight times for a total of 32 experimental units. The experiment was conducted at the Oregon State University Lewis-Brown Horticulture Research Farm near Corvallis, OR (68 m, Benton County, U.S.A.), and ran from 22 July 2011 to 30 September 2011. Thirty-two plastic tanks (94 cm L × 70 cm W × 33 cm H) were filled with ~120 L of tap water (pH = 8.0) on 1 July and were covered with weighted screen lids. On 5 July, we added 2 g of leaf litter to each tank for habitat heterogeneity. On the same day, we dosed half of the tanks with solutions of nitrogen (NH4NO3) and phosphorus (H3PO4) to achieve concentrations of 1800 μg L−1 and 200 μg L−1, respectively (Johnson et al., 2007). At the end of the experiment, a water sample of 10 mL was collected from each tank and pooled by nutrient treatment. Samples were frozen in pre-cleaned amber glass jars and shipped to Mississippi State Chemical Laboratory (Mississippi State, MS, U.S.A.) for independent analysis of nutrient concentrations. Results of these analyses indicated that nutrient-control pools had average concentrations of 510 μg L−1 N and 90 μg L−1 P and nutrient-treated pools had average concentrations of 540 μg L−1 N and 180 μg L−1 P. Thus, although the concentration of N was marginally higher in supplemented pools, our manipulation primarily elevated P concentrations. Environmental sampling has revealed nutrient concentrations as high as 2859 μg L−1 N and 348 μg L−1 P in eutrophic wetlands inhabited by amphibians, so nutrient concentrations in our experiment are ecologically relevant (Johnson et al., 2007). On 12 July, we inoculated each tank with 600 mL of water containing zooplankton, phytoplankton and periphyton from laboratory stocks. Tanks were checked after 7 days to confirm the presence of zooplankton.
Clutches of Pseudacris regilla eggs (23 masses) were collected on 23 June within 48 h of oviposition from a natural pond in the Cascade Mountains (Linn County, elevation = 1140 m). Pseudacris regilla is a typical pond-dwelling tadpole, possessing a generalised oral morphology that allows for scraping and suspension feeding on green algae, cyanobacteria, bacteria, diatoms, protozoa, and organic and inorganic debris (Wassersug, 1972, 1976). Eggs were hatched and tadpoles reared in outdoor holding tanks near the experimental site. Tadpoles were fed rabbit chow ad libitum. Bd exposure occurred in separate tanks before the experiment. On 6 July, tadpoles were split into two groups. Tadpoles in the Bd exposure group were exposed to Bd three times over a 17-day period (on 7 July, 13 July and 19 July). Bd was grown in pure culture on plastic Petri plates (10 cm diameter) with standard TGhL nutrient agar medium (Longcore, Pessier & Nichols, 1999). We inoculated plates with liquid culture of Bd isolate JEL 274, originally isolated from Anaxyrus boreas from Colorado, and incubated them at 22 °C for 8 days prior to use. Each plate was flooded with 15 mL of dechlorinated water and scraped after 5 min. The water from these plates was pooled, and Bd zoospore concentration was quantified with the use of a haemocytometer. The broth was then diluted using dechlorinated water and added to the Bd exposure tank to achieve an average zoospore concentration of 2.0 × 105 zoospores L−1. This dose is within the range of doses normally used to infect tadpoles in mesocosm and laboratory experiments (Searle et al., 2011; Buck et al., 2012, 2015; Hamilton, Richardson & Anholt, 2012). A broth containing water from flooded control plates was diluted and added to the Bd control tank. On 22 July (day 1 of the experiment), 40 tadpoles from either the Bd or the Bd-control exposure group were added to each experimental tank. The initial mass of the tadpoles (mean ± 1 SE) was 82.3 ± 8 mg, and their developmental stage (Gosner, 1960) was 25–27 (hind limb buds beginning to develop).
On day 20 of the experiment, ten tadpoles from each tank were haphazardly chosen, euthanised using an overdose of MS-222 and preserved in 90% ethanol. The mass and Gosner (Gosner, 1960) stage of these individuals was later measured. Following the methods of Boyle et al. (2004), we used real-time quantitative polymerase chain reaction (qPCR) to quantify the infection status of all Bd-exposed individuals (n = 160) and two randomly selected individuals from each control Bd tank (n = 32). Each sample was run in triplicate against a Bd standard titration from 10−1 to 102 zoospores. qPCR analysis was conducted on an Applied Biosystems StepOne Plus real-time PCR machine (Applied Biosystems, Inc., Waltham, Massachusetts, USA).
Remaining amphibians were removed from tanks upon emergence at Gosner (Gosner, 1960) stages 45–46 (metamorphosis). The first newly metamorphosed amphibian (metamorph) was observed on day 26. Following this initial observation, tanks were checked daily for metamorphs until the end of the experiment on day 71. Metamorphosed individuals were euthanised using an overdose of MS-222 and preserved in 90% ethanol for later measurement of mass. At the end of the experiment, all remaining individuals (~9% of all individuals added to mesocosms) were preserved, regardless of Gosner stage.
To determine how treatments affected the pond community, we sampled zooplankton, phytoplankton and periphyton on 28 and 29 July and every 2 weeks thereafter (four times total) following the methods of Buck et al. (2015). Briefly, zooplankton were sampled using a tube sampler and then filtered and preserved for later quantification. Chlorophyll a was extracted from phytoplankton samples, and fluorescence measurements were taken with a Turner Designs fluorometer (model TD-700; Sunnyvale, CA, U.S.A.). To quantify periphyton biomass, the periphyton was scraped from the outer side of a microscope slide mounted vertically in the tank, dried on pre-weighed filters and reweighed.
We deployed iButton temperature probes (Maxim, Sunnyvale, CA, U.S.A.) in 16 tanks at the beginning of the experiment. Each probe logged temperature every 4 h over the course of the experiment. Temperature measurements were averaged by week. Dissolved oxygen and pH measurements were taken using digital meters (Oakton Instruments, Vernon Hills, IL, U.S.A.) on day 14 of the experiment. ANOVAs were conducted to test for the effects of treatments on temperature, dissolved oxygen and pH.
Statistical analyses
Response variables for tadpoles included mass and developmental stage (Gosner, 1960). Response variables for metamorphs included mass, development (larval period) and survival to metamorphosis. We used linear mixed-effects models to determine the effects of nutrients, Bd exposure and their interaction on mass and development of amphibians. We used a Cox mixed-effects model to determine the effects of nutrients, Bd exposure and their interaction on survival of amphibians to metamorphosis. Individuals were nested by tank to avoid pseudoreplication. Mixed-effects models were constructed using the nlme and coxme packages in R version 3.0.2. (2013). We also conducted qPCR analysis on all 10 Bd-exposed animals from each tank that were preserved on day 20 of the experiment (n = 160), and two randomly selected unexposed animals from each tank (n = 32). We used an ANOVA to test for the effects of the nutrient treatment on infection load.
Abundance of cladocerans, abundance of phytoplankton and periphyton biomass were log-transformed to meet parametric assumptions. We performed a series of repeated-measures ANOVAs to determine the effects of nutrient supplementation, Bd and their interaction on community response variables. We used Mauchly’s test for sphericity to test the assumption that the variances of the differences between the repeated measurements were approximately the same, and we report Greenhouse-Geisser-corrected P-values. Repeated-measures ANOVAs were conducted using the ez package in R version 3.0.2. (2013).
We employed structural equation modelling (SEM) to test the effects of treatments on the aquatic community. SEM, which is a statistical technique based on the analysis of variance–covariance matrices (Grace, 2006), is well suited to testing web-like hypotheses because it allows variables to serve simultaneously as independent and dependent variables. For the aquatic community, measured variables included in our model were abundance of cladocerans, abundance of phytoplankton and biomass of periphyton, all measured on the last sampling occasion. All community variables were log-transformed to meet parametric assumptions. We constructed a full model, which included fourteen paths to be tested (a-n). A latent variable, tadpole performance, was associated with the indicator variables log mass, development (log larval period) and survival of amphibians to metamorphosis (arcsine square-root transformed). Using the lavaan package in R version 3.0.2 (2013), we tested among nineteen hypothesised path models that removed various sets of paths from the full model. These models were ranked using the corrected Akaike information criterion (AICc) using the AICcmodavg package in R version 3.0.2. (2013).
Results
We measured mass and quantified Gosner stage for the tadpoles preserved on day 20 of the experiment. Linear mixed-effects models revealed that mass of tadpoles was increased by nutrient supplementation (P < 0.001), but was not significantly affected by Bd exposure (P = 0.70) or the interaction between nutrients and Bd exposure (P = 0.71) (Table 1, Fig. 1a). Nutrient additions caused tadpoles to develop faster (P = 0.0088), but Gosner stage at day 20 was not significantly affected by Bd exposure (P = 0.31) or the interaction between nutrients and Bd exposure (P = 0.56) (Table 1, Fig. 1b).
Table 1.
Output of linear mixed-effects models on mass and developmental stage of tadpoles
| Coefficient | SE | d.f. | P-value | |
|---|---|---|---|---|
| Mass | ||||
| Nutrients | 0.16574 | 0.0410 | 28 | <0.001 |
| Bd | 0.01604 | 0.0410 | 28 | 0.70 |
| Nutrients*Bd | −0.02175 | 0.0580 | 28 | 0.71 |
| Development (Gosner stage) | ||||
| Nutrients | 0.03891 | 0.0138 | 28 | 0.0088 |
| Bd | 0.01481 | 0.0138 | 28 | 0.31 |
| Nutrients*Bd | 0.01152 | 0.0195 | 28 | 0.56 |
Fig. 1.
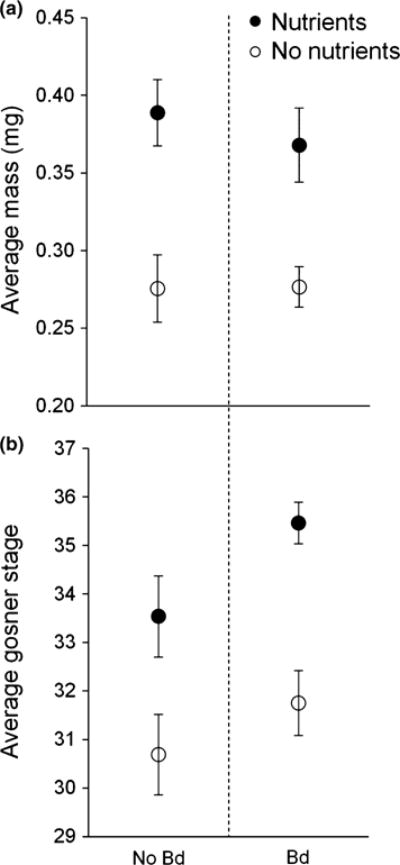
Response variables for tadpoles: (a) mass and (b) developmental stage of Pseudacris regilla tadpoles in the absence (open symbols) and presence (filled symbols) of added nutrients and not exposed (left) and exposed (right) to Bd. Values plotted are means ± SE (n = 32 tanks).
We measured mass, length of the larval period and percentage survival to metamorphosis for the individuals preserved at metamorphosis. Linear mixed-effects models revealed that mass of metamorphs was increased by nutrient supplementation (P < 0.001), but was not significantly affected by Bd exposure (P = 0.91) or the interaction between nutrients and Bd exposure (P = 0.24) (Table 2, Fig. 2a). The larval period of amphibians was reduced by nutrient supplementation (P < 0.001) and Bd exposure (P = 0.015), but was not significantly affected by their interaction (P = 0.88) (Table 2, Fig. 2b). Survival to metamorphosis was 76% overall and was increased by nutrient additions (P = 0.0095), but was not significantly affected by Bd exposure (P = 0.91) or the interaction between nutrients and Bd exposure (P = 0.54) (Table 2, Fig. 2c).
Table 2.
Output of linear mixed-effects models and a Cox mixed-effects model on mass, development and survival to metamorphosis
| Coefficient | SE | d.f. | P-value | |
|---|---|---|---|---|
| Mass | ||||
| Nutrients | 0.13947 | 0.02822 | 28 | <0.001 |
| Bd | 0.00308 | 0.02892 | 28 | 0.91 |
| Nutrients*Bd | −0.04757 | 0.03970 | 28 | 0.24 |
| Development (larval period) | ||||
| Nutrients | −0.05530 | 0.0144 | 28 | <0.001 |
| Bd | −0.03836 | 0.0148 | 28 | 0.015 |
| Nutrients*Bd | 0.00305 | 0.0202 | 28 | 0.88 |
|
| ||||
| Hazard ratio | SE | Z | P-value | |
|
| ||||
| Survival to metamorphosis | ||||
| Nutrients | 1.61 | 0.1830 | 2.59 | 0.0095 |
| Bd | 1.02 | 0.1866 | 0.11 | 0.91 |
| Nutrients*Bd | 1.17 | 0.2577 | 0.61 | 0.54 |
Fig. 2.
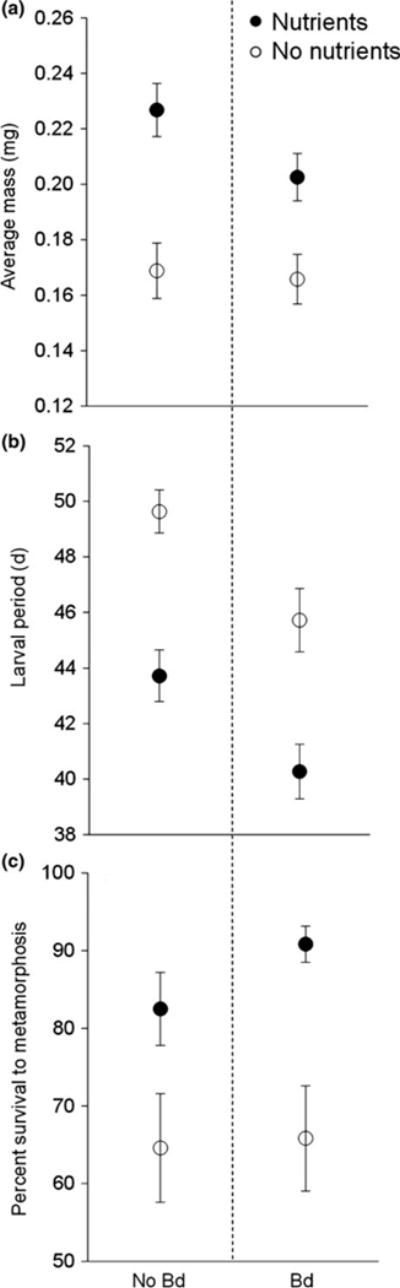
Response variables for metamorphs: (a) mass, (b) development and (c) survival of Pseudacris regilla tadpoles in the absence (open symbols) and presence (filled symbols) of added nutrients and not exposed (left) and exposed (right) to Bd. Values plotted are means ± 1 SE (n = 32 tanks).
qPCR analysis was conducted on all 10 Bd-exposed animals from each tank that were preserved on day 20 of the experiment (n = 160), and two randomly selected unexposed animals from each tank (n = 32). Seventy-eight of 160 (49%) Bd-exposed larvae were infected, and average infection intensity was 0.1–2.2 Bd genome equivalents. This infection intensity is low compared to infection loads detected in many laboratory studies (e.g. Searle et al., 2011), but is comparable to infection intensities observed in mesocosm experiments (Buck et al., 2012, 2015; Hamilton et al., 2012). All unexposed individuals that were tested were uninfected. Nutrient supplementation was not a significant predictor of infection status or load of amphibians. Bd-exposed tadpoles in nutrient-supplemented tanks (n = 80) had an average infection intensity of 0.68 ± 0.22 genome equivalents, and Bd-exposed tadpoles in non-supplemented tanks (n = 80) had an average infection intensity of 0.65 ± 0.17 genome equivalents.
We measured the abundance of cladocerans, the abundance of phytoplankton and the biomass of periphyton every 2 weeks over the course of the experiment. Repeated-measures ANOVAs revealed that the abundance of cladocerans fluctuated over time (P < 0.001), but was not significantly affected by nutrient additions (P = 0.58) or Bd exposure status of amphibians (P = 0.36) (Table 3, Fig. 3a). Neither phytoplankton nor periphyton was significantly affected by the Bd treatment, but both were affected by the interaction of time and nutrient additions (Table 3). Relative to control tanks, phytoplankton bloomed in nutrient-supplemented tanks in week 2 and then subsided for the rest of the experiment (Fig. 3b), whereas periphyton biomass in nutrient-supplemented tanks exhibited the opposite temporal pattern, with low levels early and a peak at the end of the experiment (Fig. 3c). In nutrient-supplemented tanks, periphyton biomass was negatively associated with larval period of the tadpoles (R2 = 0.25, Fig. 4).
Table 3.
Output of repeated-measures ANOVAs on cladoceran abundance, phytoplankton concentration and periphyton biomass. Included are all main effects and any significant interactions
| DFn | DFd | F | P-value | |
|---|---|---|---|---|
| Cladoceran abundance | ||||
| Nutrients | 1 | 28 | 0.32 | 0.58 |
| Bd | 1 | 28 | 0.86 | 0.36 |
| Time | 3 | 84 | 11.71 | <0.001 |
| Phytoplankton concentration | ||||
| Nutrients | 1 | 28 | 0.10 | 0.76 |
| Bd | 1 | 28 | 3.86 | 0.06 |
| Time | 3 | 84 | 100.48 | <0.001 |
| Nutrients*Time | 3 | 84 | 17.36 | <0.001 |
| Periphyton biomass | ||||
| Nutrients | 1 | 28 | 8.02 | 0.008 |
| Bd | 1 | 28 | 0.48 | 0.49 |
| Time | 3 | 84 | 19.19 | <0.001 |
| Nutrients*Time | 3 | 84 | 9.31 | <0.001 |
Fig. 3.
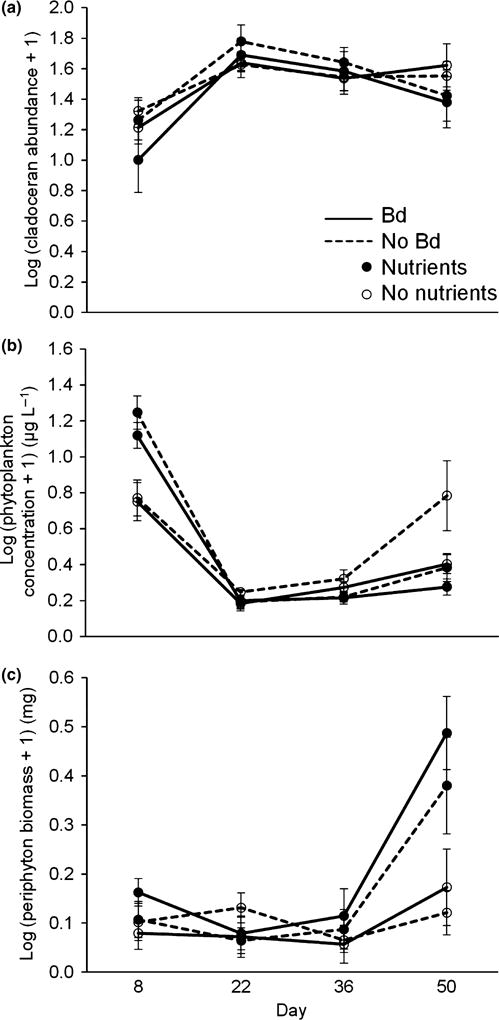
Community response variables: (a) log cladoceran abundance, (b) log phytoplankton concentration and (c) log periphyton biomass in the absence (open symbols) and presence (filled symbols) of nutrient additions and the absence (dashed lines) and presence (solid lines) of Bd over the four sampling periods. Values plotted are means ± 1 SE (n = 32 tanks).
Fig. 4.
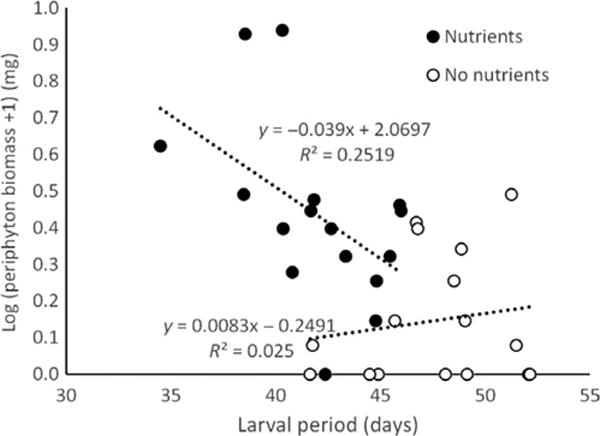
Correlation between larval period and periphyton biomass at the end of the experiment in the absence (open symbols) and presence (filled symbols) of nutrient additions.
Our structural equation model (Fig. 5a) confirmed the effects of nutrients (P < 0.001) and Bd (P = 0.008) on tadpole performance. Based on AICc, the SEM with the greatest support (Table 4, Fig. 5b) included a positive association between nutrients and periphyton (P < 0.001) and a negative association between nutrients and phytoplankton (P = 0.013). Tanks with Bd-exposed tadpoles had less phytoplankton at the end of the experiment than tanks with unexposed tadpoles (P = 0.021). We also detected negative associations between cladocerans and periphyton (P = 0.011) and between tadpoles and cladocerans (P = 0.028). This model accounted for 75% of the model weights.
Fig. 5.
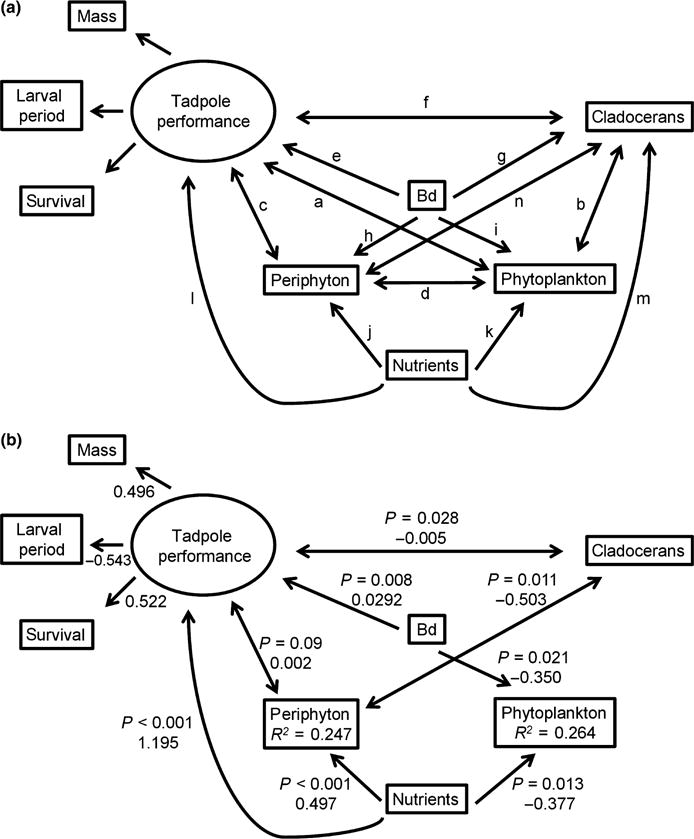
(a) Full model depicting the latent variable tadpole predation and competition pressure and paths a-m to be tested. (b) Best model for the end of the experiment (based on corrected Akaike information criterion) showing positive effects of nutrients on periphyton and amphibians and a negative effect of Bd-infected tadpoles on phytoplankton. P-values and standardised coefficients are shown next to each path. R2 values indicate total variance explained by predictor variables.
Table 4.
Comparison of the 19 competing models used to explain interactions among Pseudacris regilla tadpoles, Batrachochytrium dendrobatidis, zooplankton, periphyton, phytoplankton and nutrients. See Fig. 5a to match letters with paths
| Model | Paths | AICc | Δ AIC | ω |
|---|---|---|---|---|
| 1 | c,e-f,i-l,n | −90.44 | 0.00 | 0.75 |
| 2 | a,e-f,i-l,n | −87.03 | 3.41 | 0.14 |
| 3 | a,c,e-f,i-l,n | −85.32 | 5.12 | 0.06 |
| 4 | a,c,e,i-l,n | −85.18 | 5.26 | 0.05 |
| 5 | a,c-f,i-l,n | −79.71 | 10.73 | 0.00 |
| 6 | a-c,e-f,i-l,n | −79.54 | 10.90 | 0.00 |
| 7 | a-f,i-l,n | −73.23 | 17.21 | 0.00 |
| 8 | a-f,i-l | −70.81 | 19.63 | 0.00 |
| 9 | a-f,i-n | −67.74 | 22.70 | 0.00 |
| 10 | a-f,h-l,n | −67.70 | 22.74 | 0.00 |
| 11 | a-f,h-n | −61.23 | 29.21 | 0.00 |
| 12 | a-g,i-n | −60.03 | 30.42 | 0.00 |
| 13 | a-l,n | −59.44 | 31.00 | 0.00 |
| 14 | a-h,j-n | −56.31 | 34.13 | 0.00 |
| 15 | a-d,f-n | −55.96 | 34.48 | 0.00 |
| 16 | a-j,l-n | −55.59 | 34.85 | 0.00 |
| 17 | a-n | −51.79 | 38.65 | 0.00 |
| 18 | a-i,k-n | −48.24 | 42.20 | 0.00 |
| 19 | a-k,m-n | −14.30 | 76.14 | 0.00 |
Average weekly temperatures in the experimental tanks ranged from 13.7 to 20.5 °C, which is suitable for the growth of Bd (Piotrowski, Annis & Longcore, 2004). Dissolved oxygen and pH ranged from 7.0 to 8.3 ppm and from 7.9 to 8.0, respectively. Separate ANOVAs indicated that temperature, dissolved oxygen and pH were not affected by treatments.
Discussion
The addition of nitrogen and phosphorus to experimental mesocosms caused changes in algal growth. A bloom of phytoplankton occurred within 2 weeks of nutrient additions. No such effect was observed for periphyton, possibly because of shading by phytoplankton (Rohr et al., 2008; Staley et al., 2011; Halstead et al., 2014), or because of suppression by tadpoles. By day 20 of the experiment, positive effects of nutrient supplementation on tadpole mass and development were evident. These effects were maintained through metamorphosis; amphibians from nutrient-supplemented tanks attained higher mass, metamorphosed faster and had higher survival rates than amphibians from control tanks. These results are in accordance with previous studies. Nutrient supplementation typically benefits larval amphibians by increasing the growth of periphyton (e.g. Leibold & Wilbur, 1992). Tadpoles may have also benefitted from the phytoplankton bloom that occurred early in the experiment. Like many species of rasping tadpoles, P. regilla incorporates significant amounts of suspended algal particles into its diet, in addition to attached algal particles scraped from surfaces (Wassersug, 1972, 1976; Altig et al., 2007; Whiles et al., 2010).
In contrast to nutrient supplementation, exposure to Bd did not detectably affect tadpole mass or development or other members of the aquatic community early in the experiment. qPCR analysis revealed that about half of Bd-exposed tadpoles were infected by day 20 of the experiment, but the infection had not yet progressed far enough to cause detectable changes in mass and development. However, by metamorphosis, the effects of Bd on development were evident, with Bd-exposed tadpoles metamorphosing sooner than unexposed individuals, even though mass was not affected by Bd exposure. Although qPCR analysis detected infections in only approximately half of the tadpoles exposed to Bd, previous studies have shown that exposure can incur costs, even in the absence of detectable infections (Rohr et al., 2013), and that Bd exposure may be a better predictor of effects than Bd load (Hanlon et al., 2015). In response to Bd infection, larval amphibians generally delay metamorphosis (Parris & Baud, 2004; Parris & Cornelius, 2004). The mechanism proposed for such a delay is that Bd causes mouthpart deformities that reduce larval feeding efficiency (Venesky et al., 2009, 2010), thus reducing growth and delaying metamorphosis. While it is possible that Bd infection damaged mouthparts, making suspension feeding more obligatory than facultative, it is unlikely that this damage could cause the observed accelerated metamorphosis without a reduction in body size.
An alternative explanation for the earlier metamorphosis of Bd-exposed amphibians is that Bd exposure stressed the tadpoles. Unfavourable conditions or high mortality risk in the larval environment often induces developmental plasticity in amphibians, including earlier metamorphosis (Newman, 1992). Plasticity of amphibian development in response to pathogen exposure has been documented for ranavirus (Warne, Crespi & Brunner, 2011) and the water mould Saprolegnia (Touchon, Gomez-Mestre & Warkentin, 2006; Uller, Sagvik & Olsson, 2009). Bd infection is associated with (Gabor, Fisher & Bosch, 2013; Peterson et al., 2013) and may cause (Searle et al., 2014) elevated corticosterone levels in tadpoles, and this stress hormone can accelerate metamorphosis (Denver, 2009). Although Bd was associated with accelerated metamorphosis, it was not accompanied by smaller body size, suggesting that this hypothesis is also incapable of fully explaining the observed patterns.
It is possible that with increased statistical power, we would have detected Bd-induced changes in mass in addition to changes in development. However, the most parsimonious explanation for accelerated metamorphosis of Bd-exposed amphibians without accompanying changes in body size might be consumption of Bd zoos-pores by tadpoles. Facultative suspension-feeding tadpoles, including Hylids, have been shown to filter Bd zoospores from the water column (Venesky et al., 2014). Zoospores, which are rich in nutrients (Gleason et al., 2008), may represent a food resource that might have accelerated amphibian metamorphosis and caused a shift to increased suspension feeding. Fungi are a common food item of tadpoles (e.g. Altig et al., 2007). Thus, tadpoles might incorporate Bd into their diet because of phylogenetic inertia, in spite of the fact that this would increase contact rates between zoospores and keratinised mouthparts, increasing infection risk. This evolutionary trap hypothesis might explain why so many amphibians are infected with Bd and certainly warrants further exploration. However, this explanation assumes that sufficient quantities of zoospores were shed by infected tadpoles to serve as a resource.
Tadpoles in our experiment were exposed to Bd in separate tanks before the start of the experiment, and Bd was not added directly to experimental tanks. Therefore, any associations between Bd and other members of the aquatic community must be indirect, through its effects on amphibians. The effects of Bd on tadpoles probably explain the negative association between Bd and phytoplankton in our structural equation model. Bd-exposed tadpoles may consume more phytoplankton than unexposed tadpoles (Buck et al., 2012, 2015) because nutritious zoospores cause a shift to increased suspension feeding, or because the infection reduces feeding efficiency on attached algal particles (Venesky et al., 2009, 2010), forcing a shift to suspended algal particles. Indirect effects through amphibians may also explain the negative association between nutrients and phytoplankton detected in our structural equation model. Tadpoles in the nutrient supplementation treatment exhibited increased mass, development and survival, increasing their ability to consume algal resources, and this may have led to the negative association.
We did not detect any response by cladoceran populations to the phytoplankton bloom that occurred in nutrient-supplemented tanks near the beginning of the experiment. This result counters findings from the previous studies (Leibold & Wilbur, 1992; McMahon et al., 2012; Halstead et al., 2014). However, it is possible that larval amphibians suppressed cladoceran populations indirectly through competition for shared phytoplankton resources, as previously suggested by Buck et al. (2012, 2015). Indeed, we detected a negative association between tadpole performance and cladocerans at the end of the experiment. Competition between cladocerans and tadpoles for phytoplankton could also explain the negative association between cladocerans and periphyton at the end of the experiment: below a certain concentration of suspended algal particles, Daphnia may switch to periphyton as an alternative food source (Siehoff et al., 2009).
We also detected a periphyton bloom in nutrient-supplemented tanks at the end of the experiment. The negative correlation between larval period and periphyton biomass in nutrient-supplemented tanks suggests that this was caused by earlier metamorphosis of amphibians in nutrient-supplemented tanks and continued consumption pressure from amphibians in non-supplemented tanks.
Our results show that nutrient supplementation increased algal growth and induced higher rates of growth and development in larval amphibians, consistent with the previous studies (e.g. Halstead et al., 2014). Exposure to Bd increased development, but not mass, of amphibians, which may have been caused by a shift to a diet rich in suspended particles including algae and Bd zoospores. We hypothesised that resource supplementation would minimise negative effects of Bd on amphibians, but we did not find interactive effects of the treatments in this experiment. Instead, resource supplementation and exposure of amphibians to Bd altered the structure of the aquatic community. Pathogens and parasites are integral members of ecosystems and have been shown to play an important role in shaping community structure and function (reviewed in Hatcher et al., 2012). For example, Bd-induced population declines of amphibians have altered communities of primary producers (Connelly et al., 2008) and have even caused changes in nitrogen cycling (Whiles et al., 2013) in Neotropical streams. However, these effects are due to extinction of tadpoles (dominant grazers) in these streams, rather than sublethal effects of the pathogen on amphibians. Although previous studies have shown that Bd exposure alters the consumption of algal resources by tadpoles (Venesky et al., 2009, 2010), suggesting the potential for effects on the community, ours is the first study to demonstrate that sublethal effects of the pathogen on amphibians can alter aquatic community dynamics.
Acknowledgments
We thank E. Hunt and P. Buck for field assistance. Members of the Blaustein laboratory provided advice regarding experimental design and execution, data analysis, and comments on the manuscript. We also thank the R. Tanguay, PISCO and S. Hacker laboratories, and E. Scheessele for the use of equipment and protocol, and S. Robbins and D. Hinds-Cook for assistance at the Horticulture Farm. This material is based upon work supported by the National Science Foundation under Grant No. 1210520. Supplementary funding was provided by the OSU Zoology Research Fund and the Society of Wetland Scientists.
References
- Altig R, Whiles MR, Taylor CL. What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshwater Biology. 2007;52:386–395. [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Buck JC, Scheessele EA, Relyea RA, Blaustein AR. The effects of multiple stressors on wetland communities: pesticides, pathogens and competing amphibians. Freshwater Biology. 2012;57:61–73. [Google Scholar]
- Buck JC, Scholz KI, Rohr JR, Blaustein AR. Trophic dynamics in an aquatic community: interactions among primary producers, grazers, and a pathogenic fungus. Oecologia. 2015;178:239–248. doi: 10.1007/s00442-014-3165-6. [DOI] [PubMed] [Google Scholar]
- Buck JC, Truong L, Blaustein AR. Predation by zooplankton on Batrachochytrium dendrobatidis: biological control of the deadly amphibian chytrid fungus? Biodiversity and Conservation. 2011;20:3549–3553. [Google Scholar]
- Connelly S, Pringle CM, Bixby RJ, Brenes R, Whiles MR, Lips KR, et al. Changes in stream primary producer communities resulting from large-scale catastrophic amphibian declines: can small-scale experiments predict effects of tadpole loss? Ecosystems. 2008;11:1262–1276. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Wildlife ecology - Emerging infectious diseases of wildlife -Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Stress hormones mediate environment-genotype interactions during amphibian development. General and Comparative Endocrinology. 2009;164:20–31. doi: 10.1016/j.ygcen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Fisher MC, Bosch J. A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLoS ONE. 2013;8:e56054. doi: 10.1371/journal.pone.0056054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason FH, Kagami M, Lefevre E, Sime-Ngando T. The ecology of chytrids in aquatic ecosystems: roles in food web dynamics. Fungal Biology Reviews. 2008;22:17–25. [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Grace JB. Structural Equation Modeling and Natural Systems. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Hall SR, Becker CR, Duffy MA, Caceres CE. Variation in resource acquisition and use among host clones creates key epidemiological trade-offs. American Naturalist. 2010;176:557–565. doi: 10.1086/656523. [DOI] [PubMed] [Google Scholar]
- Hall SR, Leibold MA, Lytle DA, Smith VH. Grazers, producer stoichiometry, and the light: nutrient hypothesis revisited. Ecology. 2007;88:1142–1152. doi: 10.1890/06-0923. [DOI] [PubMed] [Google Scholar]
- Halstead NT, McMahon TA, Johnson SA, Raffel TR, Romansic JM, Crumrine PW, et al. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters. 2014;17:932–941. doi: 10.1111/ele.12295. [DOI] [PubMed] [Google Scholar]
- Hamilton PT, Richardson JML, Anholt BR. Daphnia in tadpole mesocosms: trophic links and interactions with Batrachochytrium dendrobatidis. Freshwater Biology. 2012;57:676–683. [Google Scholar]
- Hanlon SM, Lynch KJ, Kerby J, Parris MJ. Batrachochytrium dendrobatidis exposure effects on foraging efficiencies and body size in anuran tadpoles. Diseases of Aquatic Organisms. 2015;112:237–242. doi: 10.3354/dao02810. [DOI] [PubMed] [Google Scholar]
- Hatcher MJ, Dick JTA, Dunn AM. Diverse effects of parasites in ecosystems: linking interdependent processes. Frontiers in Ecology and the Environment. 2012;10:186–194. [Google Scholar]
- Johnson PTJ, Carpenter SR. Influence of eutrophication on disease in aquatic ecosystems: patterns, processes, and predictions. In: Ostfeld R, Keesing F, Eviner V, editors. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton University Press; Princeton, NJ: 2008. pp. 71–99. [Google Scholar]
- Johnson PTJ, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, et al. Aquatic eutrophication promotes pathogenic infection in amphibians. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Townsend AR, Cleveland CC, Glibert PM, Howarth RW, McKenzie VJ, et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecological Applications. 2010;20:16–29. doi: 10.1890/08-0633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Holt RD. How should environmental stress affect the population dynamics of disease? Ecology Letters. 2003;6:654–664. [Google Scholar]
- Leibold M, Wilbur H. Interactions between food-web structure and nutrients on pond organisms. Nature. 1992;360:341–343. [Google Scholar]
- Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- McKenzie VJ, Townsend AR. Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth. 2007;4:384–396. [Google Scholar]
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, et al. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecology Letters. 2012;15:714–722. doi: 10.1111/j.1461-0248.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- Newman R. Adaptive plasticity in amphibian metamorphosis. BioScience. 1992;42:671–678. [Google Scholar]
- Parris MJ, Baud DR. Interactive effects of a heavy metal and chytridiomycosis on gray treefrog larvae (Hyla chrysoscelis) Copeia. 2004;2004:344–350. [Google Scholar]
- Parris MJ, Cornelius TO. Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology. 2004;85:3385–3395. [Google Scholar]
- Peterson JD, Steffen JE, Reinert LK, Cobine PA, Appel A, Rollins-Smith L, et al. Host stress response is important for the pathogenesis of the deadly amphibian disease, chytridiomycosis, in Litoria caerulea. PLoS ONE. 2013;8:e62146. doi: 10.1371/journal.pone.0062146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9–15. [PubMed] [Google Scholar]
- R Development Core Team. Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, et al. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–U50. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Searle CL, Belden LK, Du P, Blaustein AR. Stress and chytridiomycosis: exogenous exposure to corticosterone does not alter amphibian susceptibility to a fungal pathogen. Journal of Experimental Zoology Part a-Ecological Genetics and Physiology. 2014;321:243–253. doi: 10.1002/jez.1855. [DOI] [PubMed] [Google Scholar]
- Searle CL, Gervasi SS, Hua J, Hammond JI, Relyea RA, Olson DH, et al. Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conservation Biology. 2011;25:965–974. doi: 10.1111/j.1523-1739.2011.01708.x. [DOI] [PubMed] [Google Scholar]
- Searle CL, Mendelson JR, Green LE, Duffy MA. Daphnia predation on the amphibian chytrid fungus and its impacts on disease risk in tadpoles. Ecology and Evolution. 2013;3:4129–4138. doi: 10.1002/ece3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehoff S, Hammers-Wirtz M, Strauss T, Ratte HT. Periphyton as alternative food source for the filter-feeding cladoceran Daphnia magna. Freshwater Biology. 2009;54:15–23. [Google Scholar]
- Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- Smith KF, Acevedo-Whitehouse K, Pedersen AB. The role of infectious diseases in biological conservation. Animal Conservation. 2009;12:1–12. [Google Scholar]
- Smith V. Host resource supplies influence the dynamics and outcome of infectious disease. Integrative and Comparative Biology. 2007;47:310–316. doi: 10.1093/icb/icm006. [DOI] [PubMed] [Google Scholar]
- Smith VH, Jones TP, Smith MS. Host nutrition and infectious disease: an ecological view. Frontiers in Ecology and the Environment. 2005;3:268–274. doi: 10.1890/1540-9295(2005)003[0029:ETTEID]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VH, Schindler DW. Eutrophication science: where do we go from here? Trends in Ecology & Evolution. 2009;24:201–207. doi: 10.1016/j.tree.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Staley ZR, Rohr JR, Harwood VJ. Test of direct and indirect effects of agrochemicals on the survival of fecal indicator bacteria. Applied and Environmental Microbiology. 2011;77:8765–8774. doi: 10.1128/AEM.06044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Touchon JC, Gomez-Mestre I, Warkentin KM. Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2006;84:556–563. [Google Scholar]
- Uller T, Sagvik J, Olsson M. Pre-hatching exposure to water mold reduces size at metamorphosis in the moor frog. Oecologia. 2009;160:9–14. doi: 10.1007/s00442-009-1280-6. [DOI] [PubMed] [Google Scholar]
- Venesky MD, Liu X, Sauer EL, Rohr JR. Linking manipulative experiments to field data to test the dilution effect. Journal of Animal Ecology. 2014;83:557–565. doi: 10.1111/1365-2656.12159. [DOI] [PubMed] [Google Scholar]
- Venesky MD, Parris MJ, Storfer A. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. EcoHealth. 2009;6:565–575. doi: 10.1007/s10393-009-0272-7. [DOI] [PubMed] [Google Scholar]
- Venesky MD, Wassersug RJ, Parris MJ. Fungal pathogen changes the feeding kinematics of larval anurans. Journal of Parasitology. 2010;96:552–557. doi: 10.1645/GE-2353.1. [DOI] [PubMed] [Google Scholar]
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne RW, Crespi EJ, Brunner JL. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Functional Ecology. 2011;25:139–146. [Google Scholar]
- Wassersug R. The mechanism of ultraplanktonic entrapment in anuran larvae. Journal of Morphology. 1972;137:279–287. doi: 10.1002/jmor.1051370303. [DOI] [PubMed] [Google Scholar]
- Wassersug RJ. Internal oral features in Hyla regilla (Anura: Hylidae) larvae: an ontogenic study. Occasional Papers of the Museum of Natural History. 1976;49:1–24. [Google Scholar]
- Whiles MR, Gladyshev MI, Sushchik NN, Makhutova ON, Kalachova GS, Peterson SD, et al. Fatty acid analyses reveal high degrees of omnivory and dietary plasticity in pond-dwelling tadpoles. Freshwater Biology. 2010;55:1533–1547. [Google Scholar]
- Whiles MR, Hall RO, Dodds WK, Verburg P, Huryn AD, Pringle CM, et al. Disease-driven amphibian declines alter ecosystem processes in a tropical stream. Ecosystems. 2013;16:146–157. [Google Scholar]


