Abstract
Purpose
Glioblastoma (GBM) cancer stem cells and their neural stem cell counterparts are hypothesized to contribute to tumor progression. We examined whether GBM contrast enhancement contact with neurogenic regions (NR) affect recurrence patterns, as contrast enhancement reflects regions of blood–brain barrier breakdown.
Methods
102 patients with primary GBM, treated at Johns Hopkins Hospital between 2006 and 2009, were included. All patients underwent surgical resection followed by adjuvant IMRT (60 Gy/30 fractions) and concomitant temozolomide. Initial and recurrent tumor distance from the subventricular zone (SVZ) or subgranular zone (SGZ) was measured. Tumors were categorized as NR contacting or non-contacting. The chi-square test was used to analyze the association between tumor contact and recurrence pattern.
Results
49 of 102 (48.0%, 95% CI: 0.386–0.576) tumors contacted NRs at initial presentation, and, of these tumors, 49/49 (100%) contacted NRs at recurrence. Of 53 tumors that were initially non-contacting, 37/53 (69.8%, 95% CI: 0.565–0.804) recurred contacting NRs. In total, 86/102 (84.3%, 95% CI: 0.760–0.901) recurrent GBM contacted NRs compared with 49/102 (48%, 95% CI: 0.386–0.576) at initial presentation. Of the recurrent tumors that did not contact NRs, 16/53 (30.1%, 95% CI: 0.195–0.435) recurred medially toward NRs with a significant decrease in distance between tumor contrast enhancement and NRs. 16/49 (32.6%, 95% CI: 0.212–0.466) initially NR-contacting GBMs recurred out-of field while 7/53 (13.2%, 95% CI: 0.0655–0.248) initially non-contacting recurred out of the radiation treatment field (p = 0.0315, Odds ratio: 3.19, 95% CI: 1.18–8.62).
Conclusions
GBM contrast-enhancing recurrence is significantly associated with proximity to NRs. NR-contacting initial tumors were more likely to recur out of radiation treatment fields.
Keywords: Glioblastoma, Subventricular zone, Recurrence
The prognosis of patients with glioblastoma (GBM) is poor, and most tumors recur despite maximal surgical resection and adjuvant concurrent chemo and radiation therapy [1]. GBMs have the potential to recur with widespread infiltration, and historically whole brain radiation treatment was used to ensure coverage of distant progression [2]. However, the majority of GBMs recur in close proximity to the initial tumor bed and modern radiation treatment fields have shifted to treatment of the gross tumor volume and a 1–2 cm margin that is thought to contain residual microscopic disease at high risk for local progression [3,4]. Despite inclusion of this additional volume at risk for recurrence, the majority of glioblastomas progress locally within radiation treatment fields [5], and attempts at dose escalation or hypofractionation have not improved outcomes or changed the patterns of recurrence [6,7].
One hypothesis is that GBM progression is driven by a subpopulation of cancer stem cells identified in GBMs, which are capable of propagating tumors and have chemotherapeutic and radio-resistant properties in vitro [8,9]. The origin of this cell population is unclear, but it is hypothesized that GBM cancer stem cells may represent dedifferentiated cancer cells or that they may stem from dysregulated normal neural stem cells. Normal neural stem cells reside in two areas: the subventricular zone (SVZ), a group of cells that line the lateral wall of the lateral ventricles, and the subgranular zone (SGZ) a group of cells within the subgranular layer of the hippocampus [10]. GBMs that spatially involve the SVZ have been demonstrated to have a higher propensity to recur at distant locations [11,12]. Moreover, those with subventricular involvement have been demonstrated to have more rapid progression and decreased overall survival [8,12–14].
However, the contribution of these neurogenic regions as potential sources of cancer stem cells as well as their role in GBM recurrence is controversial [15]. Stem cells within the SVZ and SGZ are thought to play functional roles in memory, neurocognition, and neuro-regeneration [9,16,17]. Radiation-induced injury to neural stem cells within the hippocampus is one mechanism that may mediate cranial irradiation neurotoxicity [18–21]. As a result, hippocampal sparing radiation treatment plans have been evaluated in pediatric populations as well as in the setting of whole brain radiation for metastases and has been prospectively correlated with memory preservation [22]. Thus, the evidence supporting neural stem cells as mediators of neurocognition and their potential for glioma initiation creates a complex challenge when examining neural stem cell region irradiation in glioblastoma.
While neurogenic regions are not typically targeted with radiation therapy, several groups have retrospectively correlated higher radiation doses to the SVZ and SGZ with improved patient survival outcome in GBM patients [23–26]. Given this potential relationship between regions containing neural stem cells and GBM recurrence, we aimed to explore this further. Specifically, we evaluated whether GBMs have a propensity to have contrast enhancing recurrence near neurogenic regions and whether spatial relationships between GBMs and neural stem cells affected recurrence with respect to radiation treatment fields.
Materials and methods
Patient selection and recorded variables
Medical charts were reviewed under institutional review board approval. Selection criteria for patients included in this analysis were patients with primary histo-pathologically diagnosed GBM, treated at Johns Hopkins University between 2006 and 2009. Only adult (age > 18 years) patients who underwent non-biopsy surgical resection [either subtotal (STR) or gross-total resection (GTR)] followed by standard of care adjuvant therapy involving IMRT (60 Gy/30 fractions) and concomitant temozolomide were included. All patients had a minimum follow-up of 7 months after completion of radiation therapy treatment.
Based on these criteria 102 patients were included in this analysis. Clinical data collected included patient demographics, treatment course, and disease course. Operative notes were reviewed for surgical resection approaches that penetrated the lateral ventricles. Cases of post-operative meningitis, encephalitis, cerebral infarcts, as well as CD4 count nadirs were also recorded. Residual disease was measured volumetrically by contouring the volume of contrast enhancing residual tumor after resection on T1 weighted MRI post-contrast, excluding the surgical resection cavity [27,28].
Tumor categorization and recurrence characterization
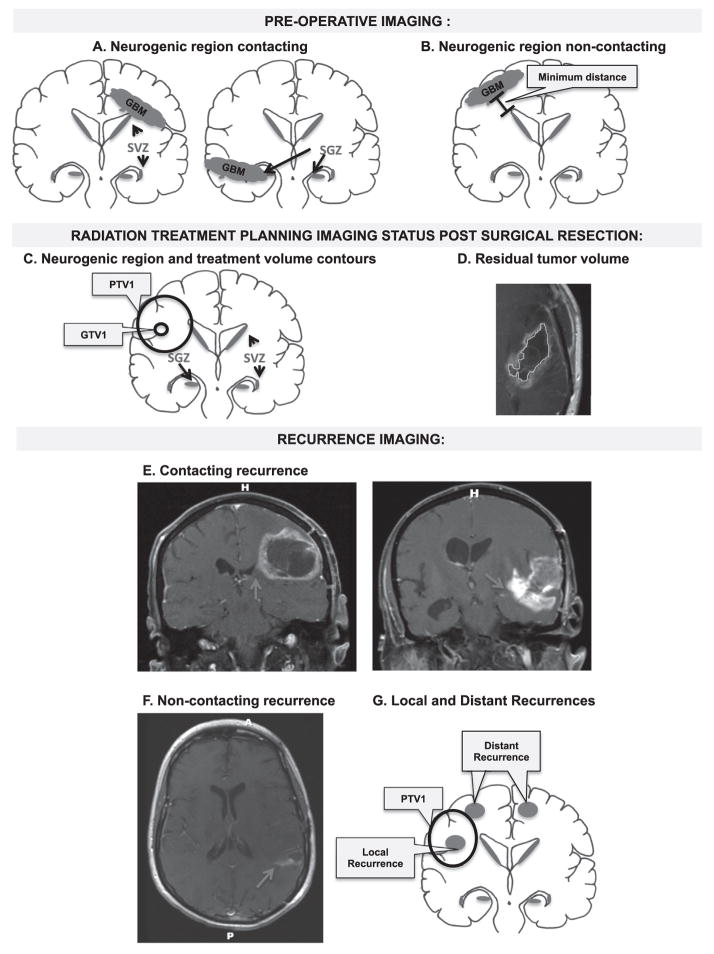
We reviewed the diagnostic imaging at a minimum of 3 time points: (1) pre-operative, (2) post-operative radiotherapy treatment planning, and (3) date of initial tumor recurrence (Fig. 1). Time of tumor recurrence was defined as the date that tumor progression was documented by both the neuro-radiologist and the treating neuro-oncologist. In instances where follow-up imaging was equivocal for recurrence vs. pseudoprogression, the date that the treating neuro-oncologist documented a change in management was defined as the date of recurrence.
Fig. 1.
Schematic of diagnostic imaging review and methodology. (A and B) Schematic depicts review of GBM imaging at initial diagnosis and neurogenic region contact categorization. GBMs are categorized into SGZ and/or SVZ contacting (A) or non-contacting (B). The minimum distance between the non-contacting GBMs and the nearest neurogenic region was also measured. (C and D) Radiation treatment planning imaging review status post surgical resection. (C) Schematic depicting neurogenic region contours as well as the GTV1 and PTV1 treatment volume contours. (D) Residual tumor volume was also measured by contouring contrast enhancement on T1-weighted MRI post gadolinium and the tumor resection cavity. (E–G). Review of imaging at the time of recurrence and categorization as neurogenic region contacting recurrence, with red arrow denoting contrast enhancement contacting a neurogenic region (E), and non-contacting recurrence (F) on T1-weighted MRI post gadolinium. (F) Also demonstrates an example of asymmetric medial enhancement toward neurogenic region in a recurrent non-contacting GBM. (G) GBM recurrence was also categorized as local or distant based on whether the majority of tumor volume occurred in-field or out-of-field with respect to PTV1.
Initial and recurrent tumors were categorized as contacting or non-contacting neurogenic regions by measuring the distance of tumor associated contrast enhancement on T1-weight MRI post gadolinium to the SGZ and the SVZ (Fig. 1). The SGZ was identified within the hippocampus, as the hypointense signal medial to the temporal horn on T1-weighted MRI axial sequences [22]. The SVZ was defined as a 5 mm region along the lateral wall of the lateral ventricle [23,29,30]. Contacting tumors were defined as tumors having a distance of 0 cm between the contrast enhancing tumor edge and either of these neurogenic regions.
Recurrent tumor on T1-weighted MR post-contrast was characterized as in-field or out-of field with respect to the planning target volume-1 (PTV1). PTV1 was defined as the gross tumor volume on T1 weighted MRI post gadolinium plus T2 FLAIR sequence MRI tumor volume including the surgical resection cavity with an additional 1–1.5 cm margin. GTV2 was defined as gross tumor volume, including the surgical resection cavity, as seen on T1 weighted MRI post gadolinium alone. PTV2 was defined as this GTV2 volume with an additional 1–1.5 cm margin. Recurrent tumors with >80% of enhancement volumes within the 95% isodose line were defined as in-field recurrence (Fig. 1). Multicentric recurrent tumors with any volume of recurrence outside of the 95% isodose line with respect to PTV1 were defined as out-of-field recurrence. Multicentric tumors were defined as those with multiple lesions without a clear path of tumor spread. All other recurrent tumors with >20% of enhancement volumes outside of the 95% isodose line were defined as out-of-field.
Contouring and dosimetry collection
Contouring and dosimetry data collected for this cohort of patients were previously described [23]. Briefly, ipsilateral, contralateral, and bilateral SVZs were contoured on treatment plans using a co-registered MRI and the radiation treatment planning computed tomography. The SVZ was defined as a 5-mm margin along the lateral wall of the LVs. Dose–volume histograms were calculated, and mean doses were extracted for ipsilateral, contralateral, and bilateral SVZ regions of interest, as well as GTV1, GTV2, PTV1, and PTV2. Contrast enhancement on post-operative T1-weighted axial MRIs was also contoured, with surgical resection cavity excluded, to obtain a volumetric measurement of residual tumor.
Statistical analysis
The collected data were reviewed and statistical analyses were performed using SPSS (version 20; SPSS; Chicago, IL). Proportions and confidence intervals of NR contacting and non-contacting recurrences at initial diagnosis relative to the number of tumors that contacted these regions at the time of recurrence were calculated. Survival analysis was completed using univariate and multivariate Cox Regression with age, performance status, and extent of resection used as covariates. Patient age was stratified as younger than 70 years versus 70 years or older. Karnofsky performance status (KPS) at diagnosis was stratified as less than 90 vs. 90 or greater. Surgical resection was categorized as subtotal resection, in which there was residual contrast enhancement on MRI or gross total resection, in which there was no residual contrast enhancement on MRI after resection. Patients lost to follow-up or those who were alive and had not progressed at the time of analysis were censored from the analysis. Patients lost to follow-up or those who were alive at the time of analysis were censored from the OS analysis. Logistic regression was used to identify if initial NR contact, age, extent of resection and KPS were predictors of NR contact at recurrence. The Student t-test was used to assess whether there were significant differences in age, residual tumor volume, and CD4 counts, between contacting and non-contacting groups. Paired t-tests were used to compare the minimum distances from initial and recurrent tumor to NR for NR-contacting and non-contacting groups. Chi-squared tests were used to analyze the association between tumor contact and recurrence patterns. Chi-squared tests were also used to compare the number of out-of-field recurrences between tumors that were contacting and non-contacting NR at the time of initial diagnosis. All p-values are two-sided.
Results
The patient cohort was 60% male and 40% female. The median patient age was 59 years (range: 29–78). At initial diagnosis 49/102 (48%, 95% CI: 0.386–0.576) of GBMs contacted NR and 53/102 (52%, 95% CI: 0.424–0.614) were non-contacting. Tumor progression occurred in 102/102 patients. Median progression free survival was 10.4 months (range: 1.23–54.9 months) for contacting tumors and 10.6 months (range: 1.50–44.5) for NR non-contacting tumors (p = 0.416, HR 1.19: 0.784–1.80). Median overall survival was 15.8 months (range 3.6–54.9 months) for NR contacting tumors and 16.9 months (range 5.87–52) for NR non-contacting tumors (p = 0.937, HR 0.984: 0.654–1.48) (Table 1). Multivariate survival analysis using age, KPS, and extent of resection as covariates did not reveal an association between NR contact and PFS or OS (Table 2). Contacting tumors had a significantly larger contrast-enhancing tumor residual volume following resection with a median of 32.5 cm3 (SD = 26.5) compared to 22.6 cm3 (SD = 17.5) in NR non-contacting tumors (p = 0.0005). When comparing NR contacting and non-contacting patients there was no significant difference in age, CD4 nadir, or post-operative meningitis (Table 1).
Table 1.
Patient demographics, disease, treatment characteristics, and clinical outcome (n = 102).
| Gender | |
| Male | 61 (60%) |
| Female | 41 (40%) |
| Age (years) | |
| Median (range) | 59 (29–78) |
| Contacting – mean (SEM) | 58 (1.67) |
| Non-contacting – mean (SEM) | 57 (1.23) |
| p-Value | 0.517 |
| Tumor relationship to neurogenic regions (NR) at initial diagnosis (%, 95% CI) | |
| Contacting/total | 49/102 (48%, 0.386–0.576) |
| Non-contacting/total | 53/102 (52%, 0.424–0.614) |
| Residual volume (mean in cm3) | |
| Contacting – mean (SEM) | 41.6 (3.79) |
| Non-contacting – mean (SEM) | 25.2 (6.25) |
| p-Value | 0.0005 |
| CD4 count nadir (cells/mm3) | |
| Contacting – mean (SEM) | 207 (15.4) |
| Non-contacting – mean (SEM) | 204 (20.2) |
| p-Value | 0.917 |
| Post-operative meningitis | |
| Contacting | 2 |
| Non-contacting | 1 |
| Progression free survival | |
| NR contacting tumor (months, range) | 10.4 (1.23–54.9) |
| NR non-contacting tumor (months, range) | 10.6 (1.50–44.5) |
| p-Value (HR: 95% CI) | 0.416 (1.19: 0.784–1.80) |
| Overall survival | |
| NR contacting tumor (months, range) | 15.8 (3.60–54.9) |
| NR non-contacting tumor (months, range) | 16.9 (5.87–52.0) |
| p-Value (HR: 95% CI) | 0.937 (0.984: 0.654–1.48) |
Abbreviations: NR = neurogenic region, HR = hazard ratio, CI = confidence interval.
Table 2.
Multivariate Cox Regression analysis of prognostic factors and survival.
| Prognostic Factor | Progression free survival
|
Overall survival
|
||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 0.763 (0.432–1.35) | 0.349 | 1.02 (0.597–1.74) | 0.944 |
| KPS | 0.750 (0.386–1.455) | 0.394 | 0.644 (0.339–1.22) | 0.178 |
| Extent of resection | 1.07 (0.684–1.67) | 0.772 | 1.14 (0.750–1.723) | 0.546 |
| NR Contact | 1.19 (0.784–1.80) | 0.416 | 0.984 (0.654–1.480) | 0.937 |
Abbreviations: HR = hazard ratio, CI = confidence interval, KPS = Karnofsky performance status, NR = neurogenic region.
At the time of initial diagnosis, 49 of 102 (48%, 95% CI: 0.386–0.576) tumors were in contact with NR (Table 1). In comparison, at the time of recurrence, 49/49 (100%, 95% CI: 0.927–1) of the respective recurrent tumors were contacting stem cell containing niches (Table 3). Of the 53 tumors that did not contact the NRs at the time of initial diagnosis, 37 of 53 (70%, 95% CI: 0.565–0.805) of these tumors recurred in contact with the NRs (Table 3, Fig. 2). In total, 86/102 (84%, 95% CI: 0.760–0.901) recurrent GBM contacted the SVZ or SGZ compared with only 49/102 (48%) at the time of initial diagnosis (Table 3). Logistic regression identified initial tumor NR contact as a significant predictor of recurrent tumor contact (p = 0.024, OR: 2.45e–10). However, age (p = 0.414, OR: 0.482), extent of resection (p = 0.216, OR: 2.258), and performance status (0.698, OR: 0.618) were not statistically significant predictors for NR contact at the time of recurrence.
Table 3.
GBM NR contact and recurrence.
| Tumor relationship to NR at recurrence (%, 95% CI) | |
| Contacting/total | 86/102 (84%, 0.760–0.901) |
| Non-contacting/total | 16/102 (16%, 0.099–0.240) |
| Recurrence pattern of contacting tumors at initial diagnosis (%, 95% CI) | |
| Recurrent contacting tumor/total | 49/49 (100%, 0.927–1) |
| Recurrent non-contacting tumor/total | 0/49 (0%, 0–0.073) |
| Recurrence pattern of non-contacting tumors at initial diagnosis (%, 95% CI) | |
| Recurrent contacting tumor/total | 37/53 (70%, 0.565–0.805) |
| Recurrent non-contacting tumor | 16/53 (30%, 0.195–0.435) |
| Mean minimum distance1 from NR to non-contacting GBM | |
| Initial (cm) | 1.73 (SEM: 0.261) |
| Recurrent (cm) | 1.08 (SEM: 0.256) |
| Paired t-test p-value | 0.0006 |
| Local tumor recurrence with respect to PTV1 (%, 95% CI) | |
| Local recurrences/total | 79 (77%, 0.684–0.845) |
| Initial contacting tumor local recurrence/contacting tumors | 33/49 (67%, 0.534–0.788) |
| Initial non-contacting tumor local recurrence/non-contacting tumors | 46/53 (87%, 0.752–0.935) |
| Distant tumor recurrence with respect to PTV1 (%, 95% CI) | |
| Distant recurrences/total | 23 (23%, 0.155–0.316) |
| Initial contacting tumor distant recurrence/contacting tumors | 16/49 (33%, 0.212–0.466) |
| Initial non-contacting tumor distant recurrence/non-contacting tumors | 7/53 (13%, 0.065–0.248) |
| Chi-squared test p-value | 0.0315 (OR2: 3.19, 1.18–8.62) |
| Clinical factors as predictors of NR recurrence | |
| Age | p = 0.414 (OR: 0.482) |
| Extent of resection | p = 0.216 (OR: 2.258) |
| Performance status | p = 0.698 (OR: 0.618) |
| Initial tumor NR contact | p = 0.024 (OR: 2.45e–10) |
Abbreviations: CI = confidence interval, OR = Odds ratio.
Mean minimum distance between neurogenic regions and tumor contrast enhancement.
Fig. 2.
Glioblastoma recurrence patterns in association with neurogenic region contact. (A) Glioblastoma initial neurogenic region contact and contacting or non-contacting recurrence pattern. In total, 84% (95% CI: 0.760–0.901) of recurrent GBMs recurred in contact with neurogenic regions. 36% (95% CI: 0.276–0.459) of recurrent GBMs in contact with neurogenic regions were initially non-contacting. 16% (95% CI: 0.099–0.240) of recurrent GBMs did not contact neurogenic regions, and there were no initially contacting tumors that were non-contacting at recurrence. (B) Glioblastomas in contact with neurogenic regions are significantly correlated with distant recurrences outside of radiation treatment fields with 7/53 non-contacting initial tumors recurring distantly in comparison to 16/49 contacting initial tumors (p = 0.0315, Odds ratio 3.19, 95% CI: 1.18–8.62).
Of the tumors that did not initially contact the NRs, 16/53 (30%, 95% CI: 0.195–0.435) recurred medially toward the SVZ. These non-contacting recurrent tumors had a mean distance of 1.73 cm (STD = 1.04) from NRs at the time of initial diagnosis vs. 1.08 cm (STD = 1.02) upon recurrence (paired t-test, p = 0.0006, Table 3).
Local recurrence within the radiation treatment field was found in 79 of 102 tumors (77%, 95% CI: 0.684–0.845). By contrast, 23 of 102 (23%, 0.195–0.435) recurred distantly (Table 3, Fig. 2). Of the tumors that initially contacted the NRs, 16 of 49 (35%, 95% CI: 0.212–0.466) recurred out-of field compared with 7 of 53 (13%, 95% CI: 0.065–0.248) tumors that did not initially contact the neurogenic niches but ultimately recurred outside of the radiation treatment volume (p = 0.0315, Odds ratio: 3.19, 95% CI: 1.18–8.62, Table 3, Fig. 2).
The mean radiation dose to NRs was compared between tumors that recurred in contact and non-contacting recurrent tumors. There was no statistically significant difference. Additionally, the patient cohort was divided into high and low radiation dose groups with a cut-off of 40 Gy, which was found to be statically significant for survival in this cohort based in a previously published study [23]. Increased radiation dose to NRs did not have a positive or negative correlation with cases of distant recurrence.
Discussion
At present, the concept of purposeful inclusion of NRs in radiation treatment for GBMs is controversial as cells within NRs may serve functional roles in cognition and memory, but may also contain cancer stem cells that contribute to GBM progression. Recently completed and ongoing clinical trials are investigating both sides of this important clinical question (NCT01478854, NCT02177578, NCT02039778). The current literature, which suggests improved patient progression free and overall survival outcomes with increased radiation to these areas, is limited by its retrospective nature and numerous confounding factors. It can be hypothesized that these results potentially reflect elimination of GBM stem cells that contribute to GBM progression, which argues for inclusion of these areas in radiation treatment planning. However, the relationship between areas containing normal neural stem cells and GBM cancer stem cells remains unclear. Here we examined the recurrence patterns of GBMs with respect to neurogenic niches to further examine the hypothesis that areas containing neural stem cells potentially contribute to GBM progression.
Our findings suggest that GBM contrast enhancing recurrence is significantly associated with SVZ or SGZ-contact. Both NR contacting and non-contacting tumors at initial diagnosis had a propensity for neurogenic contact upon recurrence, though there was an increased post-surgical residual volume within contacting tumors. In order to account for the limitations associated with dichotomizing tumors into contacting and non-contacting groups, we also evaluated the minimum distance between tumor and neurogenic regions upon recurrence. Significantly, even in this group of non-contact recurrent tumors, these tumors universally recurred closer to NRs at the time of progression. Furthermore, it was qualitatively observed that many of these recurrences had an asymmetrical enhancement pattern with a medial tropism toward the SVZ or SGZ (Fig. 1). While pathologic and physiologic conditions, may certainly produce abnormal patterns of contrast enhancement, there was no correlation between immunosuppression or infections between groups.
Notably, our findings are limited to contrast-enhancing GBM recurrence patterns. While GBM contrast enhancing components represent disruption of the blood–brain barrier, high-grade gliomas are also composed of infiltrative non-contrast enhancing components demonstrated on T2-FLAIR [31]. Here we focus on contrast-enhancement at the time of recurrence, as contrast enhancement was universally noted at the time of progression, and T2-FLAIR abnormalities can represent post-operative changes and radiation treatment effect [1,32]. Due to the multifactorial contribution of T2-FLAIR changes in the post-treatment setting our findings focus on contrast-enhancing tumor components of recurrence patterns. However, we acknowledge that this is a limitation in our study as T2-FLAIR findings can also represent tumor recurrence and that T1 contrast abnormalities can also potentially represent post-operative or radiation related changes.
Several potential etiologies confound our observation that GBMs have a tendency to recur near neurogenic niches. First, local outgrowth of residual tumor from surgical resection cavities may contribute to the proximity and decrease in minimal distance from NRs that we observed. This confounding factor is that the initially contacting tumors had a larger volume of residual contrast enhancement following the initial surgical resection than the non-contacting tumors. This increase in residual contrast enhancement may potentially be due to difficulty in surgically resecting deep-seated tumors or those with sub-ependymal spread. Additionally, cancer stem cells have been shown in vitro to have a tropism toward CSF and it is also possible that migration of treatment resistant cancer stem cells toward areas containing CSF contributes to this finding [33]. However, given that the SVZ and SGZ are thought to be a potential source of cancer stem cells, the contribution of cancer stem cells from these areas to recurrent tumors is an intriguing consideration and the tendency of GBMs to recur near NRs requires more investigation. Thus, while NR contact did not translate into differences in survival, the tendency of the majority of GBMs in this cohort to recur near NRs is hypothesis generating in terms of how cells in NRs may contribute to recurrence, and encourage further evaluation regarding the potential benefit of the NRs as clinical treatment targets.
GBMs are heterogeneous tumors and while the majority of tumors recur locally, they also have a potential to disseminate widely at autopsy as well as recur distantly and multi-focally [3,4]. Here, we observed that tumors, which contacted neurogenic niches at diagnosis, had a higher incidence of distant recurrence outside of the radiation treatment field. This finding is in congruence with Lim et al. and AdeberG et al., who also found an association between neurogenic niche contact, multifocal distant progression, and poor patient outcome [11,34]. However, there are alternative and confounding etiologies for this observation. At the time of diagnosis GBM cells may have already diffusely invaded brain parenchyma [35]. GBM is an invasive disease, and contrast-enhancing contact with SVZ or SGZ at the time of initial diagnosis may represent extensive microscopic disease that could account for what appears to be distant out of treatment field recurrence. Additionally, given that tumors which contact NRs are also in close proximity with cerebrospinal fluid (CSF), our finding is perhaps confounded by the hypothesis that CSF circulation may seed tumor cells to distant sites. However, these results also support the hypothesis that enrichment of stem-like tumor cells in NR-contacting tumors may result in GBMs with different biological behaviors leading to tumor initiation at distant locations.
A previous study published by our group in this cohort of patients with GBM treated with surgery followed by radiation and temozolomide chemotherapy demonstrated ipsilateral SVZ radiation ≥40 Gy correlated with prolonged PFS and OS [23]. Our evaluation of recurrence patterns with respect to radiation dose did not reveal a correlation between increased NR radiation dose and decreased incidence of NR contact at the time of tumor recurrence or a decreased incidence of out-of-field recurrences. However, the previously reported survival outcome correlation with dose that was a finding restricted to a sub-group of forty-one patients who had received gross total resection [23]. Moreover, we are further limited by the retrospective nature of the data, as no patients intentionally received increased radiation to NRs.
In summary, our observations demonstrate that GBM contrast enhancing recurrence has a propensity toward NRs, with eighty-four of percent GBMs contacting NR at the time of recurrence. Additionally, we demonstrate that the pattern of local and distant recurrence seems to be influenced by initial tumor contact with NRs, as NR-contacting initial tumors were associated with out-of-field recurrence. Our findings build upon previous literature by examining recurrence with respect to both the SVZ and SGZ and by examining recurrence patterns with respect to radiation treatment fields in a uniform cohort of patients. Given that these NRs are thought to contain GBM cancer stem cells, our data support the hypothesis that cancer stem cells may play a role in GBM recurrence as well as in initiation. Moreover, the observation that NR contacting tumors have a tendency to recur distantly outside of radiation treatment fields, lends credence to the hypothesis that GBMs near tumor-initiating cancer stem cell niches may have different biologic behaviors. Whether radiation dose to NRs affects patterns of GBM recurrence is uncertain and limited here by multiple confounding factors. Though these findings are observational correlations, further characterization of tumor progression with respect of neurogenic niches may allow for more insights into the role of NSCs in GBM therapeutic resistance. Future prospective studies evaluating radiation dose to the neurogenic niches, patient outcomes and recurrence patterns will be critical to better understanding this relationship and ultimately, potentially improving outcomes in patients with this devastating disease.
Acknowledgments
Data from this submission were presented at the 2013 American Society for Radiation Oncology in Atlanta, Georgia. This work was supported by R01NS070024 to AQH and HHMI Medical Research Scholars Grant to LCC.
Footnotes
Conflicts of interest notification
Dr. Redmond is a member the Elekta Oligometastasis Consortium, supported by a research grant from Elekta AB.
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Concannon JP, Kramer S, Berry R. The extent of intracranial gliomata at autopsy and its relationship to techniques used in radiation therapy of brain tumors. Am J Roentgenol Radium Ther Nucl Med. 1960;84:99–107. [PubMed] [Google Scholar]
- 3.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–11. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 4.Wallner KE, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–9. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 5.Sherriff J, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;1022:20120414. doi: 10.1259/bjr.20120414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlin C, et al. Phase II, two-arm RTOG trial (94–11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (>20 or ≤20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–8. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 7.Malmstrom A, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–26. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 8.Chaichana KL, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–24. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 9.Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Lim DA, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–9. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafri NF, et al. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15:91–6. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsa AT, et al. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg. 2005;102:622–8. doi: 10.3171/jns.2005.102.4.0622. [DOI] [PubMed] [Google Scholar]
- 14.Barami K, et al. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009;16:195–201. doi: 10.1016/j.jocn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kut C, Janson K. Redmond, new considerations in radiation treatment planning for brain tumors: neural progenitor cell-containing niches. Semin Radiat Oncol. 2014;24:265–72. doi: 10.1016/j.semradonc.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 17.Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 18.Madsen TM, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 19.Monje ML, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 20.Barani IJ, Benedict SH, Lin PS. Neural stem cells: implications for the conventional radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2007;68:324–33. doi: 10.1016/j.ijrobp.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Redmond KJ, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 2013;15:360–9. doi: 10.1093/neuonc/nos303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondi V, et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiation therapy for patients with brain metastases: primary endpoint results of RTOG 0933. Int J Radiat Oncol Biol Phys. 2013 [Google Scholar]
- 23.Chen L, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;86:616–22. doi: 10.1016/j.ijrobp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee P, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86:609–15. doi: 10.1016/j.ijrobp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gupta T, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109:195–203. doi: 10.1007/s11060-012-0887-3. [DOI] [PubMed] [Google Scholar]
- 26.Evers P, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaichana KL, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014 doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Chaichana KL, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–22. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondi V, et al. Hippocampal Contouring: A Contouring Atlas for RTOG 0933. 2011 [Google Scholar]
- 30.Redmond KJ, et al. A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage. J Neurooncol. 2011;104:579–87. doi: 10.1007/s11060-011-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 32.Champ CE, et al. Evaluating changes in radiation treatment volumes from postoperative to same-day planning MRI in High-grade gliomas. Radiat Oncol. 2012;7:220. doi: 10.1186/1748-717X-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukushima Y, et al. Induction of glioma cell migration by vitronectin in human serum and cerebrospinal fluid. J Neurosurg. 2007;107:578–85. doi: 10.3171/JNS-07/09/0578. [DOI] [PubMed] [Google Scholar]
- 34.Adeberg S, et al. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. Int J Radiat Oncol Biol Phys. 2014;90:886–93. doi: 10.1016/j.ijrobp.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Fomchenko EI, Holland EC. Platelet-derived growth factor-mediated gliomagenesis and brain tumor recruitment. Neurosurg Clin N Am. 2007;18:39–58. viii. doi: 10.1016/j.nec.2006.10.006. [DOI] [PubMed] [Google Scholar]