Abstract
The consolidation of spatial navigational memory during sleep is supported by electrophysiological and behavioral evidence. The features of sleep that mediate this ability may change with aging, as percentage of slow wave sleep is canonically thought to decrease with age, and slow waves are thought to help orchestrate hippocampal-neocortical dialogue that supports systems level consolidation. In this study, groups of younger and older subjects performed timed trials before and after polysomnographically recorded sleep on a 3D spatial maze navigational task. Although younger subjects performed better than older subjects at baseline, both groups showed similar improvement across pre-sleep trials. However, younger subjects experienced significant improvement in maze performance during sleep that was not observed in older subjects, without differences in morning psychomotor vigilance between groups. Older subjects had sleep quality marked by decreased amount of slow wave sleep and increased fragmentation of slow wave sleep, resulting in decreased slow wave activity. Across all subjects, frontal slow wave activity was positively correlated with both overnight change in maze performance and medial prefrontal cortical volume, illuminating a potential neuroanatomical substrate for slow wave activity changes with aging and underscoring the importance of slow wave activity in sleep-dependent spatial navigational memory consolidation.
Keywords: brain imaging, maze, prefrontal cortex, psychomotor vigilance, sleep fragmentation
1. Introduction
The ability to consolidate memories during sleep depends on several factors, including the type of memory being integrated and the duration and architecture of ensuing sleep (Stickgold and Walker, 2013). Spatial navigational memory has represented an appealing model memory system to investigate the role of sleep in part because of the potential mechanistic insights offered by studies on rodents. Patterns of hippocampal activity recorded during a period of exploration in an environment were subsequently replayed during non-REM sleep (Wilson and McNaughton, 1994), where the replay happened over time scales much faster than occurred during wake, and in REM sleep, where the replay happened with greater temporal fidelity to wake (Louie and Wilson, 2001). These observations raised the possibility that replay during sleep may be contributing to the strengthening of neural connections for spatial navigation formed during exploration based on Hebbian models of plasticity.
Sharp wave ripple complexes in the hippocampus that occur during slow wave sleep are temporally coordinated with neocortical slow waves and have been implicated in the transfer of information between the hippocampus and neocortex that could help support spatial memory (Siapas and Wilson, 1998; Sirota et al., 2003). When sharp wave ripple complexes were selectively suppressed in post-learning sleep, rats trained on a spatial radial arm maze task showed significantly impaired memory (Girardeau et al., 2009).
In human subjects, significantly greater improvement in performance on spatial navigational maze tasks occurred across sleep than across a similar period of wakefulness (Ferrara et al., 2008; Nguyen et al., 2013). Efforts to identify contributions of particular sleep stages have suggested roles for both slow wave and REM sleep. In a limited number of subjects, neuroimaging suggested that the degree of blood flow to the right hippocampus during slow wave sleep correlated significantly with the degree of overnight improvement in a spatial navigation task (Peigneux et al., 2004). For forms of declarative memory, the degree of overnight consolidation has been shown to be proportional to prefrontal slow wave activity in younger and older subjects, and augmenting prefrontal slow wave activity using transcranial electrical stimulation during sleep enhanced retention of declarative memories (Marshall et al., 2006). REM sleep fragmentation due to obstructive sleep apnea impaired overnight improvement in spatial navigation, and the degree of REM fragmentation correlated with poorer overnight performance improvement (Varga et al., 2014).
These findings bear relevance to the changes in sleep architecture with aging as two of the well characterized consequences of aging include increased sleep fragmentation and loss of slow wave sleep. Although sleep fragmentation is thought to be a general feature of sleep in the elderly, evidence suggests that fragmentation may be preferential to non-REM sleep (Klerman et al., 2013).
Such a combination of slow wave sleep loss and fragmentation of remaining slow wave sleep with aging could thus impact the overnight consolidation of spatial navigational memories. The overnight consolidation of memory for word pairs, a form of declarative memory thought to be dependent on the hippocampus, was impaired with aging and was correlated with slow wave activity particularly in the prefrontal leads of the EEG (Mander et al., 2013). Furthermore, this study showed that the degree of atrophy in the medial prefrontal cortex appeared to be a significant correlate of slow wave sleep loss and impaired declarative memory consolidation.
The current study set out to examine the effects of aging on sleep architecture, sleep fragmentation, and slow wave activity, and examine the relationship between medial prefrontal cortical volume and slow wave activity in aging. Based on the role of slow wave sleep in spatial navigational memory and our expectation of reduced and fragmented slow wave sleep in older subjects, we also determined whether the consolidation of spatial navigational memory that normally occurs during sleep is diminished as a function of age.
2. Materials and Methods
2.1 Participants
Both younger (ages 18-23) and older (ages 51-85) subjects were recruited from the community as part of studies on brain and glucose metabolism (younger group) and normal aging (older group). The subjects had no sleep complaints and agreed to undergo additional inlab polysomnography, maze testing, and psychomotor vigilance testing for the present study. Subjects were excluded if they were pregnant, had intellectual disability or severe learning impairment (IQ<70) (younger group), or had medical conditions or history of significant conditions that may affect brain structure or function, such as stroke, uncontrolled diabetes, traumatic brain injury, any neurodegenerative diseases, depression, or MRI evidence of intracranial mass or infarcts (older group). All included subjects completed a standard neuropsychological test battery and were cognitively normal. Subjects were excluded from analysis if polysomnography revealed more than mild obstructive sleep apnea (AHI4% > 10/hour) that was incidentally discovered (3 older subjects and 0 younger subjects) or if subjects failed to find the target in 2 or more of the maze trials before sleep (4 older subjects and 0 younger subjects). The number of older subjects included was 13 (5 men, 8 women, mean age 68.2 years), and the number of younger subjects included was 18 (8 men, 10 women, mean age 20.0 years). All subjects provided informed consent prior to their participation. All procedures were approved by the NYU School of Medicine Institutional Review Board.
2.2 Virtual Maze Performance and Psychomotor Vigilance Test (PVT)
At about 20:00 on each night of study, subjects began training on a virtual maze task: a simple 3D environment designed for this research (Nguyen et al., 2013). After a period of general familiarization with joystick controls (Thrustmaster™) in a Z-shaped corridor, subjects initially spent 3 minutes exploring a complex maze designed using “Unreal Tournamant 3 Editor” (© Epic Games, Cary, NC). Avatar walking speed and turning speed were reduced to minimize motion “cybersickness.” The game was projected onto a screen in a darkened testing room. The viewing area was 67 inches × 50 inches, and subjects sat 13 feet away with their vision corrected if they usually required it. Subjects were instructed to remember the layout of the maze environment as well as possible. Subsequently, subjects navigated through the same maze during three pre-sleep test trials, in which they were instructed to reach a specified goal point as quickly as possible. Time to reach the goal per trial was capped at 600 seconds. We retrospectively inquired about prior 3D video game experience, and used a construct whereby “novice” subjects were defined as having prior 3D video game experience of less than once per year and “experienced” subjects were defined as having prior 3D video game experience of at least once per year (Wamsley et al., 2010). We obtained responses from 12 of 18 younger and 13 of 13 older subjects.
Subjects were connected to polysomnography equipment at about 21:00, and a full night diagnostic polysomnogram (PSG) was performed following standard AASM criteria (Iber et. al, 2007).
To control for attention effects, subjects performed a standardized twenty-minute psychomotor vigilance test beginning one hour after awakening from each night in the sleep laboratory. Thereafter, subjects performed three final test trials on the 3D virtual maze, again instructed to reach the same specified goal point as quickly as possible.
The primary performance metric on the virtual maze was completion time (CT). Overnight change was calculated as the difference between the averages across the 3 trials before and after sleep, normalized to the average of the 3 trials before sleep for each metric. Positive values represent improvement and negative values represent worsening. Performance metrics on the psychomotor vigilance test included reaction time (in milliseconds) and number of lapses (no response after 500 milliseconds). A transform of the number of lapses was done to enable parametric testing (lapses transform = sqrt (#lapses) + sqrt (#lapses+1)) (Dinges et al., 1997).
2.3 Polysomnography
All polysomnograms consisted of a full night spent in the NYU Sleep Disorders Center. Monitoring included sleep by EMG, EOG, and EEG, respiration by a nasal cannula/pressure transducer and oral thermistor, effort by rib/abdomen impedance plethysmography; single lead EKG, and oxygen saturation by finger oximeter. Polysomnograms were scored in 30-second epochs according to standard criteria (AASM) for sleep and EEG arousals. Total sleep time and percent time spent in wake, non-REM stage 1 (NREM 1), non-REM stage 2 (NREM 2), non-REM stage 3 (NREM 3) (slow wave sleep), and REM sleep were determined. Respiratory events were scored from the airflow signal using AASM criteria and stage specific (REM, non-REM and Total) apnea indices were calculated. Apneas were defined as absence of airflow for ≥10 seconds. Hypopnea4% was defined as a reduction in the amplitude of breathing by 30% or more for ≥10 seconds with ≥ 4% decline in blood oxygen saturation, irrespective of the presence of an arousal. Hypopnea (3% or arousal) was defined as a reduction in the amplitude of breathing by 30% or more for ≥10 seconds accompanied by ≥ 3% decline in blood oxygen saturation or an arousal indicated by a sudden increase in the cortical EEG frequency or by sudden increase in the motor tone in the chin or anterior tibealis by EMG.
The apnea-hypopnea index with 4% oxygen desaturation (AHI4%) is defined as the sum of all apneas and hypopneas with 4% desaturation divided by the total sleep time in hours. AHI-all is defined as the sum of apneas and hypopneas (with 3% desaturation or arousal) divided by the total sleep time in hours.
Continuity of sleep was assessed as duration of sleep runs, defined as the duration of consecutive 30-second epochs of sleep scored as that stage, terminated by one or more epochs scored as another stage, including wake (Kishi et al., 2011; Norman et al., 2006).
EEG data from the experimental night were imported into Matlab and epoched into 30 sec bins. Epochs containing artifacts were rejected, and the remaining epochs were filtered between 0.4 and 50 Hz. A fast Fourier transform was then applied to the filtered EEG signal at 30 sec intervals with 50% overlap employing a Hamming window. Absolute slow wave activity in an EEG lead was defined as the spectral power between 0.5 and 4.0 Hz during non-REM sleep. Relative slow wave activity was defined as the absolute slow wave activity divided by the absolute spectral power across all frequencies (0.5-50 Hz) for a given EEG lead, allowing normalization of spectral power across subjects that may otherwise be influenced by inter-individual anatomical differences.
2.4 Brain Imaging
All subjects received structural volumetric MRI scans on a 3T system (Trio; Siemens, Erlangen, Germany) using standardized imaging procedures described previously (Glodzik et al., 2011) with the exception of one older subject, whose MRI was acquired at 1.5T and was not included in the subsequent analysis. Gray matter cortical volumes were calculated using Freesurfer image analysis software, version 5.1 (http://surfer.nmr.mgh.harvard.edu/) from MPRAGE sequences (Yau et al., 2014). Motion correction, skull stripping, Talairach transforms, atlas registration, spherical surface maps and parcellations were performed automatically using a within-subject template (Reuter et al., 2012). Gray matter volumes were first used to create a medial prefrontal cortex region of interest (ROI) using the sum of the following bilateral ROIs: caudal anterior cingulate cortex, medial orbitofrontal cortex, rostral anterior cingulate cortex and superior frontal gyrus (Desikan et al., 2006) and were then normalized to the intracranial volume. The MR images and overlying cortical map were inspected visually for accuracy using the Freeview software included in the Freesurfer package.
2.5 Data Analysis
Data were analyzed using SigmaPlot version 11.0, IBM SPSS 23, and Matlab (R 2013b). Comparison of sleep and psychomotor vigilance data was performed between subjects using unpaired t-tests for normally distributed data and mean values +/− the standard error of the mean are reported. For data not normally distributed, analysis consisted of Mann-Whitney rank sum tests and median values are reported. For maze performance data, analysis consisted of using a two-way mixed factorial ANOVA with age group as the between-subjects independent variable and time as the within-subjects independent variable. Average completion time data were natural-log transformed to overcome violation of Levene's test of equality of variances prior to analysis. Correlations between normally distributed variables (mPFC volume and frontal relative SWA) were calculated using a Pearson product moment, and the correlation coefficient r is reported. Correlations between variables where one or more variables were not normally distributed (% change in maze performance) were calculated using a Spearman rank order correlation, and the correlation coefficient rho is reported.
For sleep continuity data, a bootstrap-based analysis that accounted for the number of runs contributed by each subject was performed to determine a cumulative duration probability distribution for each stage (REM, NREM 1, NREM 2, and NREM 3). Thus, striking a balance between equivalence among subjects and retaining a sufficient number of data points, for those subjects with more than the median number of runs, runs were randomly sampled up to the median number. Log rank tests were used to compare younger versus older subjects on the survival curves derived from the sampling procedure. This procedure was repeated 1,000 times. At each step of the iteration, a p value was estimated from the history of prior p values, yielding an increasingly stable result as the number of iterations increases. Results for all statistical analyses were considered significant at p < 0.05.
3. Results
3.1 Effects of Aging on Sleep Architecture and Sleep Continuity
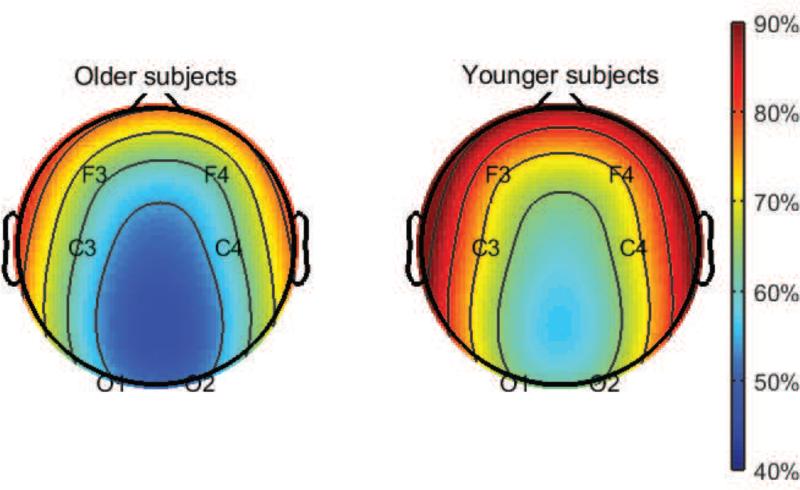
There were no significant differences between younger and older subjects in total sleep time or sleep efficiency. Similarly, there were no significant differences in severity of obstructive sleep apnea measured by the AHI4% or the AHI-all. We observed the expected significant decrease in non-REM stage 3 sleep (slow wave sleep) in older subjects, which was compensated by numerically smaller but statistically significant increases in non-REM stage 2 sleep and REM sleep. There were no significant differences between groups in non-REM stage 1 sleep. In line with the decreases in slow wave sleep in older subjects, we observed decreases in the relative slow wave activity in older subjects that was particularly prominent in the frontal leads (F3 and F4) (Figure 1). These results are summarized in Table 1.
Figure 1.
Slow wave activity in the brain diminishes with aging. EEG topographic maps of relative slow wave activity (0.5-4 Hz) in older and younger subjects expressed as a percent of the power across all frequency bands (0.5-50 Hz).
Table 1.
Differences in Sleep Architecture Between Older and Younger Subjects
| Sleep Measure | Younger subjects (n=18) | Older subjects (n=13) |
|---|---|---|
| Total sleep time | 392.3 min ± 11.2 | 367.8 min ± 11.0 |
| Sleep efficiency | 85.7% (median) | 85.0% (median) |
| %NREM 1 | 13.2% ± 1.1 | 16.5% ± 1.9 |
| %NREM2 | 39.5% ± 1.8 | 46.9% ± 2.7* |
| %NREM 3 (SWS) | 32.3% ± 2.1 | 16.2% ± 2.8** |
| %REM | 15.1% ± 1.3 | 20.3% ± 2.3* |
| AHI4% | 0.6/hour (median) | 0.9/hour (median) |
| AHI3%all | 4.0/hour (median) | 5.4/hour (median) |
| NREM 1 mean bout length | 1.0 min (median) | 1.0 min (median) |
| NREM 2 mean bout length | 2.6 min ± 0.2 | 2.6 min ± 0.2 |
| NREM 3 (SWS) mean bout length | 5.9 min ± 0.7 | 2.0 min ± 0.3** |
| REM mean bout length | 8.8 min ± 1.0 | 6.5 min ± 0.7 |
Key: NREM1 = non-REM stage 1, NREM2 = non-REM stage 2, NREM3 = non-REM stage 3, SWS = slow wave sleep.
= p < 0.05
= p < 0.001.
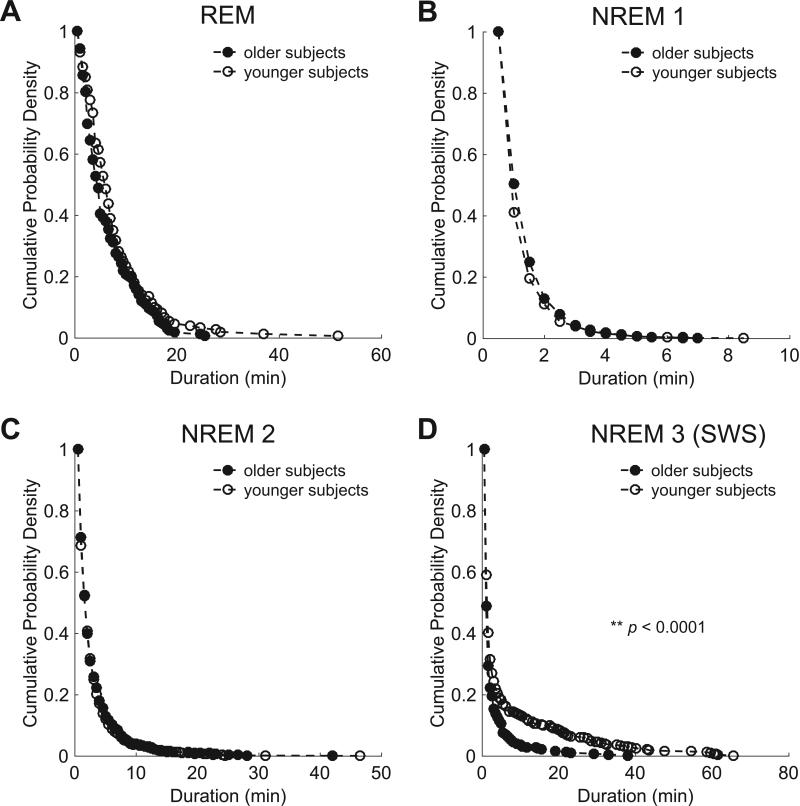
In order to evaluate the degree of fragmentation of individual sleep stages across groups, we examined the cumulative duration probability distribution of each stage. The cumulative duration probability distribution for slow wave sleep was significantly left-shifted in older subjects compared to younger subjects (p < 0.001), indicating slow wave sleep occurred in shorter bouts in older subjects. There were no significant differences in the duration probability distribution of non-REM stage 1, non-REM stage 2, or REM sleep between groups (Figure 2). In summary, older subjects have significantly less slow wave sleep with less relative slow wave activity in the frontal leads. Increased fragmentation of sleep in the older subjects is limited to slow wave sleep without increased fragmentation of the other sleep stages.
Figure 2.
Sleep fragmentation in aging is specific to slow wave sleep. Continuity of sleep manifested by survival curves (cumulative probability distributions) of stage-specific sleep runs in older (black) and younger (white) subjects. No significant difference between subjects was observed in the survival curves for REM (A), NREM 1 (B), or NREM 2 (C) sleep. The survival curve for older subjects showed a significant shift toward shorter runs of NREM 3 (slow wave) sleep compared with younger subjects, p < 0.001, log rank test (D). SWS = slow wave sleep.
3.2 Effects of Aging on Spatial Navigational Memory and Psychomotor Vigilance
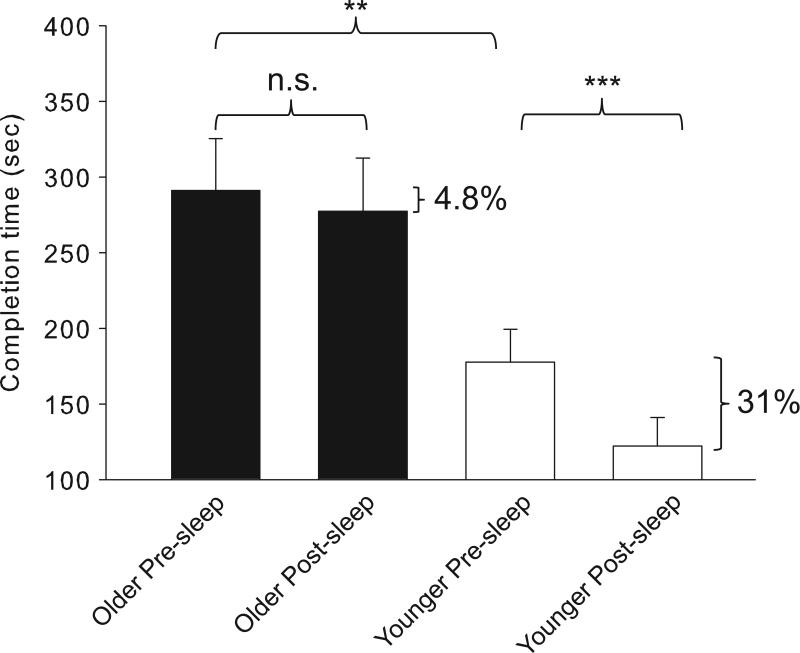
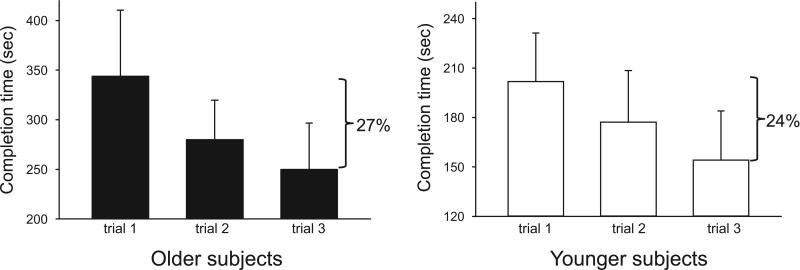
With regard to prior 3D video game experience, among younger respondents, 9 of 12 qualified as novice and 3 of 12 as experienced. In the older subjects, 12 of 13 qualified as novice and 1 of 13 qualified as experienced (chi square = 1.39, df = 1, p = 0.24). We tracked subjects’ average maze performance before and after sleep by measure of completion time, and observed a significant difference across time (F(1,,29) = 8.2, p = 0.008) and age group (F(1,29) = 18.4, p < 0.001), with a significant interaction between time and age group (F(1,29) = 5.4, p = 0.027). In the three pre-sleep trials, older subjects took significantly longer to complete the maze than younger subjects (291.2 +/− 34.3 sec in older subjects vs. 177.7 +/− 21.5 sec in younger subjects, p = 0.008, simple main effect) (Figure 3). Despite this difference in baseline performance, there was no significant interaction between pre-sleep trial number and age group (F(1,29) = 0.03, p = 0.88), and the degree of improvement from the first trial to third trial before sleep was similar between the groups, suggesting that younger and older subjects encode the information similarly (Figure 4). Across a night of sleep, there was significant improvement in completion time in younger subjects on morning trials compared to evening trials (177.7 +/− 21.5 sec before sleep vs. 122.2 +/− 18.8 sec after sleep, p < 0.001, simple main effect), representing a 31% improvement. This phenomenon was not observed in older subjects (291.2 +/− 34.3 sec before sleep vs. 277.2 +/− 35.4 sec after sleep, p = 0.73, simple main effect), where the improvement was only 4.8%.
Figure 3.
Gains in virtual maze performance across sleep are age dependent. Older subjects take longer than younger subjects to complete pre-sleep maze trials (**p = 0.008, simple main effect, mixed factorial ANOVA). Across sleep, younger subjects improve by 31% (177.7 +/− 21.5 sec before sleep vs. 122.2 +/− 18.8 sec after sleep, ***p < 0.001, simple main effect, mixed factorial ANOVA) whereas older subjects improve by only 4.8% (291.2 +/− 34.3 sec before sleep vs. 277.2 +/− 35.4 sec after sleep, p = 0.73, simple main effect, mixed factorial ANOVA). n.s. = non-significant.
Figure 4.
Rate of initial improvement across the pre-sleep maze trials is similar between younger and older subjects.
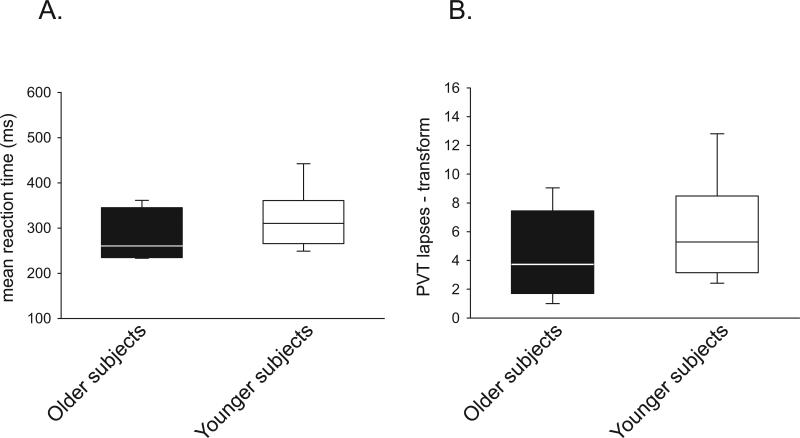
In order to control for possible effects on attention affecting spatial navigation performance in the morning, subjects performed a twenty-minute psychomotor vigilance test immediately prior to completing the three morning test trials on the virtual maze. We observed no significant differences between groups in mean reaction times (276.7 ms (median) in older subjects, 310.4 ms (median) in younger subjects, p = 0.327, rank sum test) or in number of lapses (3.7 (median) in older subjects, 5.3 (median) in younger subjects, p = 0.335, rank sum test) (Figure 5).
Figure 5.
Morning psychomotor vigilance is not different between older subjects and younger subjects. There is no difference in (A) mean reaction time between older and younger subjects (310.4 ms in younger subjects, 276.7 ms in older subjects, p = 0.327, rank sum test) or (B) in a transform of the number of lapses (5.3 (median) in younger subjects, 3.7 (median) in older subjects, p = 0.335, rank sum test)
3.3 Sleep Associations with Memory and Medial Prefrontal Cortical Volume
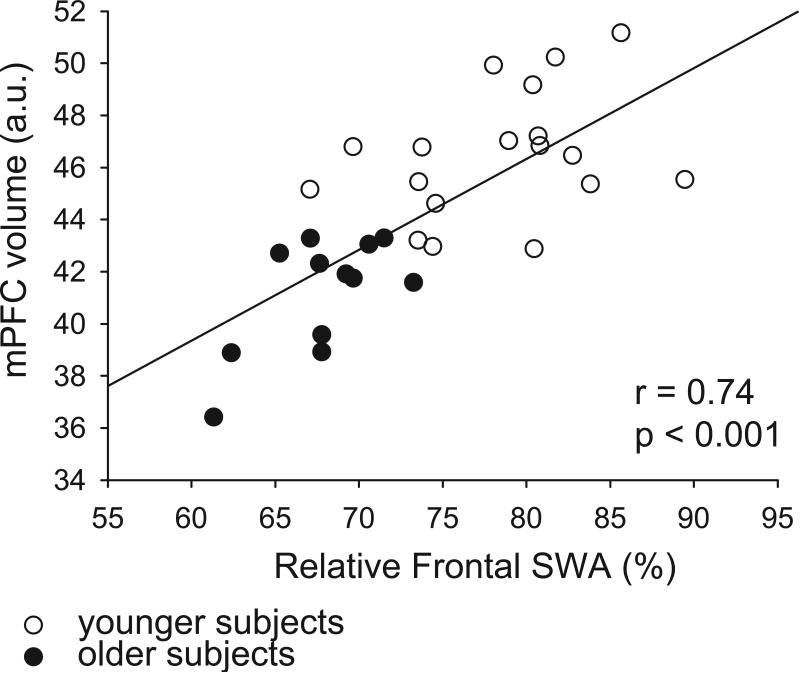
Cortical slow waves in sleep are generated primarily frontally and travel posteriorly (Murphy et al., 2009), and in adults, slow wave activity is greatest over frontal EEG leads (Ringli and Huber, 2011). Prior studies have suggested that medial prefrontal cortical atrophy in older subjects is associated with reduced slow wave activity (Mander et al., 2013). Across all subjects we observed a significant positive correlation between medial prefrontal cortical volume and average relative frontal slow wave activity (r = 0.74, p < 0.001) (Figure 6). Using a partial correlation to control for age, we showed a continued significant positive partial correlation between medial prefrontal cortical volume and average relative frontal slow wave activity (r = 0.52, p = 0.004), suggesting that controlling for age had little effect on the strength of the relationship between these variables.
Figure 6.
Relative frontal slow wave activity positively correlates with medial prefrontal cortical volume across all subjects. Scatter plot of the relationship between relative frontal slow wave activity and medial prefrontal cortical volume for older subjects (black circles) and younger subjects (white circles). r = 0.74, p < 0.001, Pearson's correlation coefficient.
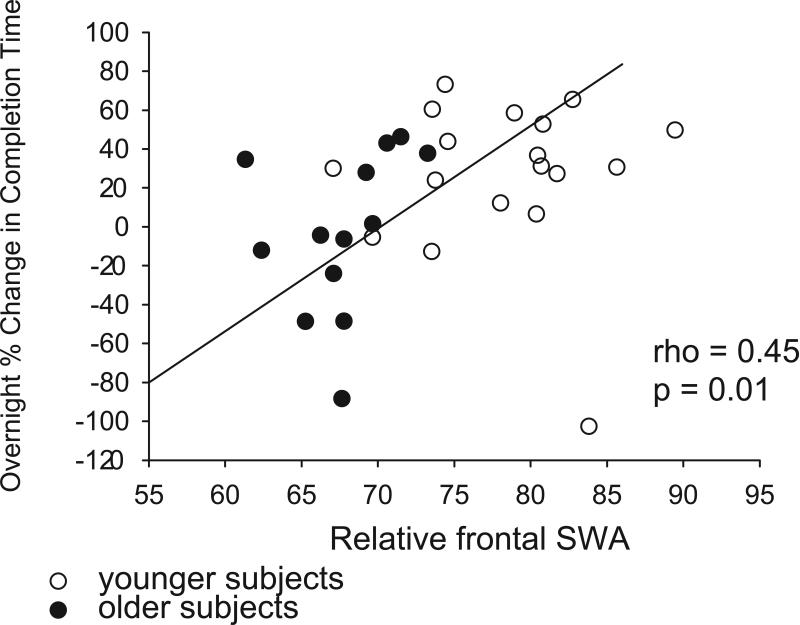
Due to the importance of slow wave sleep in the consolidation of spatial navigational memories, we examined the relationship between prefrontal slow wave activity and overnight change in maze performance. Across all subjects, there was a significant positive correlation between average relative frontal slow wave activity and the overnight percent change in maze completion time (rho = 0.45, p = 0.01) (Figure 7). A partial correlation controlling for age demonstrated a reduced strength of association between average relative frontal slow wave activity and the overnight percent change in maze completion time (rho = 0.3, p = 0.056), suggesting that age has some mediating effect on the relationship between relative frontal slow wave activity and spatial navigational memory. Notably, we did not observe a significant correlation between medial prefrontal cortical volume and overnight change in maze performance (rho = 0.19, p = 0.31) (Supplemental Figure 1).
Figure 7.
Relative frontal slow wave activity positively correlates with overnight spatial navigational memory consolidation across all subjects. Scatter plot of the relationship between relative frontal slow wave activity and overnight change in virtual maze completion time for older subjects (black circles) and younger subjects (white circles). rho = 0.45, p = 0.01, Spearman rank order correlation.
4. Discussion
In this study we demonstrate that overnight sleep acts to benefit spatial navigational memory performance in younger subjects, but this benefit is significantly attenuated in older subjects. The 31% improvement in completion time in the younger group in this study is similar to what has been observed in other studies of overnight improvement in completion time on related virtual mazes in college-aged subjects (Ferrara et al., 2008; Nguyen et al., 2013). The observation that older subjects take longer to complete the initial trials on the virtual maze before sleep is consistent with prior findings in which older and younger subjects performed consecutive trials on a comparable virtual maze (Moffat et al., 2001). However, consolidation of this spatial information during sleep has not previously been examined in older individuals. Studies have demonstrated deficits in sleep-dependent memory consolidation for declarative memories in middle aged (Backhaus et al., 2007) and older subjects (Mander et al., 2013), and for visuospatial object location memory in older subjects (Cherdieu et al., 2014), although there are some discrepancies showing no differences (Wilson et al., 2012). Sleep-dependent consolidation of motor sequence memories also appears to be detrimentally affected with aging (Brown et al., 2009; Spencer et al., 2007), and the degree of the deficit may depend on task demands (Gudberg et al., 2015).
The lack of overnight maze performance improvement may depend on factors not strictly related to sleep. For example, it may be related to poor initial encoding of the spatial information or morning sleepiness or inattention related to differences in sleep architecture. However, these possibilities are not likely playing a substantial role in the age group differences because both groups showed similar rates of improvement in completion time across the first three maze trials before sleep, suggesting similar encoding efficiency. That said, older subjects have been shown to rely less on allocentric strategies that utilize the hippocampus during the encoding of spatial navigational information (Bohbot et al., 2012; Konishi et al., 2013), which may then have implications for how these memories are subsequently consolidated in sleep. Initial raw performance did not predict subsequent overnight consolidation as there was no correlation between average pre-sleep completion time and subsequent overnight percent change in completion time in all subjects. Differences in attention appear unlikely to account for differences in morning maze performance as there were no significant differences between the groups in morning reaction time or number of lapses on the psychomotor vigilance test, suggesting similar levels of alertness. Prior 3D video game experience may influence spatial navigational memory consolidation across sleep (Wamsley et al., 2010). Although we cannot completely rule out this potential confound, we did not observe any significant difference in prior 3D video game experience between the younger and older responding subjects.
Assuming that sleep is playing an active role in the consolidation of spatial navigational memories, there are several potential qualities of sleep differing between younger and older subjects that could be contributing. Older subjects typically have reduced total sleep time and sleep efficiency compared to younger subjects. Additionally, the degree of sleep apnea increases with age, resulting in increased sleep fragmentation (Ware et al., 2000). However, in our populations, there were no significant differences in total sleep time, sleep efficiency, or severity of sleep apnea, a phenomenon possibly achieved by excluding subjects with greater than mild obstructive sleep apnea. Evidence from both animal models (Bjorness et al., 2005; Smith and Rose, 1996) and humans (Varga et al., 2014) suggests that REM sleep may be important in the consolidation of spatial navigational memory. The differences in REM sleep in our groups are unlikely to explain the performance differences observed as the older subjects actually had a slightly greater percentage of REM sleep than younger subjects, and there were no differences in the degree of REM fragmentation.
The differences in slow wave sleep, and in particular, in relative slow wave activity in the prefrontal region, offer the most parsimonious explanation for the differences in observed consolidation of spatial navigational memory between the younger and older groups. Percent of slow wave sleep positively correlated with memory for word pairs in younger, but not older subjects (Scullin, 2013). Slow wave-specific sleep disruption via auditory stimulation without changes in total sleep have been shown to impair declarative memory (Van Der Werf et al., 2011). Conversely, enhancement of neocortical slow waves via auditory stimulation in phase with the ongoing rhythmic occurrence of slow oscillation up states (Ngo et al., 2013) or via transcranial electrical stimulation in younger (Marshall et al., 2006) and older subjects (Westerberg et al., 2015) enhanced declarative memory across sleep. While the effects of similar bidirectional manipulations of slow wave sleep on spatial navigational memory have not been completed, imaging studies have demonstrated that the degree of blood flow to the right hippocampus during slow wave sleep correlated with the degree of improvement across sleep in a spatial navigational task (Peigneux et al., 2004).
Sleep slow oscillations are thought to generate from an interaction between thalamic and cortical circuits. Slow waves themselves are traveling waves that originate frontally over prefrontal and orbitofrontal cortex and travel anterioposteriorly (Massimini et al., 2004). Brain atrophy with aging disproportionately affects frontal cortex and may represent one mechanism by which slow wave sleep diminishes with age. Our observation that relative frontal slow wave activity correlates with medial prefrontal cortical volume is consistent with this theory. Our data cannot exclude the possibility that the reduced medial prefrontal cortical volume with aging contributes to impaired spatial navigational memory consolidation independent of effects on slow wave sleep, however, we did not observe a significant correlation between medial prefrontal cortical volume and overnight change in maze performance. This is in contrast to the negative correlation observed between orbital prefrontal cortical volume and working memory performance in older subjects, although this study also found larger orbital prefrontal cortical volumes with advanced age (Salat et al., 2002). This is also in contrast to the positive correlation observed between medial prefrontal cortical volume and overnight declarative memory performance (Mander et al., 2013). Notably though, in this study neither age nor medial prefrontal cortical gray matter volume significantly predicted the extent of overnight declarative memory retention when frontal slow wave activity was included in the statistical model.
In sum we have demonstrated that the sleep of older subjects does not impart the same benefits toward the consolidation of spatial navigational memory as the sleep of younger subjects. This effect is likely attributable to reduced slow wave activity in older subjects, the consequence of both decreased amount of and increased fragmentation of slow wave sleep. It remains possible that impoverished spatial navigational memory, reduced slow wave activity and prefrontal brain atrophy are independent hallmarks of aging. However, our data support the emerging model in which these variables are related and open avenues to explore the causal interrelationships between these factors further. Our data highlight the importance of sleep quality that is challenged in aging not only from the natural decreases in slow wave sleep, but also from factors such as the increased incidence of OSA with aging and the societal tendency to accumulate sleep debt volitionally.
Supplementary Material
Aging is associated with fragmentation of slow wave sleep but spares other stages
Spatial memory consolidation during sleep is seen in younger but not older subjects
Initial rate of spatial learning and morning vigilance are similar in young and old
Degree of overnight memory performance change correlates with slow wave activity
Slow wave activity correlates with medial prefrontal cortical volume
Acknowledements
We thank Rakhil Kanevskaya and Boris Opancha for expert sleep scoring and Justin Lu for IT support. This work was supported by the philanthropy of the James Kuhn Friends of Sleep Medicine, the Leon Levy Foundation Neuroscience Fellowship (A.W.V.), the NYU CTSA grant UL1TR000038 from the National Center for the Advancement of Translational Science (NCATS) (A.W.V), the American Sleep Medicine Foundation Junior Faculty Research Award (A.W.V.), NIEHS Training Grant T32-ES007267 (Principal Investigator William N. Rom), K24-HL109156 (I.A.), R01- DK083537 (A.C), and R01- HL118624 (R.S.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem. 2005;12:352–359. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, Boivin M, Robaey P. Virtual navigation strategies from childhood to senescence: evidence for changes across the life span. Frontiers in aging neuroscience. 2012;4:28. doi: 10.3389/fnagi.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PloS one. 2009;4:e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherdieu M, Reynaud E, Uhlrich J, Versace R, Mazza S. Does age worsen sleep-dependent memory consolidation? Journal of sleep research. 2014;23:53–60. doi: 10.1111/jsr.12100. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Ferrara M, Iaria G, Tempesta D, Curcio G, Moroni F, Marzano C, De Gennaro L, Pacitti C. Sleep to find your way: the role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–851. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nature neuroscience. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, Mistur R, Pirraglia E, de Santi S, Li Y, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. doi: 10.1038/jcbfm.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudberg C, Wulff K, Johansen-Berg H. Sleep-dependent motor memory consolidation in older adults depends on task demands. Neurobiology of aging. 2015;36:1409–1416. doi: 10.1016/j.neurobiolaging.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- Kishi A, Natelson BH, Togo F, Struzik ZR, Rapoport DM, Yamamoto Y. Sleep-stage dynamics in patients with chronic fatigue syndrome with or without fibromyalgia. Sleep. 2011;34:1551–1560. doi: 10.5665/sleep.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Wang W, Duffy JF, Dijk DJ, Czeisler CA, Kronauer RE. Survival analysis indicates that age-related decline in sleep continuity occurs exclusively during NREM sleep. Neurobiology of aging. 2013;34:309–318. doi: 10.1016/j.neurobiolaging.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi K, Etchamendy N, Roy S, Marighetto A, Rajah N, Bohbot VD. Decreased functional magnetic resonance imaging activity in the hippocampus in favor of the caudate nucleus in older adults tested in a virtual navigation task. Hippocampus. 2013;23:1005–1014. doi: 10.1002/hipo.22181. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature neuroscience. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiology of aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Nguyen ND, Tucker MA, Stickgold R, Wamsley EJ. Overnight Sleep Enhances Hippocampus-Dependent Aspects of Spatial Memory. Sleep. 2013;36:1051–1057. doi: 10.5665/sleep.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RG, Scott MA, Ayappa I, Walsleben JA, Rapoport DM. Sleep continuity measured by survival curve analysis. Sleep. 2006;29:1625–1631. doi: 10.1093/sleep/29.12.1625. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Progress in brain research. 2011;193:63–82. doi: 10.1016/B978-0-444-53839-0.00005-3. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28:105–114. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiology & behavior. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nature neuroscience. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Vis JC, Koene T, Van Someren EJ. Reduction of nocturnal slow-wave activity affects daytime vigilance lapses and memory encoding but not reaction time or implicit learning. Progress in brain research. 2011;193:245–255. doi: 10.1016/B978-0-444-53839-0.00016-8. [DOI] [PubMed] [Google Scholar]
- Varga AW, Kishi A, Mantua J, Lim J, Koushyk V, Leibert DP, Osorio RS, Rapoport DM, Ayappa I. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14571–14577. doi: 10.1523/JNEUROSCI.3220-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Payne JD, Stickgold R. A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learn Mem. 2010;17:332–336. doi: 10.1101/lm.1828310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–170. [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, Paller KA. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiology of aging. 2015 doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22:1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.