Abstract
Scedosporium apiospermum, a ubiquitous environmental mold, is increasingly reported as causing invasive fungal disease in immunocompromised hosts. It poses a therapeutic challenge due to its intrinsic resistance to traditional antifungals and ability to recur despite demonstrating susceptibility. We present an immunocompromised patient with a cutaneous S. apiospermum infection that disseminated despite treatment with voriconazole, the drug of choice. Adding echinocandins and GM-CSF provided partial recovery, indicating a potential synergistic role of dual-antifungal and immunotherapeutic agents.
Keywords: Scedosporium, Synergy, GM-CSF, Immunocompromised, Corticosteroid
1. Introduction
Scedosporium apiospermum, once considered the asexual form of Pseudoallescheria boydii, is a filamentous fungus found worldwide in soil, sewage, and polluted waters [1]. Previously considered exceedingly rare, S. apiospermum is increasingly reported as a cause of opportunistic infection, as use of corticosteroids, immunosuppressants, antineoplastics, and broad-spectrum antibiotics have become more widespread [2]. Furthermore, it is thought that increased use of antifungals in immunocompromised patients with agents that have activity against Candida spp. and Aspergillus fumigatus but only modest or no activity against Scedosporium (e.g. amphotericin B and echinocandins), may exert a selective pressure and contribute to the increased incidence of Scedosporium infections [3].
S. apiospermum infections most commonly occur in the paranasal sinuses, lungs, skin, soft tissue, central nervous system, and bones, but disseminated disease is also common and often fatal [1]. Herein we report a case of disseminated infection from a cutaneous source in a patient exposed to steroids in which progression of disease was observed despite adequate treatment with voriconazole, the current drug of choice. S. apiospermum poses a therapeutic challenge due to its intrinsic resistance to commonly used antifungal agents and its ability to recur even when susceptibility to these medications is demonstrated. In our case, the addition of echinocandins and granulocyte macrophage colony-stimulating factor (GM-CSF) to the patient's treatment regimen provided partial recovery. This case demonstrates a potential synergistic role for dual-antifungal treatment with adjunctive immunotherapeutic agents in the treatment of S. apiospermum infections. As S. apiospsermum infections become increasingly prevalent, further consideration and investigation of this combination therapy is necessary to combat this highly fatal and aggressive organism.
2. Case
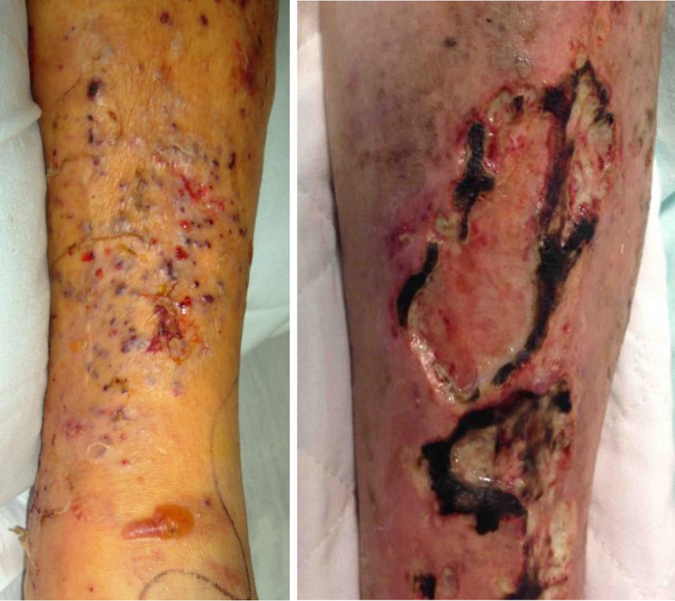
A 77-year-old man on high dose steroids for presumed temporal arteritis presented on day 0 with a 10 day history of progressive swelling, erythema, and pain of the left leg. He denied fever, chills, nausea, vomiting or diarrhea and denied any history of trauma or travel inside or outside the United States. The patient's past medical history included hypercholesterolemia, hypertension, congestive heart failure, coronary artery disease, chronic obstructive pulmonary disease, benign prostatic hypertrophy and sphenoid sinusitis. He was admitted to the hospital on day 0 for treatment of leg cellulitis. Physical exam revealed diffuse, circumferential, macular erythema and warmth extending from the left ankle to the popliteal fossa. There were three, 5 mm pink papules with fine scale at the superior-most aspect of the erythema. On the anterior tibia there were two 1 cm flaccid bullae. The leg was non-tender. The patient received vancomycin and cefepime and after 7 days of therapy there was partial improvement of the erythema; however, on day +7, new diffuse, non-tender, 0.5–1.5 cm subcutaneous nodules emerged (Fig. 1A). Histopathologic examination revealed a dermal nodular infiltrate of neutrophils with surrounding histiocytes, some of which were multinucleated. A periodic acid-Schiff-diastase (PAS-D) stain revealed small, narrow angled, branching hyphae. Wound cultures were positive for mold, subsequently identified as S. apiospermum. Identification was based on phenotypic characteristics and sequencing of the intertranscribed spacer (ITS) region, which was compared to reference data available at GenBank using the basic local alignment search tool (BLAST) [4]. The isolate was susceptible to voriconazole and posaconazole, but resistant to amphotericin B, 5-fluorocytosine, itraconazole, and caspofungin using the Sensititre YeastOne kit [5], as shown in Table 1. Other cultures for bacteria and acid-fast bacilli were negative. On day +10, the patient was started on intravenous (IV) voriconazole 6 mg/kg for 1 day followed by 4 mg/kg for 4 days, and he continued on oral voriconazole 200 mg q12 hours as an outpatient. Therapeutic trough levels were measured and maintained in a range of 4–6 mg/L. The prednisone, which had been started on day −60 at a dose of 60 mg, had been tapered to 40 mg at day 0 and was tapered off completely by day +84.
Fig. 1.
A. Left shin on initial presentation after treatment with antibiotic therapy. B. Left shin lesion after addition of micafungin and GM-CSF. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
Table 1.
Sensitivities of Scedosporium apiospermum isolate.
| Antifungal | First admission MICa (μg/ml) | Second admissions MICb (μg/ml) | Synergyb | MICb (μg/ml) | Interpretation |
|---|---|---|---|---|---|
| AMB | 2 | >2 | AMB+CAS | >2+>4 | Indifferent |
| MON | – | 0.5 | AMB+MICA | >2+>4 | Indifferent |
| ITC | >16 | – | |||
| VRC | 0.12 | 1 | AMB+POS | 2+4 | Indifferent |
| POS | 0.5 | >4 | AMB+VRC | 2+0.5 | Indifferent |
| MICA | – | 0.5 | MICA+TRB | 0.5+<0.015 | Indifferent |
| CAS | >8 | 1 | |||
| ANID | – | 4 | |||
| KTC | – | 1 | |||
| TRB | – | >2 | |||
| 5FC | >64 | – |
AMB, amphotericin B; MON, miconazole; ITC, itraconazole; 5FC, flucytosine; VRC, voriconazole; TRB, terbinafine; CAS, caspofungin; MICA, micafungin; POS, posaconazole; ANID, anidulafungin; KTC, ketoconazole
Obtained by CLSI adapted method based on breakpoints available for pathogenic yeasts, Wadsworth Center Laboratories, Albany NY.
Obtained by M38-A2 CLSI broth dilution antifungal susceptibility testing, University of Texas Health Science Center at San Antonio, South Texas reference laboratories.
On day +99, the patient was admitted for acute decompensated heart failure. At that time, voriconazole was discontinued given improvement of his left lower extremity lesion. By day +114 (15 days since discontinuing therapy), recurrence of disease was observed with new metastatic nodules in the upper extremities, one of which was incised and drained and again grew S. apiospermum with sensitivities and synergy studies shown in Table 1. IV voriconazole was restarted at 6 mg/kg for 1 day followed by 4 mg/kg for 5 days and was continued orally at 200 mg q12 hours as an outpatient. The patient was subsequently re-admitted on day +155 with septic shock thought to be due to bacterial superinfection of the left leg lesions given an increased area of ulceration, oozing and eschar formation. New metastatic lesions in his upper extremities were also observed at that time. Computed tomography scans of the left leg throughout all three admissions were consistent with progressing cellulitis without evidence of osteomyelitis. The margin of the left leg ulcer was biopsied and a PAS-D stain revealed fungal elements. Wound culture again grew S. apiospermum. In addition to IV voriconazole 4 mg/kg, Micafungin 100 mg and GM-CSF 250 μg IV daily were started as salvage therapy. An improvement in the left leg lesion was noted (Fig. 1B), no new nodules emerged, and the existing nodules regressed. He remained stable and was discharged to physical rehabilitation on oral voriconazole 200 mg q12 hours and micafungin 100 mg daily for an indefinite period of time. The patient remained on dual-antifungal suppressive therapy until he presented on day +256 with sepsis secondary to pneumonia from which he was not able to recover with antibiotic therapy, and he expired on day +266.
3. Discussion
This case report draws attention to the importance of having increased awareness of S. apiospermum, particularly in immunocompromised patients. However, it is unlikely for patients to provide a history that raises suspicion for this organism in particular. For example, while most cases of cutaneous S. apiospermum infection follow surgery or trauma, particularly when there is contact with soil where this organism is ubiquitious, most patients will specifically deny any history of trauma [2]. However, even minor lesions on extremities that go unnoticed can provide a portal of entry for this organism, as suggested by Uenotsuchi et al.’s review of 20 cases of cutaneous S. apiospermum infection that all happened to have occurred on extremities [2]. In line with this, our patient's lesion was located on the left leg and he did not report any history of trauma. Interestingly, he was a resident of the Rockaway Peninsula of New York City, which was severely affected by Hurricane Sandy approximately 6 months prior to his initial presentation. We hypothesize that environmental destruction may have led to increased epidemiologic risk in this immunosuppressed host after he returned to his home in the aftermath of the hurricane.
Accurate and prompt diagnosis of S. apiospermum is essential, as this organism can be often misidentified as other molds with different resistance profiles, such as Aspergillus spp. and Fusarium spp [1]. These three species are angioinvasive and have non-pigmented, slender, septate hyphae 2–4 µm in width. Scedosporium spp. can be differentiated by their more irregular branching pattern compared to the orderly dichotomous 45-° angle branching observed in Aspergillus and by their production of ovoid conidia with truncated bases, which can be confused with yeast [6]. These distinguishing features, however, are often absent from histological specimens [1], rendering a diagnosis by histopathological means alone unreliable. Shah et al. found that the diagnosis of Aspergillus by histopathologic and cytopathalogic examination was only 78% accurate [6]. The erroneous diagnosis of Aspergillus was often favored over both common (Scedosporium, Fusarium, and Paecilomyces spp.) and uncommon mimickers (Trichosporon loubieri). Culture is routinely used for diagnosis and speciation but even its utility is limited by slow growth, false-negatives, and contaminations with other organisms [1]. In our case, the early detection of fungal elements in the dermis, phenotypic characteristics on culture, and sequencing of the ITS region were all necessary to make a timely and accurate diagnosis.
Treatment of Scedosporium spp. is often difficult, particularly when numerous small abscesses are not amenable to surgical intervention, as inadequate debridement impedes the penetration of systemic antifungals. Scedosporium spp. are inherently resistant to amphotericin B and frequently, though unpredictably, resistant to azoles [7]. The broad-spectrum azole voriconazole is the drug of choice for S. apiospermum infections. It has demonstrated fungicidal activity against S. apiospermum in vitro, and it has been shown to be effective in mouse and guinea pig models [8] as well as in case reports in humans [9], [10]. Duration and efficacy of therapy is not widely known due to a lack of large studies. However, in one of the largest studies on the treatment of scedosporiosis with voriconazole, 57% of patients achieved response at a median of 103 days [11]. Recurrences are common, as seen in our patient, and might happen even without interruption of therapy or development of resistance to voriconazole.
A marked clinical response was observed when micafungin and GM-CSF were started and no further metastatic nodules were observed. The combination of micafungin and voriconazole has been demonstrated to have a synergistic effect against several fungi in vitro including Scedosporium spp [12]. The underlying mechanism is thought to involve cell wall reorganization and exposure of β-glucan, resulting in enhanced immune recognition [13]. In clinical practice, combination antifungal therapy is more often necessary with the highly resistant S. prolificans, and treatment response has been demonstrated in several cases [14], [15] as well as for other invasive filamentous fungal infections [16]. While antifungal therapy is important as an adjuvant, the containment and ultimate clearance of Scedosporium infections is dependent on innate host defense, particularly the numeric and functional activity of polymorphonuclear cells (PMNs). The addition of GM-CSF and interferon-gamma (IFN-γ) have been shown to increase antifungal activity of PMNs in vitro [17], which is thought to be due to enhancement of phagocytosis and oxidative burst leading to increased hyphal damage [18]. There has been one reported case of GM-CSF and IFN-γ used in concert with several antifungals, including itraconazole and amphotericin B, as salvage therapy, which ultimately resulted in clinical cure in a 10-year-old HIV-positive patient who presented with S. apiospermum otomastoiditis [19]. However, to our knowledge, this is the first reported case of dual-antifungal therapy with adjunctive cytokine therapy in the treatment of refractory S. apiospermum in the non-pediatric literature.
Although the cause of this patient's death was not suspected to be due to scedosporiosis, a clear clinical cure was not achieved. Therapeutic considerations may have included more prolonged treatment with GM-CSF or the addition of IFN-γ, however no standardized regimens for dual-antifungal or cytokine therapies currently exist. Given the importance of this emerging area and a paucity of guidelines to guide management, more studies are needed to outline appropriate administration of combination antifungals and cytokines as salvage therapies for the management of refractory scedosporiosis.
Conflict of interest
There are none.
Acknowledgements
There are none.
References
- 1.Shinohara M.M., George E. Scedosporium apiospermum: an emerging opportunistic pathogen that must be distinguished from Aspergillus and other hyalohyphomycetes. J. Cutan. Pathol. 2009;36:39–41. doi: 10.1111/j.1600-0560.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 2.Uenotsuchi T., Moroi Y., Urabe K., Tsuji G., Koga T., Matsuda T. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient and a review of the literature. Acta Derm. Venereol. 2005;85(2):156–159. doi: 10.1080/00015550410024553. [DOI] [PubMed] [Google Scholar]
- 3.Lamaris G.A., Chamilos G., Lewis R.E., Safdar A. Raad II, Kontoyiannis DP. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989 to 2006. Clin. Infect. Dis. 2006;43(12):1580–1584. doi: 10.1086/509579. [DOI] [PubMed] [Google Scholar]
- 4.Castelli M.V., Buitrago M.J., Bernal-Martinez L., Gomez-Lopez A., Rodriguez-Tudela J.L., Cuenca-Estrella M. Development and validation of a quantitative PCR assay for diagnosis of scedosporiosis. J. Clin. Microbiol. 2008;46(10):3412–3416. doi: 10.1128/JCM.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linares M.J., Charriel G., Solís F., Rodriguez F., Ibarra A., Casal M. Susceptibility of filamentous fungi to voriconazole tested by two microdilution methods. J. Clin. Microbiol. 2005;43(1):250–253. doi: 10.1128/JCM.43.1.250-253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah A.A., Hazen K.C. Diagnostic accuracy of histopathologic and cytopathologic examination of Aspergillus species. Am. J. Clin. Pathol. 2013;139(1):55–61. doi: 10.1309/AJCPO8VTSK3HRNUT. [DOI] [PubMed] [Google Scholar]
- 7.Lackner M., de Hoog G.S., Verweij P.E., Najafzadeh M.J., Curfs-Breuker I., Klaassen C.H. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob. Agents Chemother. 2012;56(5):2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capilla J., Guarro J. Correlation between in vitro susceptibility of Scedosporium apiospermum to voriconazole and in vivo outcome of scedosporiosis in guinea pigs. Antimicrob. Agents Chemother. 2004;48(10):4009–4011. doi: 10.1128/AAC.48.10.4009-4011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girmenia C., Luzi G., Monaco M., Martino P. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 1998;36(5):1436–1438. doi: 10.1128/jcm.36.5.1436-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanafani Z.A., Comair Y., Kanj S.S. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23(11):836–840. doi: 10.1007/s10096-004-1233-2. [DOI] [PubMed] [Google Scholar]
- 11.Troke P., Aguirrebengoa K., Arteaga C., Ellis D., Heath C.H., Lutsar I. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyn K., Tredup A., Salvenmoser S., Müller F.M. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob. Agents Chemother. 2005;49(12):5157–5159. doi: 10.1128/AAC.49.12.5157-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdar A. Fungal cytoskeleton dysfunction or immune activation triggered by beta-glucan synthase inhibitors: potential mechanisms for the prolonged antifungal activity of echinocandins. Cancer. 2009;115(3):2812–2815. doi: 10.1002/cncr.24323. [DOI] [PubMed] [Google Scholar]
- 14.Steinbach W.J., Schell W.A., Miller J.L., Perfect J.R. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 2003;41(8):3981–3985. doi: 10.1128/JCM.41.8.3981-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howden B.P., Slavin M.A., Schwarer A.P., Mijch A.M. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22(2):111–113. doi: 10.1007/s10096-002-0877-z. [DOI] [PubMed] [Google Scholar]
- 16.Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin. Infect. Dis. 2002;34(10):1415–1417. doi: 10.1086/340260. [DOI] [PubMed] [Google Scholar]
- 17.Gil-Lamaignere C., Winn R.M., Simitsopoulou M., Maloukou A., Walsh T.J., Roilides E. Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparison with Aspergillus spp. Med. Mycol. 2005;43(3):253–260. doi: 10.1080/13693780412331271072. [DOI] [PubMed] [Google Scholar]
- 18.Roilides E., Simitsopoulou M., Katragkou A., Walsh T.J. Host immune response against Scedosporium species. Med. Mycol. 2009;47(4):433–440. doi: 10.1080/13693780902738006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abzug M.J., Walsh T.J. Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr. Infect. Dis. J. 2004;23(8):769–773. doi: 10.1097/01.inf.0000134314.65398.bf. [DOI] [PubMed] [Google Scholar]