Abstract
Background
Information on socioeconomic determinants in the management of diabetes mellitus is scarce in lower middle income countries. The aim of this study is to describe the socioeconomic determinants of management and complications of diabetes mellitus in a lower middle income setting.
Methods
Cross sectional descriptive study on a stratified random sample of 1300 individuals was conducted by an interviewer administered questionnaire, clinical examinations and blood investigations. A single fasting venous blood sugar of ≥126 mg/dl was considered diagnostic of new diabetics and poor control of diabetes mellitus as HbA1C > 6.5 %.
Results
There were 202 (14.7 %) with diabetes mellitus. Poor control was seen in 130 (90.7 %) while 71 (49.6 %) were not on regular treatment. Highest proportions of poor control and not on regular medication were observed in estate sector, poorest social status category and poorest geographical area. The annual HbA1C, microalbuminuria, retinal and neuropathy examination were performed in less than 6.0 %. Social gradient not observed in the management lapses. Most (76.6 %) had accessed private sector while those in estate (58.1 %) accessed the state system.
The microvascular complications of retinopathy, neuropathy and microalbuminuria observed in 11.1 %, 79.3 % and 54.5 % respectively. Among the macrovascular diseases, angina, ischaemic heart disease and peripheral arterial disease seen in 15.5 %, 15.7 % and 5.5 % respectively. These complications do not show a social gradient.
Conclusions
Diabetes mellitus patients, irrespective of their socioeconomic status, are poorly managed and have high rates of complications. Most depend on the private healthcare system with overall poor access to care in the estate sector.
Keywords: Diabetes mellitus complications, Diabetes mellitus management, Socioeconomic determinants
Background
Diabetes mellitus is a global public health problem with a majority in developing countries [1]. In 2008 the age-standardised prevalence of diabetes mellitus for men and women was 9.8 % (8.6–11.2) and 9.2 % (8.0–10.5) respectively, with 40 % of these residing in China and India [1]. The projected increase over the period from 1995-2025 for developed and developing countries was 42 % and 170 % respectively [2]. In 2025 it is expected that 75 % of diabetics will reside in developing countries [3].
Diabetes mellitus is a progressive disease requiring effective lifelong medical care for the prevention of secondary and tertiary complications. The optimal control of blood glucose has clearly demonstrated a significant decrease in the development of complications [4]. Control of diabetes mellitus requires the combination of treatment and preventive action, taking into account biological and health behavioural factors, health service responsiveness, and socioeconomic conditions [5].
The relationship between socioeconomic positions and the prevalence, management and complications of diabetes mellitus are well known in High Income Countries (HICs) with low socioeconomic status associated with unhealthy behaviours, poor access to care, deficient processes of care and high rate of diabetes complications [6–19].
In Low Income Countries (LICs)/Lower Middle Income Countries (LMICs) the socioeconomic factors are mostly explored in relation to the prevalence of diabetes mellitus while little is known on social determinants of complications and management of this condition [3, 20–24]. According to the World Bank LICs are defined as those with a gross national income per capita of $1,045 or less in 2014 while for HICs the figure is $12,736 or more [25].The few studies which investigate the complications and management of diabetes mellitus in low and lower middle income settings are usually restricted to hospital attendees [22, 26, 27]. The available data on complications and management of diabetes mellitus in Sri Lanka are limited to state hospital attendees, excluding many patients who obtain services from the private healthcare sector [28–34]. In Sri Lanka the out-patient care for more than 50 % of the population is provided by the private healthcare sector while in-patient care for more than 90 % is provided by the state healthcare sector [35]. State healthcare in Sri Lanka is free at the point of delivery to all citizens of Sri Lanka. This includes all visits/consultations (out-patient as well as in-patient care including intensive care services and surgical care), medications, investigations and procedures including ambulance transportation/transfers and hospital meals.
Understanding the socioeconomic determinants in the management and complications of diabetes mellitus would help to focus measures to address existing issues related diabetes mellitus [3, 36]. This would further aid to design specific strategies aimed at reducing inequalities and shed more light as to why control measures of diabetes mellitus have failed in South Asian region.
This study is a community based survey to describe the socioeconomic determinants of diabetes mellitus management in a representative sample from a suburban area, in Sri Lanka.
Methods
A detailed description of the study method is already published [37]. A sample of 1300 individuals between the ages of 35 to 64 years was randomly selected representing the urban, rural and estate sector. The estate sector mainly consists of tea, rubber and coconut plantation sectors and housed to approximately 6 % of Sri Lankans. Compared to urban and rural sectors it is the least resourced and poorest. In this study setting tea and rubber plantation sectors were included.
Data were collected using trained data collectors with validated questionnaires (social status index questionnaire, Rose questionnaire for angina detection and questionnaire to assess neuropathy symptom score & modified neuropathy disability score) [29, 30, 38, 39]. When administering the questionnaire the participants were simply asked whether they received any information regarding the diseases and if so from whom.
All participants were investigated for diabetes mellitus by conducting fasting plasma glucose (FPG) level using a venous blood sample. The blood samples were obtained after an overnight fast of at least 12 hours. The collected blood samples were analysed at the Public Health Laboratory of National Institute of Health Sciences (Kalutara), using Clini Check Plus Mini Analyser. FPG of ≥126mg/dl or those who were currently (within the past four weeks) on insulin/hypoglycaemics were considered as having diabetes mellitus [40].
The Unsatisfactory Basic Needs Index (UBNI) developed by Satharasinghe [41] and the social status index developed by De Silva [39] were utilized as measures of socioeconomic matrices. The UBNI is an area level deprivation index calculated taking into consideration the level of education, occupation, housing conditions (wall, roof and floor), source of lighting and cooking. It has a correlation coefficient of 0.62 with the Headcount ratio [41]. The social status index composed of education, occupation, income, assets and social networking. The reliability revealed a Cohen’s kappa coefficient >0.78 for each item category. The criterion (level of agreement 65 %) and construct validity (Root Mean Square Error of Approximation = 0.056) were satisfactory [39]. The highest level of education attained was recorded. Those who have passed the General Certificate of Education - Ordinary Level examination (which is held at the end of Grade 10) but has failed to complete the General Certificate of Education -Advanced Level examination was grouped as Ordinary Level to Grade 12 (which includes Grade 11 as well). Those who have passed General Certificate of Education -Advanced Level (which was held at the end of Grade 12) was grouped as Advanced Level and above.
All participants (old and new) diagnosed with diabetes mellitus were assessed by the first author who was trained on clinical examinations of diabetic complications and calibrated against a specialist (Cohen’s kappa coefficient 0.7 for diabetic retinopathy). Measures were taken to maintain the quality and accuracy of the data [37].
Diabetic retinopathy was diagnosed by clinical examination. Visual acuity was tested in corrected state, using standard 6 m Snellen chart for each eye. Patients were asked to bring their spectacles for the eye test. Pinhole correction was used when spectacles were not brought. Fundus examination was carried out by direct ophthalmoscopy performed after pupils were dilated. The patients gaze was fixed away from light except when examining the macular areas when they were asked to look directly at the light. Those in the stage of mild nonproliferative retinopathy or above were considered as having diabetic retinopathy. Neuropathy was assessed by a validated neuropathy symptom score and modified neuropathy disability score [30, 33]. A spot urine albumin was measured in the absence of urinary tract infection, with turbidimetric method using Rx Daytona machine. The presence of urine albumin >30mg/l was considered as microalbuminuria. The presence of diabetic retinopathy, neuropathy or microalbuminuria was considered as having microvascular disease.
Ischaemic Heart Disease (IHD) was diagnosed by 12 lead electrocardiography (ECG) using the Minnesota coding system [42]. Evidence of large Q or QS waves, those with complete left bundle branch block, presence of small Q waves, ST segment abnormalities and T wave abnormalities were considered as existence of IHD. Angina was diagnosed using the Rose questionnaire [38] and Peripheral Arterial Disease (PAD) was diagnosed using the ankle-brachial pressure index (ABI) [43]. The ABI of 0.91–1.30 was considered as normal. The presence of IHD, angina or PAD was considered as having macrovascular disease.
Diabetic patients who have discontinued medication for more than seven days, on one or more occasions, during the preceding year, were categorized as those who were not on continuous treatment. Visit to a medical professional (state or private sector) on every month during the preceding year was identified as successful monthly follow-ups [44]. Failure to visit on two or more consecutive months was regarded as failure in monthly follow-ups. Self reported impotence, early ejaculation or late ejaculation was considered as having sexual problems. We also documented self reported hypoglycaemic attacks. The control of diabetes was assessed by the levels of glycosylated haemoglobin [45]. Those with glycosylated haemoglobin of more than 6.5 % were considered as having poor control of diabetes mellitus. The use of Benedict’s solution at home for detection of reducing substance in urine was inquired from the study participants [46].
Data were analysed using STATA 13. Findings were weighted to make a correction for the over sampling of urban and estate sectors. Results were also adjusted for age and sex of the Sri Lankan population. Standard descriptive statistics was performed. All percentages given in the results sections are expressed as weighted values. Chi square was used to compare discrete variables.
Ethics approval was obtained from the Ethics Review Committee of Faculty of Medicine, University of Colombo (EC/08/119). Informed written consent was obtained from the study participants.
Results
From the 1,300 selected individuals 1,234 (94.9 %) participated (Fig. 1). Our previous publication showed that among the participants who were screened (628 males), 202 (14.7 %) had diabetes mellitus [37]; 22.8 % of diabetics (56 individuals) were newly diagnosed.
Fig. 1.
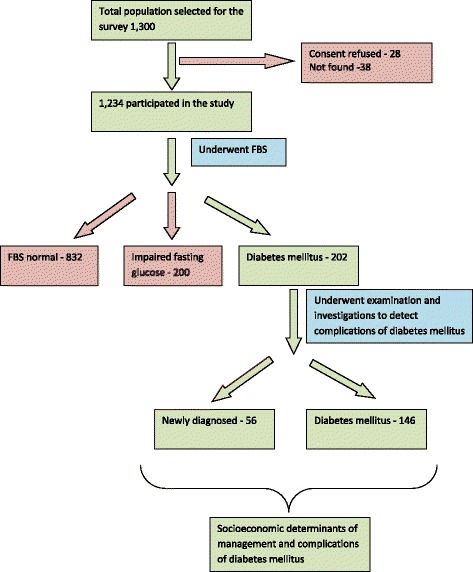
The flow chart of the study
Of the 146 (77.2 %) known to have diabetes mellitus, there were 12 (12.1 %) insulin users, and 133 (86 %) on oral medication for diabetes mellitus while one (1.9) was exclusively on diet control. While most (130, 67.1 %) received information on diet from their treating medical doctor, few (21, 13.2 %) received information on the importance of tight control of blood sugar. Table 1 describes management of already diagnosed individuals with diabetes mellitus (n = 146) and the complications among all diabetics (n = 202).
Table 1.
Management and complications of individuals with diabetes mellitus
| Management and complications | Number | Percentagea |
|---|---|---|
| Place of regular visit for treatment (n = 146) | ||
| State | 41 | 23.4 % |
| Private | 100 | 76.6 % |
| Missing | 5 | |
| Management activities (n = 146)b | ||
| Monthly follow-ups done | 53 | 38.6 % |
| Following a diabetic diet | 16 | 14.4 % |
| Diet control | 16 | 14.4 % |
| Referred to a dietician | 4 | 3.0 % |
| Diet plan by dietician | 1 | 0.1 % |
| Referred to an ophthalmology clinic | 6 | 6.6 % |
| Annual retinal examination | 3 | 4.2 % |
| Neuropathy examination | 0 | 0.0 % |
| Frequency of investigations conducted (n = 146)b | ||
| Monthly fasting plasma glucose | 62 | 47.2 % |
| Annual plasma lipids | 25 | 13.9 % |
| Annual electrocardiogram | 7 | 4.7 % |
| Annual HbA1C | 4 | 2.5 % |
| Annual urine microalbumin | 5 | 5.2 % |
| Benedict’s test at home (at least monthly) | 7 | 4.8 % |
| Capillary blood sugar at home (at least monthly) | 5 | 3.6 % |
| Complications (n = 202)b | ||
| Diabetic retinopathy | 30 | 9.8 % |
| Neuropathy | 159 | 82.9 % |
| Microalbuminurea | 61 | 33.8 % |
| Angina | 35 | 14.4 % |
| Ischaemic heart disease | 29 | 31.7 % |
| Peripheral arterial disease | 8 | 5.1 % |
| Foot ulcer | 22 | 9.0 % |
| Amputation | 2 | 0.1 % |
| Sexual problems (among males only, n = 98) (impotence, early/late ejaculation) | 43 | 61.6 % |
aAll percentages were weighted values, bBased on multiple responses
Significant differences (p < 0.05) in the frequency of diabetic retinopathy and ischaemic heart disease were observed between the newly diagnosed (diabetic retinopathy 4.1 %, ischaemic heart disease 11.1 %) and already known diabetes mellitus (diabetic retinopathy 11.5 %, ischaemic heart disease 37.6 %) groups. Almost all complications were higher except neuropathy, among the already known diabetes when compared to the newly diagnosed.
As many as 71 (49.6 %) adults with diabetes mellitus were not on regular medication. Glycaemic control, as defined by HbA1C, was poor in 130 (90.7 %) adults. Table 2 describes the distribution of diabetes management aspects by socioeconomic characteristics.
Table 2.
The distribution of diabetes mellitus (DM) management aspects by socioeconomic characteristics
| Socioeconomic characteristic | Newly diagnosed with aDM (n = 56) | Already diagnosed of aDM (146) | Poor Control of aDM (n = 130) | Not on continuous treatment (n = 71) | Monthly follow-ups not done (n = 91) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | %b | Number | %b | Number | %b | Number | %b | Number | %b | |
| Sex | ||||||||||
| Male | 34 | 27.1 % | 64 | 72.9 % | 58 | 95.8 % | 36 | 55.7 % | 48 | 75.7 % |
| Female | 22 | 19 % | 82 | 81 % | 72 | 86.5 % | 35 | 44.6 % | 43 | 49.5 % |
| Age (years) | ||||||||||
| 35 to 39 | 5 | 49.9 % | 6 | 50.1 % | 6 | 100 % | 4 | 51.0 % | 5 | 52.1 % |
| 40 to 44 | 10 | 35.2 % | 19 | 64.8 % | 17 | 89.0 % | 11 | 66.0 % | 13 | 56.4 % |
| 45 to 49 | 13 | 28.7 % | 26 | 71.3 % | 21 | 80.2 % | 13 | 31.4 % | 15 | 50.4 % |
| 50 to 54 | 4 | 0.4 % | 36 | 99.6 % | 35 | 93.5 % | 20 | 65.9 % | 25 | 79.2 % |
| 55 to 59 | 13 | 22 % | 30 | 78 % | 26 | 94.0 % | 11 | 29.5 % | 15 | 49.8 % |
| 60 to 64 | 11 | 22.7 % | 29 | 77.2 % | 25 | 92.3 % | 12 | 52.9 % | 18 | 65.4 % |
| Ethnicity | ||||||||||
| Sinhalese | 40 | 23.9 % | 116 | 76.1 % | 103 | 91.9 % | 59 | 51.2 % | 72 | 63.6 % |
| Tamil | 11 | 7.3 % | 12 | 92.7 % | 11 | 99.0 % | 6 | 4.2 % | 8 | 5.6 % |
| Muslim | 5 | 3.3 % | 18 | 96.7 % | 15 | 67.1 % | 6 | 33.1 % | 11 | 37.0 % |
| Education | ||||||||||
| No schooling | 4 | 2.2 % | 2 | 97.8 % | 1 | 41.0 % | - | - | - | - |
| Grade 5 or below | 9 | 35.2 % | 23 | 64.8 % | 21 | 99.3 % | 9 | 24.0 % | 9 | 47.6 % |
| Grade 6 to Grade 10 | 24 | 21.3 % | 55 | 78.7 % | 52 | 97.5 % | 25 | 47.4 % | 33 | 52.6 % |
| Ordinary Level to Grade 12 | 14 | 8.3 % | 30 | 91.7 % | 26 | 90.5 % | 17 | 53.0 % | 23 | 73.5 % |
| Advanced Level and above | 4 | 23.4 % | 27 | 76.6 % | 24 | 93.4 % | 17 | 64.8 % | 19 | 70.8 % |
| Missing | 1 | 9 | 3 | 3 | 7 | |||||
| Occupation | ||||||||||
| Professional | 1 | 34 % | 2 | 66 % | 2 | 100 % | 2 | 100 % | 2 | 100 % |
| Technical & clerical | 3 | 3 % | 9 | 97 % | 9 | 100 % | 7 | 79.5 % | 8 | 98.9 % |
| Vendors and sellers | 10 | 18.7 % | 23 | 81.3 % | 23 | 100 % | 11 | 43.7 % | 18 | 74.2 % |
| Skilled manual workers | 8 | 35.3 % | 15 | 64.7 % | 10 | 82.5 % | 11 | 64.6 % | 9 | 63.8 % |
| Unskilled manual workers | 13 | 34.6 % | 10 | 65.4 % | 10 | 100 % | 6 | 58.6 % | 8 | 99.5 % |
| Retired | 2 | 1.2 % | 11 | 98.8 % | 10 | 99.4 % | 4 | 47.5 % | 6 | 48.2 % |
| Unemployed | 2 | 40.8 % | 9 | 59.2 % | 9 | 100 % | 4 | 62.3 % | 6 | 63.6 % |
| Housewife | 16 | 21.4 % | 66 | 78.6 % | 56 | 83.9 % | 26 | 43.3 % | 33 | 46.5 % |
| Missing | 1 | 1 | 0 | 0 | 1 | |||||
| Sector | ||||||||||
| Urban | 25 | 26.5 % | 65 | 73.5 % | 57 | 89.0 % | 31 | 47.6 % | 40 | 62.6 % |
| Rural | 20 | 22.6 % | 68 | 77.4 % | 61 | 90.8 % | 32 | 49.6 % | 41 | 61.3 % |
| Estate | 11 | 46.4 % | 13 | 53.6 % | 12 | 89.4 % | 08 | 59.4 % | 10 | 75.8 % |
| Income Category (Monthly Income) | ||||||||||
| < Sri Lankan Rupees 10,000 | 16 | 23.4 % | 37 | 76.6 % | 33 | 99.5 % | 13 | 35.8 % | 18 | 52.0 % |
| Sri Lankan Rupees 10,000 to 30,000 | 32 | 24.4 % | 78 | 75.6 % | 70 | 89.6 % | 44 | 59.9 % | 50 | 66.9 % |
| > Sri Lankan Rupees 30,000 | 5 | 9.8 % | 29 | 90.2 % | 26 | 85.1 % | 14 | 40.6 % | 23 | 66.7 % |
| Missing | 3 | 2 | 1 | 0 | 0 | |||||
| Social status index | ||||||||||
| 1st quintile (richest) | 8 | 18.2 % | 39 | 81.8 % | 33 | 92.5 % | 20 | 43.2 % | 23 | 60.8 % |
| 2nd quintile | 13 | 28.2 % | 38 | 71.8 % | 37 | 99.8 % | 13 | 30.1 % | 18 | 37.7 % |
| 3rd quintile | 14 | 23.9 % | 31 | 76.1 % | 25 | 79.0 % | 20 | 69.2 % | 23 | 66.5 % |
| 4th quintile | 12 | 21.4 % | 30 | 78.6 % | 27 | 92.0 % | 14 | 57.3 % | 22 | 81.0 % |
| 5th quintile (poorest) | 9 | 53 % | 8 | 47 % | 8 | 100.0 % | 4 | 51.5 % | 5 | 60.9 % |
| Unsatisfactory Basic Needs Index | ||||||||||
| 1 (poorest) | 6 | 99.1 % | 2 | 0.9 % | 2 | 100.0 % | 1 | 50.8 % | 1 | 50.8 % |
| 2 | 5 | 94.9 % | 8 | 5.1 % | 8 | 100.0 % | 6 | 82.6 % | 7 | 90.6 % |
| 3 | 8 | 15.1 % | 20 | 84.9 % | 16 | 81.9 % | 12 | 60.2 % | 11 | 56.0 % |
| 4 | 17 | 28.4 % | 47 | 71.6 % | 44 | 99.6 % | 19 | 52.5 % | 31 | 67.3 % |
| 5 (richest) | 20 | 22 % | 69 | 82 % | 60 | 91.0 % | 33 | 41.1 % | 41 | 61.2 % |
| Place of management | ||||||||||
| State | - | - | 41 | 23.4 % | 33 | 79.9 % | 12 | 23.7 % | 10 | 9 % |
| Private | - | - | 100 | 76.6 % | 93 | 93.8 % | 56 | 57 % | 77 | 76.8 % |
| Missing | - | - | 5 | 4 | 3 | 4 | ||||
aDM, diabetes mellitus
bAll percentages were weighted values
There were 42 (25.1 %) individuals describing at least one hypoglycaemic attack. One or more microvascular and macrovascular complication was seen in 186 (92.1 %) and 56 (27.7 %) respectively. Table 3 describes distribution of micro and macrovascular diseases by selected socioeconomic characteristics while Table 4 demonstrates the socioeconomic aspects by place of treatment.
Table 3.
The distribution of micro and macrovascular diseases by socioeconomic characteristics
| Socioeconomic characteristic | Complications of diabetes mellitus (n = 202)a | |||
|---|---|---|---|---|
| Microvascular (n = 186) | Macrovascular (n = 56) | |||
| Number | %b | Number | %b | |
| Sex | ||||
| Male | 92 | 91.0 % | 20 | 12.5 % |
| Female | 94 | 93.1 % | 36 | 35.3 % |
| Age (years) | ||||
| 35 to 39 | 11 | 100 % | 1 | 0.8 % |
| 40 to 44 | 26 | 99.4 % | 2 | 7.0 % |
| 45 to 49 | 34 | 85.6 % | 13 | 42.4 % |
| 50 to 54 | 34 | 80.4 % | 14 | 20.6 % |
| 55 to 59 | 43 | 100 % | 18 | 35.0 % |
| 60 to 64 | 38 | 94.6 % | 8 | 22.3 % |
| Ethnicity | ||||
| Sinhalese | 144 | 93.1 % | 37 | 23.4 % |
| Tamil | 20 | 97.6 % | 15 | 93.9 % |
| Muslim | 22 | 69.0 % | 4 | 32.8 % |
| Education | ||||
| No schooling | 5 | 42.4 % | 4 | 58.5 % |
| Grade 5 or below | 29 | 92.8 % | 17 | 43.2 % |
| Grade 6 to Grade 10 | 73 | 99.4 % | 17 | 24.7 % |
| Ordinary Level to Grade 12 | 42 | 88.4 % | 7 | 11.8 % |
| Advanced Level and above | 28 | 86.2 % | 7 | 18.5 % |
| Missing | 9 | 4 | ||
| Occupation | ||||
| Professional | 2 | 46.0 % | 1 | 34.0 % |
| Technical & clerical | 11 | 71.6 % | 1 | 0.6 % |
| Vendors and sellers | 31 | 86.4 % | 6 | 11.2 % |
| Skilled manual workers | 22 | 99.9 % | 2 | 11.5 % |
| Unskilled manual workers | 22 | 99.8 % | 11 | 32.9 % |
| Retired | 13 | 100 % | 1 | 0.7 % |
| Unemployed | 11 | 100 % | 3 | 21.6 % |
| Housewife | 72 | 92.1 % | 30 | 37.3 % |
| Missing | 2 | 1 | ||
| Sector | ||||
| Urban | 83 | 91.1 % | 20 | 20.7 % |
| Rural | 82 | 92.2 % | 22 | 24.6 % |
| Estate | 21 | 85.0 % | 14 | 53.6 % |
| Income Category (Monthly Income) | ||||
| < Sri Lankan Rupees 10,000 | 47 | 92.1 % | 20 | 30.4 % |
| Sri Lankan Rupees 10,000 to 30,000 | 101 | 90.4 % | 28 | 22.6 % |
| > Sri Lankan Rupees 30,000 | 33 | 99.7 % | 5 | 1.1 % |
| Missing | 5 | 3 | ||
| Social status index | ||||
| 1st quintile (richest) | 44 | 95.1 % | 14 | 36.6 % |
| 2nd quintile | 48 | 93.9 % | 12 | 23.8 % |
| 3rd quintile | 44 | 94.7 % | 9 | 15.1 % |
| 4th quintile | 35 | 83.5 % | 9 | 20.2 % |
| 5th quintile (poorest) | 15 | 87.9 % | 12 | 66.9 % |
| Unsatisfactory Basic Needs Index | ||||
| 1 (poorest) | 6 | 99.1 % | 4 | 1.4 % |
| 2 | 12 | 99.5 % | 7 | 5.0 % |
| 3 | 26 | 92.0 % | 8 | 19.7 % |
| 4 | 58 | 89.3 % | 12 | 16.5 % |
| 5 (richest) | 84 | 93.5 % | 25 | 34.3 % |
| Place of management (n = 146) | ||||
| State | 36 | 91.8 % | 21 | 50.2 % |
| Private | 94 | 90.4 % | 17 | 18.7 % |
| Missing | 5 | 5 | ||
aPresence of one or more complications were considered; bAll percentages were weighted values
Table 4.
The distribution of socioeconomic characteristics by place of treatment
| Socioeconomic characteristic | State (n = 41) | Private (100) | ||
|---|---|---|---|---|
| Number | %a | Number | %a | |
| Sex | ||||
| Male | 14 | 16.6 % | 50 | 83.4 % |
| Female | 27 | 29.2 % | 50 | 70.8 % |
| Age (years) | ||||
| 35 to 39 | 1 | 47.9 % | 5 | 52.1 % |
| 40 to 44 | 2 | 21.5 % | 16 | 78.5 % |
| 45 to 49 | 6 | 20.1 % | 19 | 79.9 % |
| 50 to 54 | 15 | 20.9 % | 21 | 79.1 % |
| 55 to 59 | 10 | 28.1 % | 19 | 71.9 % |
| 60 to 64 | 7 | 19.2 % | 20 | 80.8 % |
| Ethnicity | ||||
| Sinhalese | 28 | 21.8 % | 84 | 78.2 % |
| Tamil | 8 | 97.6 % | 3 | 2.4 % |
| Muslim | 5 | 34.7 % | 13 | 65.3 % |
| Education | ||||
| No schooling | 1 | 59.0 % | 1 | 41.0 % |
| Grade 5 or below | 11 | 19.4 % | 10 | 80.6 % |
| Grade 6 to Grade 10 | 21 | 42.4 % | 33 | 57.6 % |
| Ordinary Level to Grade 12 | 4 | 0.6 % | 26 | 99.4 % |
| Advanced Level and above | 2 | 11.1 % | 25 | 88.9 % |
| Missing | 2 | 5 | ||
| Occupation | ||||
| Professional | - | - | 2 | 100 % |
| Technical & clerical | - | - | 9 | 100 % |
| Vendors and sellers | 2 | 5.6 % | 21 | 94.4 % |
| Skilled manual workers | 4 | 35.0 % | 11 | 65.0 % |
| Unskilled manual workers | 3 | 0.8 % | 5 | 99.2 % |
| Retired | 5 | 37.4 % | 6 | 62.6 % |
| Unemployed | 4 | 37.5 % | 5 | 62.5 % |
| Housewife | 23 | 31.9 % | 40 | 68.1 % |
| Missing | 0 | 1 | ||
| Sector | ||||
| Urban | 19 | 28.8 % | 44 | 71.2 % |
| Rural | 15 | 23.2 % | 51 | 76.8 % |
| Estate | 7 | 58.1 % | 5 | 41.9 % |
| Income Category (Monthly Income) | ||||
| < Sri Lankan Rupees 10,000 | 16 | 31.6 % | 17 | 68.4 % |
| Sri Lankan Rupees 10,000 to 30,000 | 20 | 20.3 % | 57 | 79.7 % |
| > Sri Lankan Rupees 30,000 | 4 | 16.6 % | 15 | 83.4 % |
| Missing | 1 | 11 | ||
| Social status index | ||||
| 1st quintile (richest) | 9 | 21.1 % | 30 | 78.9 % |
| 2nd quintile | 14 | 44.3 % | 23 | 55.7 % |
| 3rd quintile | 5 | 13.7 % | 26 | 86.3 % |
| 4th quintile | 8 | 15.6 % | 18 | 84.4 % |
| 5th quintile (poorest) | 5 | 61.2 % | 3 | 38.8 % |
| Unsatisfactory Basic Needs Index | ||||
| 1 (poorest) | 1 | 49.2 % | 1 | 50.8 % |
| 2 | 4 | 40.3 % | 3 | 59.7 % |
| 3 | 5 | 30.2 % | 13 | 69.8 % |
| 4 | 14 | 16.8 % | 32 | 83.2 % |
| 5 (richest) | 17 | 23.1 % | 51 | 76.9 % |
aAll percentages were weighted values
Those attending the private healthcare sector had significantly higher rates of, poor control, poor compliance and poor monthly follow-ups (p < 0.05). Meanwhile those who are attending the state healthcare sector had higher proportion with already diagnosed hypertension and ischaemic heart disease (22, 53.7 % and 10, 31.3 % respectively) compared to those visiting the private health sector (37, 44.1 % and 4, 3.6 % respectively).
Discussion
Our findings show patients with diabetes mellitus in a suburban region in Sri Lanka continue to be poorly managed with high proportions having microvascular and macrovascular complications, irrespective of socioeconomic factors. These figures, though high when compared to high income countries [10, 11, 13, 14], appear to be comparable to similar settings in Asia and Africa [21, 22, 27].
All previous studies on the management and complications of diabetes mellitus in Sri Lanka over the last three decades were on hospital attendees and demonstrated poor management with high complications [28–34]. Our survey, on the contrary captures all hospital attendees, non-hospital attendees and irregular hospital attendees, for a condition predominantly managed in the community, and shows that over the last two decades there have been no improvements in the care of patients (in hospital settings) with diabetes mellitus compared to previous studies. This is true across all socioeconomic categories.
The hospital based studies in Sri Lanka reported lesser proportions with macro and microvascular complications compared to our findings since these have failed to capture non clinic attendees and private sector patients [28–34]. Similar to previous studies commonest macrovascular complication is ischaemic heart disease while the commonest microvascular is peripheral neuropathy [28, 30, 31, 33, 47–49].
Past studies on government hospital based populations in Sri Lanka, have highlighted poor management and control of diabetes mellitus among clinic attendees of these hospitals [48–50]. This study further establishes that those who were managed by the state as well as the private sector are poorly managed.
We demonstrate that the screening of diabetic complications is being performed only in a minority even when the disease is well established, irrespective of the socioeconomic background of the patient. The reasons for such low levels of screening are unclear and needs further exploration. The possible reasons include lack of awareness of doctors, moving between doctors, poor record keeping, poor patient compliance, costs of investigations amongst others.
The poor screening is reinforced by the fact that the already diagnosed with diabetes mellitus have high proportions with complication such as diabetic retinopathy; conditions which arise due to prolonged uncontrolled state of the disease [4].
Perhaps the most surprising new finding from our study is that overall poor management and complications of diabetes mellitus seem to cut across all socioeconomic groups and do not appear to show a detectable social gradient. This is in contrast to high income countries, where prevalence, poor management and complications of diabetes show a social gradient with higher proportion observed among the lower socioeconomic groups [10, 11, 13, 14, 17–19]. Our previous publication, meanwhile, showed an inverse social gradient in the prevalence of diabetes mellitus, which is also in contrary to high income settings [37]. These disparities in diabetes mellitus prevalence, management and its compilations may indicate that Sri Lanka is in a transitional stage. Studies from Sri Lanka including the present study, do not show clear evidence of the increased prevalence of diabetes and cardiovascular disease in lower socio-economic groups compared to higher socio-economic groups [37, 49, 51–53]. This contrasts with experience from the UK, Western Europe and North America where a gradient in mortality across socio-economic groups was observed, i.e. poorer groups affected more by ill-health compared to more affluent groups [54–58]. However, a pattern similar to the current situation in Sri Lanka was observed in England and Wales prior to 1960 when poorer social classes had lower risk of death from coronary heart disease than the higher classes [59]. It is therefore hypothesized that Sri Lanka too is undergoing this transition and the current status is a point in time when the gradient in adverse outcomes appears to be equal across socio-economic groups.
Interestingly higher proportion of microvascular diseases was observed among higher socioeconomic groups while higher proportion of macrovascular diseases was seen among the lower socioeconomic groups. A social gradient is only observed for macrovascular complications within sector and income category. These observations may be due to the co-existing socioeconomic inequality of cardiovascular diseases, which requires further investigation.
The estate sector (the most disadvantaged setting in the country) [60], poorest SSI category and the poorest UBNI category, had the highest proportion of poor control, poor follow-up and not on continuous treatment for diabetes mellitus. Macrovascular complications were seen again most among the estate sector, lowest income group and the poorest SSI category.
Access to private healthcare was high for all socioeconomic strata, except those in the estate sector, despite universal free health care at the point of delivery in the country. In most LICs/LMICs and HICs majority with diabetes mellitus accessed the state sector [22, 26, 27, 61, 62]. Possible reasons for accessing the private healthcare sector are due to likelihood of follow-up by same doctor, reduced waiting times, fewer queues and less congestion, easy accessibility to the private sector specialists due to the absence of a referral system and convenience in terms time of consulting without disruption to work.
However those living in the estate sector seem to utilize mostly the state sector. The main reasons likely to be are the economic and geographical constraints in accessing the private healthcare sector. Perhaps surprisingly the prevalence of microvascular complications in this group is not very different to urban and rural sector. However the high proportion of macrovascular complications can be attributed to co-existing cardiovascular diseases.
Diabetes mellitus patients utilising government healthcare appear to be better managed compared to the private sector even though the microvascular complications were equal in both groups. Although in the state sector there are different levels of care, within each level it is likely to be uniform with less variation in care. Staffing is similar at these levels and is from the same pool of people, whereas in the private sector it is more variable. The quality of state sector services are probably better (though it may be lower than the standard care) due to a number of reasons. One reason could be because the staffing of the state sector hospitals and clinics are administratively accountable to minimum standards or record keeping and documentation. In contrast, the private sector has a wide range or personnel who service their needs. They range from part-time government employed staff or those who work as locums. This tends to fragment their services and follow-up as records are rarely kept. The short-term management as evidenced by HbA1C levels may therefore appear to be different between the two sectors. However, in the long-term, the difference may be blurred by patients shifting from one to the other sector and therefore microvascular outcomes may become similar. Also the participants may make return visits to the government sector for follow-up as it is free. Further investigations are required to explore reasons for these observations. In addition strict adherence to the protocols given by the Ministry of Health, Sri Lanka may also help to reduce the high complication rates [44].
Another important finding of this study was that the undetected diabetes mellitus proportion is decreasing. Previous studies in the country have reported undetected proportions higher than 35 % [51, 52]. In South Asia and Africa regions the undetected proportion exceeds more than half the diabetes population [21, 22]. This can be attributed to the opportunistic screening done in both private and state healthcare sectors. High proportion of newly detected in estates, 5th SSI group (lowest social status index category) and 1st UBNI group (poorest UBNI group) indicates that these groups may get less prospects for opportunistic screening. The estate sector had the highest proportion of newly diagnosed suggesting that screening should be targeted to this population.
In most European and Northern American states, the prevalence of diabetes mellitus and its complications show a social gradient with higher rates in those with lower socioeconomic status, lower income levels and poorer educational achievements [5, 7, 13, 17, 19, 48, 63, 64]. In these HICs the socioeconomic differences often also exist in accessing health care, though there are exceptions [8, 16].
Major limitation of the study was the cross sectional nature to investigate a disease with long-term complications. The potential confounding variables that were not measured were psychosocial work characteristics, psychiatric morbidity and life events is another limitation.
Interestingly our findings suggest a crucial difference with high prevalence of complications and poor control of diabetes mellitus across all socioeconomic strata possibly because of the poor quality of screening and deficiencies in adhering to guidelines and protocols. However the reasons contributing to this merit further study including those of service improvements targeting groups such as estates. In addition, it should be explored why patients are discontinuing treatment and not regularly attending the follow-up clinics.
Conclusion
The vast majority of patients with diabetes mellitus are poorly managed and most had poor control of the disease. Greater proportion of them has microvascular and macrovascular complications and during the medical management most are not screened for these complications. Although majority of poor management and complications observed in the poor geographical and poor social status groups overall socioeconomic gradient seems to be absent with regard to the management and complications of diabetes mellitus.
Acknowledgements
The study was funded by the National Health Research Council of Sri Lanka.
The authors are thankful to the staff of the National Institute of Health Sciences, Kalutara and all Primary Healthcare Staff of Kalutara Regional Director of Health Services for the immense support rendered.
A. Pubudu De Silva, S.H. Padmal De Silva and Isurujith K. Liyanage were supported by the ASCEND Program (www.med.monash.edu.au/ascend) funded by the Fogarty International Centre, National Institutes of Health, under Award Number: D43TW008332. The contents of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the ASCEND Program.
Abbreviations
- ABI
Ankle-Brachial pressure Index
- ECG
Electrocardiography
- HIC
High Income Country
- IHD
Ischaemic Heart Disease
- LIC
Low Income Country
- LMIC
Lower Middle Income Country
- PAD
Peripheral Arterial Disease
- UBNI
Unsatisfactory Basic Needs Index
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
APDS gave the original idea, developed the methodology, conducted the training and validation of data collection, data analysis and writing of the manuscript. SHPDS developed the methodology, supervised data collection, conducted analysis and writing the manuscript. RH developed the methodology, data analysis and writing of the manuscript. IKL developed the methodology, supervised data collection, conducted analysis and writing the manuscript. KSAJ also gave the original idea, developed the methodology, data analysis and writing of the manuscript. PK developed the methodology, data analysis and writing of the manuscript.CNW developed the methodology, data analysis and writing of the manuscript. SW developed the methodology, data analysis and writing of the manuscript. LCR also gave the original idea, developed the methodology, data analysis and writing of the manuscript. All authors read and approved the final manuscript.
References
- 1.Danaei G, Finucane MM, Lu Y, Singhe GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numericl estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Dagogo-Jack S. Primary prevention of type-2 diabetes in developing countries. J Natl Med Assoc. 2006;98:415–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann MO, Eachus J, Hopper CD, Davey Smith G, Propper C, Pearson NJ, et al. Socio-economic inequalities in diabetes complications, control, attitudes and health service use: a cross-sectional study. Diabet Med. 2003;20:921–9. doi: 10.1046/j.1464-5491.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsushima M, Shimizu K, Maruyama M, Nishimura R, LaPorte RE, Tajima N. Socioeconomic and behavioural risk factors for mortality of individuals with IDDM in Japan: population-based case-control study. Diabetes Epidemiology Research International (DERI) US-Japan Mortality Study Group. Diabetologia. 1996;39:710–6. doi: 10.1007/BF00418543. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi N, Jarrett J, Shipley MJ, Fuller JH. Socioeconomic gradient in morbidity and mortality in people with diabetes: cohort study findings from the Whitehall study and the WHO multinational study of vascular disease in diabetes. BMJ. 1998;316:100–105. doi: 10.1136/bmj.316.7125.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koskinen SV, Martelin TP, Valkonen T. Socioeconomic differences in mortality among diabetic people in Finland: five year follow up. BMJ. 1996;313:975–978. doi: 10.1136/bmj.313.7063.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson N, Lloyd CE, Stevens LK. Social deprivation and mortality in adults with diabetes mellitus. Diabet Med. 1998;15:205–12. doi: 10.1002/(SICI)1096-9136(199803)15:3<205::AID-DIA519>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Wild SH, McKnight JA, McConnachie A, Lindsay RS. Socioeconomic status and diabetes-related hospital admissions: a cross-sectional study of people with diagnosed diabetes. J Epidemiol Community Health. 2010;64:1022–4. doi: 10.1136/jech.2009.094664. [DOI] [PubMed] [Google Scholar]
- 11.Weng C, Coppini DV, Sonksen PH. Geographic and social factors are related to increased morbidity and mortality rates in diabetic patients. Diabet Med. 2000;17:612–7. doi: 10.1046/j.1464-5491.2000.00352.x. [DOI] [PubMed] [Google Scholar]
- 12.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ. 2001;322:1389–93. doi: 10.1136/bmj.322.7299.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi N, Stephenson JM, Fuller JH. The relationship between socioeconomic status and diabetes control and complications in the EURODIAB IDDM Complications Study. Diabetes Care. 1996;19:423–30. doi: 10.2337/diacare.19.5.423. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Jensen SC, Moss SE. The relation of socioeconomic factors to the incidence of proliferative diabetic retinopathy and loss of vision. Ophthalmology. 1994;101:68–76. doi: 10.1016/S0161-6420(94)31354-6. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Hazuda HP, Stern MP, Patterson JK, Heuven WAJV, Fong D. Effects of socioeconomic status on hyperglycemia and retinopathy levels in Mexican Americans with NIDDM. Diabetes Care. 1989;12:128–34. doi: 10.2337/diacare.12.2.128. [DOI] [PubMed] [Google Scholar]
- 16.Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24:454–9. doi: 10.2337/diacare.24.3.454. [DOI] [PubMed] [Google Scholar]
- 17.Ricci-Cabello I, Ruiz-Pérez I, Olry de Labry-Lima A, Márquez-Calderón S. Do social inequalities exist in terms of the prevention, diagnosis, treatment, control and monitoring of diabetes? A systematic review. Health Soc. Care Community. 2010;18:572–587. doi: 10.1111/j.1365-2524.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 18.Mold F, While A, Forbes A. The management of type 2 diabetes care: the challenge within primary care. Practical Diabetes Int. 2008;25:28–36. doi: 10.1002/pdi.1195. [DOI] [Google Scholar]
- 19.Hsu CC, Lee CH, Wahlgvist ML, Huang HL, Chang HY, Chen L, et al. Poverty increases type 2 diabetes incidence and inequality of care despite universal health coverage. Diabetes Care. 2012;35:2286–92. doi: 10.2337/dc11-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhowmik B, Afsana F, Diep LM, Munir SB, Wright E, Mahmood S, et al. Increasing Prevalence of Type 2 Diabetes in a Rural Bangladeshi Population: A Population Based Study for 10 Years. Diabetes Metab J. 2013;37:46–53. doi: 10.4093/dmj.2013.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow CK, Raju PK, Raju R, Reddy KS, Cardona M, Celermajer DS, et al. The prevalence and management of diabetes in rural India. Diabetes Care. 2006;29:1717–8. doi: 10.2337/dc06-0621. [DOI] [PubMed] [Google Scholar]
- 22.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran A, Jali MV, Mohan V, Snehalatha C, Viswanathanet M. High prevalence of diabetes in an urban population in south India. BMJ. 1988;297:587–90. doi: 10.1136/bmj.297.6648.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran A, Snehalatha C, Baskar AD, Mary S, Kumar CK, Selvam S, et al. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–5. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 25.The World Bank. Country and Lending Groups [Internet]. 2016. Available from: http://data.worldbank.org/about/country-classifications/country-and-lending-groups#Lower_middle_income.
- 26.Chuang LM, Tsai ST, Huang BY, Tai TY. The status of diabetes control in Asia—a cross-sectional survey of 24 317 patients with diabetes mellitus in 1998. Diabet Med. 2002;19:978–985. doi: 10.1046/j.1464-5491.2002.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Levitt NS. Diabetes in Africa: epidemiology, management and healthcare challenges. Heart. 2008;94:1376–1382. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 28.Fernando DJ, Siribaddana S, Perera N, Perera S, de Silva D. The prevalence of macrovascular disease and lipid abnormalities amongst diabetic patients in Sri Lanka. Postgrad Med J. 1993;69:557–561. doi: 10.1136/pgmj.69.813.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernando DJ, Siribaddana S, De S, Subasinge Z. Prevalence of retinopathy in a Sri Lankan diabetes clinic. Ceylon Med J. 1993;38:120–123. [PubMed] [Google Scholar]
- 30.Fernando DJ. The prevalance of neuropathic foot ulceration in Sri Lankan diabetic patients. Ceylon Med J. 1996;41:96–98. [PubMed] [Google Scholar]
- 31.Weerasuriya N, Siribaddana S, Dissanayake A, Subasinghe Z, Wariyapola D, Fernando DJ. Longterm complications in newly diagnosed Sri Lankan patients with type 2 diabetes mellitus. Q J Med. 1998;91:1–5. doi: 10.1093/qjmed/91.6.439. [DOI] [PubMed] [Google Scholar]
- 32.Weerarathna TP, Kariyawasam BDI, Welgamage T. Profile of foot problems detected by standard annual screening at a diabetic foot clinic. Galle Medical Journal. 2006;11:72. [Google Scholar]
- 33.Weerasuriya N, Siribaddana S, Wijeweera I, Dissanayeka A, Wijesekera J, Fernando DJ. The prevalence of peripheral neuropathy in newly diagnosed patients with non-insulin-dependent diabetes mellitus. Ceylon Med J. 1998;43:19–21. [PubMed] [Google Scholar]
- 34.Jayaweera-Bandara C. Diabetic retinopathy, its incidence in Sri Lanka and its treatment. Transactions of the Ophthalmic Society. 1987;29:4–16. [Google Scholar]
- 35.Engelgau M, Okamoto K, Navaratne KV, Gopalan S. Prevention and Control of Selected Chronic NCDs in Sri Lanka: Policy Options and Action. Washington DC: The World Bank; 2010. [Google Scholar]
- 36.Marmot M. Social determinants of health inequalities. Lancet. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)74234-3. [DOI] [PubMed] [Google Scholar]
- 37.De Silva AP, De Silva SHP, Liyanage IK, Rajapakse LC, Jayasinghe KS, Katulanda P, et al. Social, cultural and economical determinants of diabetes mellitus in Kalutara district, Sri Lanka: a cross sectional descriptive study. Int. J. Equity Health. 2012;11:76. doi: 10.1186/1475-9276-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull. World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 39.De Silva AP. Social Determinants of Diabetes Mellitus in Kalutara District, in Postgraduate Institute of Medicine. Colombo: University of Colombo; 2010. [Google Scholar]
- 40.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033-46. [DOI] [PMC free article] [PubMed]
- 41.Satharasinghe A. Census Department Classifies GN Divisions by Poverty. 1. Department of Census and Statistics: Colombo; 2008. [Google Scholar]
- 42.Luepker RV, Rosamond WD, Murphy R, Sprafka JM, Folsom AR, McGovern PG, et al. Socioeconomic status and coronary heart disease risk factor trends. The Minnesota Heart Survey. Circulation. 1993;88:2172–2179. doi: 10.1161/01.CIR.88.5.2172. [DOI] [PubMed] [Google Scholar]
- 43.Migliacci R, Nasorri R, Ricciarini P, Gresele P. Ankle-brachial index measured by palpation for the diagnosis of peripheral arterial disease. Fam Pract. 2008;25:228–32. doi: 10.1093/fampra/cmn035. [DOI] [PubMed] [Google Scholar]
- 44.Ministry of Healthcare and Nutrition. National Guidelines: Diabetes Mellitus . Colombo. Ministry of Healthcare and: Nutrition; 2007. [Google Scholar]
- 45.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 46.Benedict SR. A reagent for the detection of reducing sugars. J Biol Chem. 1909;5:485–487. [PubMed] [Google Scholar]
- 47.Jayawardena MHDS, Stenson D, Bjurman E, De Almeida TKR, Fernando DJS. The quality of care at a diabetes clinic: an audit In: Abstract book of the Sri Lanka Medical Association 115th anniversary academic sessions. Sri Lanka Medical Association: Colombo; 2002.
- 48.Herath HMM, Weerarathna TP, Welgamage T, Kariyawasam BDI. An audit of diabetic management in a tertiary care hospital. Galle Medical Journal. 2006;11:88. [Google Scholar]
- 49.Weerasuriya N. Diabetes mellitus in Sri Lanka. Sri Lanka Family Physician. 2000;23:18–20. [Google Scholar]
- 50.Wijesinghe MACL, Prabath API, Weerarathna TP. A study of indications for hospitalization and quality of self-care in diabetic patients admitted to Teaching Hospital Galle. Galle Medical J. 2000;2:Ab-23. [Google Scholar]
- 51.Katulanda P, Constantine GR, Mahesh JG, Sheriff R, Seneviratne RD, Wijeratne S, et al. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka - Sri Lanka Diabetes, Cardiovascular Study (SLDCS) Diabet Med. 2008;25:1062–1069. doi: 10.1111/j.1464-5491.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 52.Pinidiyapathirage MJ, Kasturiratne A, Ranawaka UK, Gunasekara D, Wijekoon N, Medagoda K, et al. The burden of diabetes mellitus and impaired fasting glucose in an urban population of Sri Lanka. Diabet Med. 2013;30:326–332. doi: 10.1111/dme.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Silva AP, De Silva SHP, Haniffa R, Liyanage IK, Jayasinghe KSA, Katulanda P, Wijeratne CN, Wijeratne S, Rajapakse LC. A cross sectional survey on social, cultural and economic determinants of obesity in a low middle income setting. Int. J. Equity Health. 2015;14:6. doi: 10.1186/s12939-015-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marmot MG, Shipley MJ, Rose G. Inequalities in death-specific explanations of a general pattern. The Lancet. 1984;323:1003–6. doi: 10.1016/S0140-6736(84)92337-7. [DOI] [PubMed] [Google Scholar]
- 55.Marmot M. Self esteem and health. Br Med J. 2004;327:574–575. doi: 10.1136/bmj.327.7415.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackenbach JP, Stirbu I, Roskam AJR, Schaap MM, Menvielle G, Leinsalu M, Kunst AE. Socioeconomic inequalities in health in 22 European countries. N. Engl. J. Med. 2008;358:2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 57.Navarro V, Muntaner C. Political and Economic Determinants of Population Health and Well-Being: Controversies and Developments. Baywood Publishing Company: New York; 2004. [Google Scholar]
- 58.Townsend P, Davidson N. Inequalities in Health, the Black Report and the Health Divide. 2. London: Penguin Books; 1992. [Google Scholar]
- 59.Rose G, Marmot MG. Social class and coronary heart disease. Br Heart J. 1981;45:13–19. doi: 10.1136/hrt.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samarasinghe V. Puppets on a String: Women's Wage Work and Empowerment among Female Tea Plantation Workers of Sri Lanka. J Developing Areas. 1993;27:329–340. [PubMed] [Google Scholar]
- 61.Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, et al. Diabetes Care Quality in the Veterans Affairs Health Care System and Commercial Managed Care: The TRIAD Study. Ann Intern Med. 2004;141:272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 62.Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, et al. Improvements in Diabetes Processes of Care and Intermediate Outcomes: United States, 1988–2002. Ann Intern Med. 2006;144:465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 63.Giachello AL, Arrom JO, Davis M, Sayad JV, Ramirez D, Nandi C, et al. Reducing diabetes health disparities through community-based participatory action research: the Chicago Southeast Diabetes Community Action Coalition. Public Health Rep. 2003;118:309–23. doi: 10.1016/S0033-3549(04)50255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landon BE, Hicks LS, O'Malley AJ, Lieu TA, Keegan T, McNeil BJ, et al. Improving the Management of Chronic Disease at Community Health Centers. N Engl J Med. 2007;356:921–934. doi: 10.1056/NEJMsa062860. [DOI] [PubMed] [Google Scholar]


