Abstract
Objectives:
The treatment of diabetic ulceration of the lower extremities is a complicated task due to the nature of the ulcer and potential underlying comorbidities. This report describes the case of a 61-year-old male patient with Type 2 Diabetes who presented with an ulcerative leg wound. The objective of this study was to evaluate the outcome of a topical compounded treatment.
Methods:
The patient applied a compounded medicine containing 2% mupirocin in a topical anhydrous silicone base containing fatty acids from pracaxi oil directly to the ulcer for 63 days, 3 times daily. This regimen was supplemented with exercise and an additional compounded medicine applied to the wound margins in order to increase circulation.
Results:
By the end of the application period, the patient’s ulcer was fully closed.
Conclusion:
A topical anhydrous silicone compounding base containing fatty acids from pracaxi oil may be useful in the treatment of patients with diabetic ulcers.
Keywords: Dermatology, wound healing, drug compounding, diabetes complications
Introduction
Diabetes currently affects 25.8 million people and 8.3% of the population of the United States.1 Ulceration of the lower extremities is a frequent medical complication for patients with diabetes. Chronic ulcers develop in diabetic patients as a result of several factors, including peripheral neuropathy, decreased blood flow, and local trauma,2 and these same factors also lead to delayed healing of ulcerative wounds. This decreased ability to heal can lead to increased rates of infection and, ultimately, may lead to amputation of affected extremities. Therefore, the treatment of chronic ulcers in patients with diabetes is important in order to reduce the risk of infection and amputation, as well as to lessen the socioeconomic burden of these outcomes on the health-care system.2
Pracaxi oil, an oil extracted from the seeds of Pentaclethra macroloba, a tree native to the Amazon, has several long-standing medicinal applications including treatment of ulcers, stretch marks, and snake bites.3 Pracaxi oil contains high amounts of oleic, linoleic, and behenic acids, fatty acids which are known to enhance wound closure and improve healing in wounding models.4,5 Additionally, these fatty acids can enhance permeation of the outer layers of the skin, allowing for faster delivery of topical active pharmaceutical ingredients.
This case report describes the use of fatty acids from pracaxi oil in a topical anhydrous silicone compounding base in a patient with a diabetic ulcer. Compounding, the preparation of customized medications to meet the specific needs of individual patients, allows patients the benefit of a personalized treatment and represents a therapeutic alternative in the treatment of dermatological conditions.6 Compounded medications may be personalized to include special combinations of active substances in particular strengths, and can be adjusted to the patient’s skin type and underlying comorbidities.7 In this case report, the outcome of a patient with a diabetic ulcer on the lower leg, who applied a topical anhydrous silicone base containing fatty acids from pracaxi oil compounded with 2% mupirocin, is presented.
Case
Professional Compounding Centers of America (PCCA) does not require ethics approval for reporting individual cases. Written consent was obtained from the patient for the use and publication of information and photographs. A 61-year-old Caucasian male with a 20-year history of type 2 diabetes (T2D) presented with an ulcer on the inner right leg caused by impact with a metal rod. The patient’s ulcer was 4 mm in diameter and was located approximately 8–10 mm above the right ankle (Figure 1). He led a sedentary lifestyle and had an occupation in which he was seated for the majority of the day. The patient reported that the wound was present for approximately 30 days during which time he applied over-the-counter medication to the ulcer which only worsened the wound. He was prescribed several antibiotic treatments by his physician, with no improvement in the ulcer over time. He discontinued the use of antibiotics and over-the-counter treatments when his wound did not improve. The patient did not disclose the type and duration of previous treatments. His physician also recommended scrubbing the wound daily with soap and water several times per day, but this was also unsuccessful in helping to heal the ulcer. The patient reported that the ulcer was painful and continued to worsen in severity over time.
Figure 1.
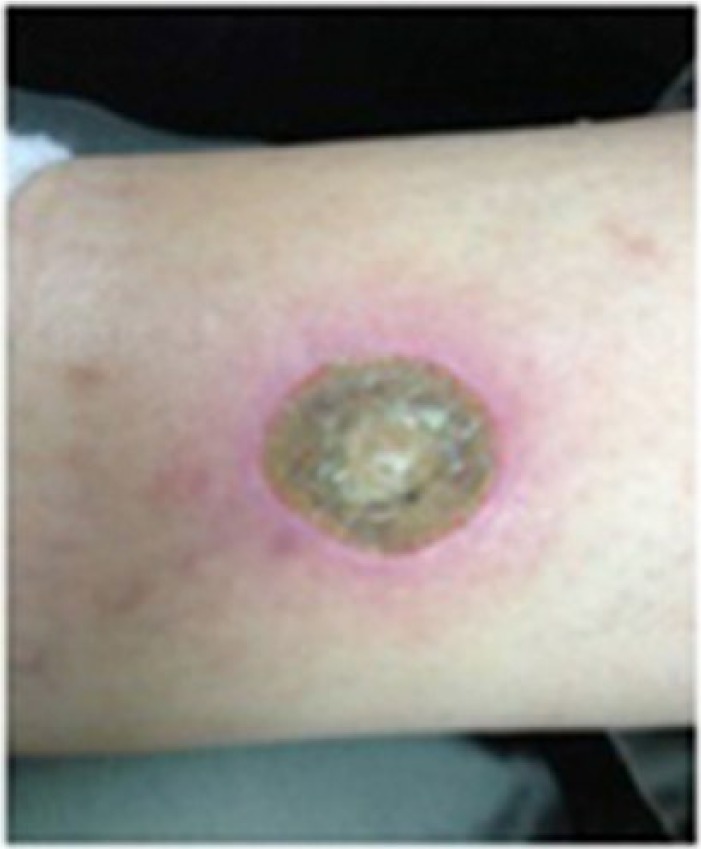
A 4-mm diabetic ulcer approximately 8–10 mm from the right ankle in a 61-year-old male patient with type 2 diabetes (T2D).
The patient was prescribed a compounded medicine containing the antibacterial agent mupirocin (2%)8 in an anhydrous silicone base containing pracaxi oil, applied topically directly to the ulcer three times daily. Additionally, because diminished peripheral blood flow and decreased angiogenesis impair wound healing in diabetic patients,9 a second compounded medicine containing 3% nifedipine8,10 and 3% pentoxifylline11 in a transdermal base was applied topically to the marginal area of the ulcer three times daily in order to increase blood flow to the ulcerated area. The patient was also advised to exercise every hour with an elastic band while sedentary to improve blood flow to the ulcerated area.12 He was not offloaded during the application period, nor was compression bandaging required.
The patient applied compounded medicine to the ulcer for 63 days, and at the conclusion of the application period, the ulcer was completely closed (Figure 2) and the patient reported a significant reduction in pain. The patient did not report any adverse reactions during the use of the compounded medicine. The overall cost of this treatment for the patient was US$255 (US$180 for 100 g of mupirocin 2% compounded in the silicone base plus US$75 for 100 g of pentoxifylline 3%/nifedipine 3% compounded in the transdermal base).
Figure 2.
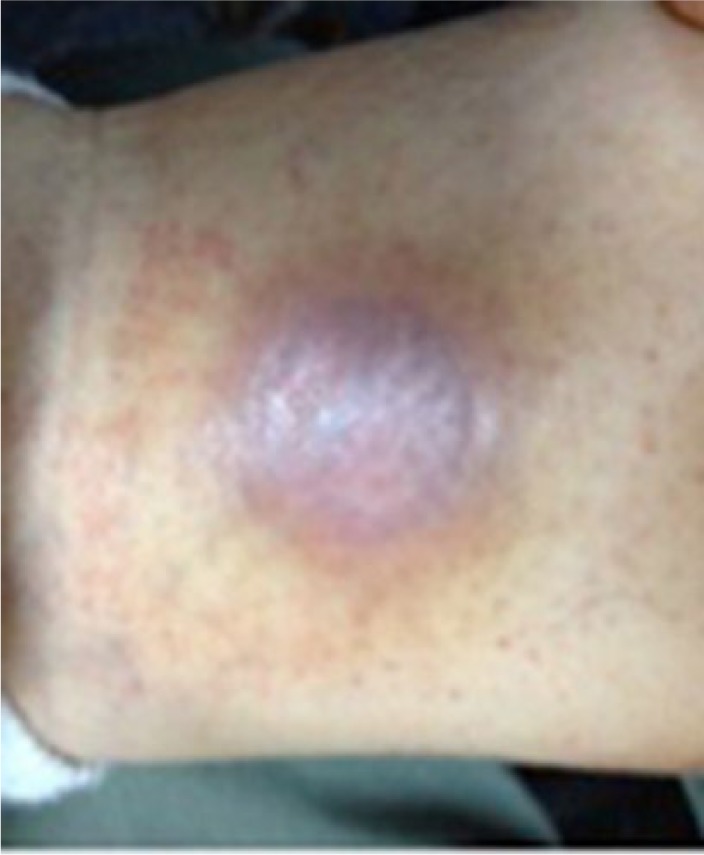
After 63 days of compounded medicine application, the diabetic ulcer was fully closed and the patient reported a significant decrease in pain associated with the ulcer.
Discussion
The annual incidence of diabetic ulceration is reported to be approximately 2.2% in diabetic patients.13 Patients with T2D frequently experience ulceration of the lower limbs due to peripheral neuropathy, peripheral vascular disease, and metabolic syndrome.2 These same risk factors also contribute to the decreased ability of diabetic patients to heal chronic wounds, such as ulcers, which in turn leads to an increased risk of infection. Diabetic ulceration and infection are the largest causes of nontraumatic lower limb amputation in the United States.1 Therefore, the development of successful treatments for diabetic ulcers is of utmost importance.
Compounded medicines are indicated for treatment of diabetic patients as these medicines can be tailored to the location, size, and nature of the wound, as well as to the underlying diabetic complications and comorbidities of a specific patient. The formulation of topical compounding bases allows for the delivery of compounded medication to all areas of the wound, promoting the healing of chronic wounds like ulcers.14
In this case report, a patient with a diabetic leg ulcer safely and successfully used 2% mupirocin in a topical anhydrous silicone compounding base containing pracaxi oil. Although this case report demonstrates a successful treatment, the lack of clinical data and the self-reported nature of the information limit the interpretation of the results.
Pracaxi oil contains high amounts of oleic, linoleic, and behenic acids, which have beneficial effects on skin structure and permeability, and are also used as targeted delivery vehicles in topical preparations.15 These fatty acids have also been shown to enhance wound closure and improve healing by altering the migration of cells in and around the wound, aiding in re-epithelialization of the wound, and altering the local inflammatory response.4,5 A recent study of seven patients examining the use of a topical compounding base containing pracaxi oil, either alone or compounded with additional active ingredients, on wounds and scars of varying etiologies, including burn wounds, moisture-associated wounds, and traumatic wounds and scars, demonstrated that this compounding base was effective in aiding wound healing and preventing or lessening scar formation. All patients rated their level of satisfaction with the treatment very highly, and all wounds were considered “improved” or “much improved” after application as assessed by an independent dermatological reviewer. Therefore, a compounding base containing pracaxi oil with high levels of beneficial fatty acids has been shown to positively influence wound healing and lessen scar formation.16 Moreover, the ability to add active ingredients to this base allows for the treatment of more severe and more complex lesions, such as diabetic ulcers, as demonstrated in the case presented here.
Conclusion
The authors suggest that compounded medicines using a topical anhydrous silicone base containing fatty acids from pracaxi oil may be valuable in the treatment of diabetic ulcers.
Acknowledgments
The authors are grateful for the information and photographs shared by the patient. The authors would like to thank Dr Chantelle Rein-Smith for providing assistance in the drafting and editing of the article.
Footnotes
Declaration of conflicting interests: CV Simmons, F Banov, and D Banov are all full-time employees of Professional Compounding Centers of America (PCCA), which manufactures the topical compounding bases described in this case report. The authors have no additional conflicts of interest.
Funding: Sponsorship for this study and article processing fees were provided by Professional Compounding Centers of America (PCCA).
References
- 1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. [Google Scholar]
- 2. Cook JJ, Simonson DC. Epidemiology and health care cost of diabetic foot problems. In: Veves A, Giurini JM, LoGerfo FW. (eds) The diabetic foot. New York: Humana Press, 2012, pp. 17–32. [Google Scholar]
- 3. Dos Santos Costa MNF, Muniz MAP, Negrão CAB, et al. Characterization of Pentaclethra macroloba oil. J Therm Anal Calorim 2014; 115(3): 2269–2275. [Google Scholar]
- 4. Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr 1999; 129(10): 1791–1798. [DOI] [PubMed] [Google Scholar]
- 5. Cardoso CRB, Souza MA, Ferro EAV, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen 2004; 12(2): 235–243. [DOI] [PubMed] [Google Scholar]
- 6. Carvalho M. Extemporaneously compounded oral medicines in European hospital pharmacies. PhD Thesis, University College London, London, 2012. [Google Scholar]
- 7. Carvalho M, Taylor K, Tuleu C. Why do we need hospital pharmacy preparation? Eur J Hosp Pharm 2012; 19(5): 467–468. [Google Scholar]
- 8. Sweetman S. Martindale: the complete drug reference. 35th ed. London: Pharmaceutical Press, 2006. [Google Scholar]
- 9. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117(5): 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torsiello MJ, Kopacki M. Transdermal nifedipine for wound healing: case reports. Int J Pharm Compd 2000; 4(5): 356–358. [PubMed] [Google Scholar]
- 11. Helmke CD. Current topical treatments in wound healing, part 1. Int J Pharm Compd 2004; 8(4): 269–274. [PubMed] [Google Scholar]
- 12. Maiorana A, O’Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 2001; 38(3): 860–866. [DOI] [PubMed] [Google Scholar]
- 13. Abbott C, Carrington A, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002; 19(5): 377–384. [DOI] [PubMed] [Google Scholar]
- 14. Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009; 49(10): 1541–1549. [DOI] [PubMed] [Google Scholar]
- 15. Patel MC, Patel Hardik K, Suthar Rajnikant M. Liposomes: as a topical drug delivery system. Int J Pharm Chem Sci 2012; 1(1): 1–10. [Google Scholar]
- 16. Banov D, Banov F, Bassani AS. Case series: the effectiveness of fatty acids from pracaxi oil in a topical silicone base for scar and wound therapy. Dermatol Ther 2014; 4: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]


