Abstract
Introduction:
Ornithine transcarbamylase deficiency is the most common inherited disorder of the urea cycle, has a variable phenotype, and is caused by mutations in the OTC gene. We report three cases of ornithine transcarbamylase deficiency to illustrate the late-onset presentation of this disorder and provide strategies for diagnosis and treatment. The patients were maternal first cousins, presenting with hyperammonemia and obtundation. Urea cycle disorder was not initially suspected in the first patient, delaying diagnosis.
Results:
Sequencing of the OTC gene showed a novel missense mutation, c.563G > C (p.G188A). Numerous family members were found to carry this mutation, which shows a trend toward later onset. Each urea cycle disorder has its own unique pattern of biochemical abnormalities, which differ from non-metabolic causes of critical illness.
Conclusion:
Regardless of age, clinical suspicion of a urea cycle disorder is important in encephalopathic patients to ensure quick diagnosis and definitive treatment of the underlying inborn error of metabolism.
Keywords: Ornithine transcarbamylase deficiency, hyperammonemia, urea cycle disorders, inborn errors of metabolism, metabolic encephalopathy
Introduction
Ornithine transcarbamylase (OTC) deficiency is the most common genetic disorder of the urea cycle, occurring in 1 in 14,000 people.1 It is inherited in an X-linked fashion with variable expressivity and is caused by mutations in the OTC gene.2 It is variable in severity ranging from asymptomatic to severe neonatal hyperammonemia. Hyperammonemia may be prompted by metabolic stressors, such as infection or protein consumption. Significant clinical heterogeneity may exist, even within families.
Later onset forms of OTC deficiency are diagnostically challenging and can go unrecognized. They often present with acute and rapidly progressive encephalopathy. We report a large extended family in which three male maternal first cousins carry a novel OTC mutation and developed later onset OTC deficiency. These patients underscore the need to suspect OTC deficiency in patients with unexplained hyperammonemia or encephalopathy.
Case reports
Patient 1 was a 20-year-old male who presented after being found unresponsive. He had been feeling unwell for the previous 2 months with weight loss, nausea, and vomiting. He had been engaging in a strength-conditioning regimen for several months in preparation for football and was taking a whey-based protein supplement, creatine monohydrate, an arginine-containing supplement, and stanozolol. On admission, he had elevated serum transaminases and ammonia of 323 µmol/L (normal: 16–60 µmol/L). He developed cerebral edema and seizures. A urea cycle disorder was suspected after consultation with a metabolist, and intravenous (IV) sodium phenylacetate/benzoate was started, after which the ammonia level normalized. Confusion persisted for 1 week after normalization of the ammonia level. Urinary orotic acid was 3.6 mmol/mol creatinine (normal: 0.4–1.2 mmol/mol creatinine), and plasma glutamate was elevated at 871 µmol/L (normal: 410–700 µmol/L). Aside from mild to moderate right-sided hearing loss, he made a full recovery. The patient is in good health, but does not comply with a protein-restricted diet, but has refrained from performance-enhancing substances.
Patient 2 was a 3-year 10-month old male who presented with altered mental status, seizures, and hyperammonemia. In the 3 days preceding admission, he had suffered from diarrhea and low-grade fever and had been diagnosed with viral gastroenteritis. Upon admission, he became obtunded and required intubation. Caregivers were aware of his cousin’s diagnosis, so he was started on IV dextrose, sodium phenylacetate/benzoate, and arginine hydrochloride, with normalization of ammonia within 24 h. Urine organic acids showed elevated orotic acid, and plasma amino acids showed elevated glutamine. He has no sequelae from his hyperammonemic episodes and is clinically well. He receives oral sodium phenylbutyrate, complies with modest dietary protein restriction, and has had only one episode of hyperammonemia for which he was briefly hospitalized.
Patient 3 was a 16-year-old male who developed emesis, fatigue, and poor appetite several days prior to an acute episode of confusion during an athletic event, which occurred on a particularly hot day. He had been eating a normal diet and not taking any supplements. Upon admission, his ammonia was 104 µmol/L. Glutamate measured 3 days after admission was slightly elevated at 769 µmol/L. He had a good response to administration of IV glucose, sodium phenylacetate/benzoate, and arginine. He recovered fully and has been symptom free after following a protein-restricted diet and taking oral sodium phenylbutyrate.
Results and family history
After Patient 1 presented with marked hyperammonemia, OTC deficiency was suspected. Sequencing of the OTC gene showed a novel mutation (c.563G > C, p.G188A). This variant was not listed as a polymorphism in the dbSNP database. Two mutations at the same nucleotide position have been described in the literature in symptomatic patients3,4 and is highly conserved with glycine present in all vertebrate species. SIFT (Sorting Intolerant From Tolerant, located at http://sift.jcvi.org/) and polyphen-2 predict that the mutation will be “not tolerated” and “probably damaging,” respectively. These lines of evidence suggest that the p.G188A mutation is the cause of OTC deficiency in this family.
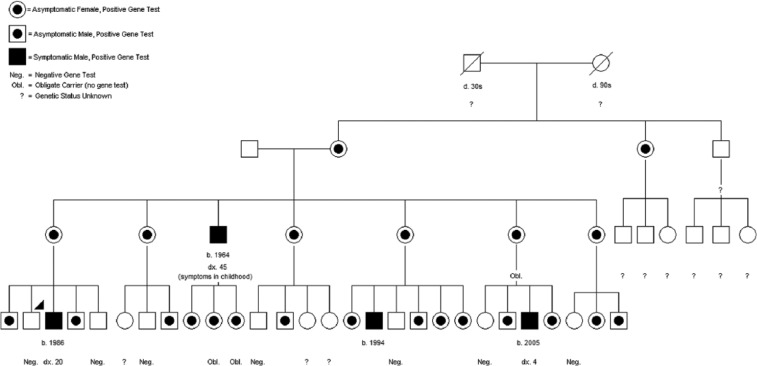
Patient 1’s mother was found to be heterozygous for this mutation. All five of the first patient’s maternal aunts are heterozygous for the mutation: four underwent testing and one is an obligate heterozygote. All are asymptomatic. Two of his brothers were hemizygous for the mutation, but had no history suggestive of hyperammonemia. The patients’ maternal uncle was hospitalized as a child for several episodes of illness and disorientation of unknown cause, with diagnosis of OTC deficiency made in middle age. Refer to Figure 1 for pedigree of the extended family.
Figure 1.
Pedigree of extended family, with arrowhead denoting Patient 1.
Discussion
Patients with OTC deficiency present with symptoms including confusion, lethargy, anorexia, hyperventilation, and hypothermia followed by ataxia, behavioral disturbances, and obtundation.5 This may mimic intoxication or central nervous system (CNS) infection. If untreated, the symptoms can progress to seizures, cerebral edema, and potentially death.6 Between episodes, patients are often completely asymptomatic, but can show symptoms such as nausea, vomiting, and anorexia. Patients who recover from critical illness caused by OTC deficiency can have residual neurological deficits, and there is an increased risk of cognitive impairment. The severity and number of hyperammonemic episodes is associated with the degree of deficit.7
Hyperammonemic episodes can be brought on by a number of factors. Our first patient was consuming excessive amounts of protein and engaging in strenuous exercise, both of which likely contributed to his hyperammonemia. Interestingly, his arginine supplement may have partially protected him. Our second patient likely had a viral gastroenteritis, which would increase his catabolism and also cause dehydration, both predisposing to hyperammonemic episodes. Our third patient presented after strenuous activity on a hot day, suggesting again that metabolic stress and dehydration contributed to his hyperammonemia.
Urea cycle disorders have a predictable pattern of laboratory abnormalities which can greatly assist in diagnosis. Hyperammonemia caused by other types of critical illness is frequently associated with additional laboratory abnormalities such as elevated lactic acid8 and prominent acidosis. In contrast, hyperammonemia without acidosis should prompt consideration of a urea cycle disorder. Patients will typically present with respiratory alkalosis caused by ammonia stimulating the respiratory center in the brain,9 and an inappropriately low serum blood urea nitrogen (BUN), which is a reflection of the impaired ureagenesis.
The biochemical evaluation of a urea cycle disorder includes plasma amino acids, urine orotic acid, and urine organic acids. Each of the urea cycle disorders has a unique pattern of biochemical abnormalities, which are summarized in Table 1. The pattern is decidedly different from that observed in patients with acute illness from sepsis11 or hepatic failure.12
Table 1.
Common patterns of plasma amino acid and orotic acid disturbance by disorder.
| Disorder | Amino acid abnormalities | Urine orotic acid |
|---|---|---|
| Ornithine transcarbamylase deficiency | Decreased citrulline, elevated glutamine, elevated alanine, decreased arginine10,11 | Markedly elevated |
| N-acetylglutamate synthetase deficiency | Decreased citrulline, elevated glutamine, elevated alanine11 | Normal |
| Carbamoyl phosphate synthetase deficiency | Decreased citrulline, elevated glutamine, elevated alanine, decreased arginine10,11 | Normal |
| Arginosuccinic acid synthetase deficiency | Markedly elevated citrulline, decreased arginine10–12 | Elevated |
| Arginosuccinic acid lyase deficiency | Elevated citrulline, elevated arginosuccinic acid, decreased arginine 11,13 | Elevated |
| Arginase deficiency | Elevated arginine11 | Elevated |
| Sepsis | Elevated aromatic and sulfur containing amino acids, decreased branched-chain amino acids14 | Normal |
| Hepatic dysfunction | Elevated tyrosine and methionine, decreased branched-chain amino acids15 | Normal |
Treatment for a presumptive urea cycle disorder should not wait for definitive diagnosis to be made. Consultation with a metabolic specialist should occur as early as possible in the patient’s clinical course. Protein intake should be discontinued temporarily and protein catabolism suppressed by dextrose-containing fluid replenishment. Protein intake should be restarted within 24–48 h to avoid further catabolism. Patients should be monitored for the cerebral edema. For severe hyperammonemia, hemodialysis to remove ammonia can be lifesaving.16 IV 10% arginine supplementation should be initiated. IV sodium benzoate/phenylacetate is effective in lowering hyperammonemia by conjugating and eliminating two non-essential amino acids, glycine and glutamine, that otherwise contribute ammonia to the urea cycle.
Conclusion
Urea cycle disorders should be considered in the differential diagnosis of patients who present with symptoms of acute hyperammonemia. If a urea cycle disorder is being considered, treatment should be started prior to confirmation of the diagnosis. Only by clinical suspicion and careful investigation will inborn errors of metabolism be uncovered and treated in a timely manner.
Acknowledgments
The authors would like to thank our patients and the extended family. We thank Drs Kristina Bailey, Candace Huebert, and Matthew Haack for their insight into the critical care aspects of these cases. Finally, we thank Dr Gary Beck and the Pediatric Writing Group for a thoughtful critique of a draft of this article.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Research ethics: This research was approved as exempt by the University of Nebraska Medical Center Institutional Review Board and has been assigned IRB# 561-13-EX. Per local guidelines, written consent was not required, but verbal consent was obtained from patients and their families for inclusion of their case information and pedigree in this article.
References
- 1. Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr 1996; 43: 127–170. [PubMed] [Google Scholar]
- 2. Lanpher BC, Gropman A, Chapman KA, et al. Urea cycles disorder overview. In: Pagon RA, Bird TD, Dolan CR, et al. GeneReviews™. Seattle, WA: University of Washington, Seattle; http://www.ncbi.nlm.nih.gov/books/NBK1217/ (1993, accessed 5 January 2012) [Google Scholar]
- 3. Gilbert-Dussardier B, Segues B, Rozet JM, et al. Partial duplication [dup. TCAC (178)] and novel point mutations (T125M, G188R, A209V, and H302L) of the ornithine transcarbamylase gene in congenital hyperammonemia. Hum Mutat 1996; 8(1): 74–76. [DOI] [PubMed] [Google Scholar]
- 4. Climent C, García-Pérez MA, Sanjurjo P, et al. Identification of a cytogenetic deletion and of four novel mutations (Q69X, I172F, G188V, G197R) affecting the gene for ornithine transcarbamylase (OTC) in Spanish patients with OTC deficiency. Hum Mutat 1999; 14(4): 352–353. [DOI] [PubMed] [Google Scholar]
- 5. Fernandes J, Saudubray J-M, Van den Berghe G, et al. (eds). Inborn Metabolic diseases: diagnosis and treatment. 4th ed. Berlin: Springer-Verlag, 2006, pp. 263–272. [Google Scholar]
- 6. Summar ML, Dobbelaere D, Brusilow S, et al. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr 2008; 97(10): 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gropman A. Brain imaging in urea cycle disorders. Mol Genet Metab 2010; 100(Suppl. 1): S20–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Knegt RJ, Groeneweg M, Schalm SW, et al. Encephalopathy from acute liver failure and from acute hyperammonemia in the rabbit. A clinical and biochemical study. Liver 1994; 14(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 9. Gordon N. Ornithine transcarbamylase deficiency: a urea cycle defect. Eur J Paediatr Neurol 2003; 7(3): 115–121. [DOI] [PubMed] [Google Scholar]
- 10. Rodney S, Boneh A. Amino Acid Profiles in Patients with Urea Cycle Disorders at Admission to Hospital due to Metabolic Decompensation. JIMD Rep 2013; 9: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deignan JL, Cederbaum SD, Grody WW. Contrasting features of urea cycle disorders in human patients and knockout mouse models. Mol Genet Metab 2008; 93(1): 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quinonez SC, Thoene JG. Citrullinemia type I. In: Pagon RA, Bird TD, Dolan CR, et al. (eds) GeneReviews™. Seattle, WA: University of Washington, Seattle; http://www.ncbi.nlm.nih.gov/books/NBK1458/ (1993, updated accessed 14 August 2013). [Google Scholar]
- 13. Panlaqui OM, Tran K, Johns A, et al. Acute hyperammonemic encephalopathy in adult onset ornithine transcarbamylase deficiency. Intensive Care Med 2008; 34(10): 1922–1924. [DOI] [PubMed] [Google Scholar]
- 14. Jiménez Jiménez FJ, Ortiz Leyba C, Morales Mendez S, et al. Variations in plasma amino acids in septic patients subjected to parenteral nutrition with a high proportion of branched-chain amino acids. Nutrition 1992; 8(4): 237–244. [PubMed] [Google Scholar]
- 15. Harada E, Nishiyori A, Tokunaga Y, et al. Late-onset ornithine transcarbamylase deficiency in male patients: prognostic factors and characteristics of plasma amino acid profile. Pediatr Int 2006; 48(2): 105–111. [DOI] [PubMed] [Google Scholar]
- 16. Panlaqui OM, Tran K, Johns A, McGill J, White H. Acute hyperammonemic encephalopathy in adult onset ornithine transcarbamylase deficiency. Intensive Care Med 2008; 34(10): 1922–1924. [DOI] [PubMed] [Google Scholar]