Abstract
Chronic tophaceous gout is the end stage of gout. We employed a blockade of interleukin-6 signaling therapy by tocilizumab instead of anakinra, an interleukin-1 receptor antagonist, for a 61-year-old Japanese woman diagnosed with tophaceous gout. Laboratory data showed that serum interleukin-6 concentration was elevated. Serum interleukin-1β concentration was under the detectable level, although serum uric acid was elevated due to renal dysfunction. The secretion patterns of interleukin-1β, tumor-necrosis factor-α, interleukin-6, and interleukin-8 from peripheral mononuclear cells isolated from the patient exhibited no remarkable differences compared with those of healthy volunteers. After treatment with the interleukin-6 receptor antagonist tocilizumab, serum interleukin-6 concentration decreased followed by improved clinical symptoms, such as reduced size of the subcutaneous nodules, no fever, and no acute gouty attacks during the treatment. Our case suggests that tocilizumab markedly improves clinical and laboratory manifestations in tophaceous gout with arthritis and fever as well as interleukin-1 blockade therapy.
Keywords: Tophaceous gout, tocilizumab, interleukin-6, interleukin-1β, inflammasome
Introduction
Gout is characterized by the formation and deposition of monosodium urate (MSU) crystals in the connective tissue and synovium of joint.1 Acute joint pain due to focal acute inflammation against the deposition of MSU crystals in the synovial fluid is a major symptom. Chronic tophaceous gout is the end stage of gout, which is directly related to hyperuricemia, and major joints such as hands, elbows, the spine, knees, and feet are affected by granulomatous nodule formation.2 Recent studies revealed that the major cause of gout is MSU crystals, being recognized by cryopyrin inflammasome, an inflammatory cytokine interleukin (IL)-1β processing platform.3 Cryopyrin, also known as nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing proteins-3 (NLRP3), is a member of the Nod-like receptor family that is composed of the N-terminal pyrin- or caspase-recruitment domain, the central part of the nucleotide-binding oligomerization domain and C-terminal leucine-rich repeats.4 Cryopyrin is reported to recognize various pathogen-associated molecular patternmolecules or damage-associated molecular pattern molecules, including MSU, and interacts with an adopter protein, apoptosis-associated speck-like protein containing a CARD (ASC), through their pyrin domains; then, ASC interacts with pro-caspase-1, leading to activation of the cryopyrin inflammasome that processes pro-IL-1β.5 4 Therefore, clinical blockade of IL-1β signaling is thought to be one of the best therapies for gout.6 Acute gouty attack has also been treated with colchicine, and its function was recently proved to involve inhibition of acetylated α-tubulin or mitochondrial transport, which resulted in inhibition of binding between ASC and cryopyrin, components of inflammasome.7 On the other hand, there were reported to be patients with tophaceous gout who could not receive these treatments due to renal dysfunction. In addition, anakinra, a recombinant human IL-1 receptor antagonist, has not been approved in Japan.8 Here, we report an elderly Japanese woman with chronic tophaceous gout with renal dysfunction accompanied by serum IL-6 elevation. Treatment of tocilizumab, an IL-6 receptor antagonist, improved clinical symptoms including tophaceous arthritis and reduced nodule size without subsequent IL-1β or other cytokine secretion.
Case report
A 61-year-old Japanese woman was admitted to our hospital complaining of fever and polyarthralgia. She had had arthralgia of her proximal interphalangeal joints and ankles for the past 3 years. Two years before admission, she was diagnosed with gouty arthritis because her excided left elbow node showed tophus with sodium urate crystals. She had suffered from polyarthritis, and serum creatinine concentration was already elevated due to renal dysfunction.
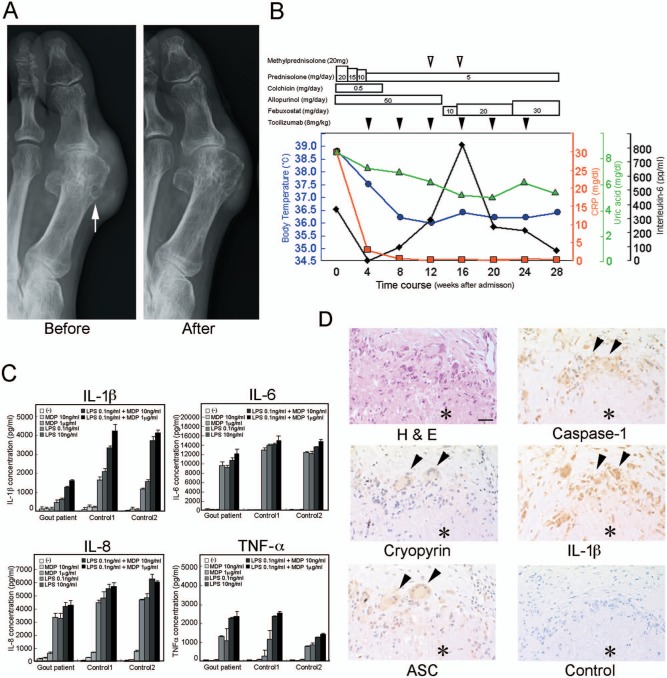
She was found to have symmetric polyarthritis of the elbows, knees, ankles, and metatarsophalangeal (MTP) joints of both feet. In addition, subcutaneous granulomatous nodules were detectable in her hands, ankles, and feet. Her serum laboratory data showed C-reactive protein (CRP), 29.8 mg/dL; ferritin, 2660 mg/L; creatinine, 2.46 mg/dL; blood urea nitrogen (BUN), 23.6 mg/dL; uric acid (UA), 8.7 mg/dL; aspartate aminotransferase (AST), 152 IU/L; and alanine aminotransferase (ALT), 245 IU/L. Her serum concentrations of cytokines measured by enzyme-linked immunosorbent assay (ELISA) were as follows: IL-1β, under the detectable level; IL-6, 371 pg/mL; and tumor-necrosis factor (TNF)-α, 3.6 pg/mL (Table 1). Her synovial fluid IL-6 concentration was 62,800 pg/mL. An X-ray image of her feet showed subcutaneous crystals around the first MTP joints and the overhanging margin in her right first metatarsal bone (Figure 1(a), Before, arrow).
Table 1.
Laboratory data of the patient’s serum before and after tocilizumab treatment.
| Before treatment | 24 weeks after treatment | |
|---|---|---|
| C-reactive protein (CRP) | 29.8 (mg/dL) | 0.04 (mg/dL) |
| Ferritin | 2660 (mg/L) | 103 (mg/L) |
| Creatinine | 2.46 (mg/dL) | 2.02(mg/mL) |
| Blood urea nitrogen (BUN) | 23.6 (mg/dL) | 53.9(mg/mL) |
| Uric acid (UA) | 8.7 (mg/dL) | 5.6 (mg/dL) |
| Aspartate aminotransferase (AST) | 152 (IU/L) | 34 (IU/L) |
| Alanine aminotransferase (ALT) | 245 (IU/L) | 20 (IU/L) |
| Interleukin-1β(IL-1β) | Not detected | Not detected |
| Interleukin-6 (IL-6) | 371 (pg/mL) | 78 (pg/mL) |
| Tumor-necrosis factor-α (TNF-α) | 245 (IU/L) | 20 (IU/L) |
Figure 1.
(a) X-ray images of the left toe from the patient with tophaceous gout. The tophus of the left first MTP joint and the overhanging margin (arrows) before tocilizumab treatment (Before) are shown. The tophus of the left first MTP joint decreased in size after tocilizumab treatment for 24 weeks (After). (b) Medications are indicated, and concomitant body temperature, serum CRP, serum UA and serum IL-6 concentrations are plotted by blue circle, red square, green triangle and black diamond, after admission. (c) IL-1β, TNF-α, IL-6, and IL-8 secretions by peripheral blood mononuclear cells (PBMCs) derived from the patient with tophaceous gout (Gout patient) and PBMCs from two healthy volunteers (Control1 and Control2). Isolated PBMCs were incubated with the indicated amount of muramyl dipeptide (MDP) (10 ng/mL or 1 µg/mL), lipopolysaccharide (LPS) (0.1 ng/mL or 10 ng/mL), MDP (10 ng/mL or 1 µg/mL) combined with LPS (0.1 ng/mL) or were left untreated (−) for 8 h. The concentrations of IL-1β, TNF-α, IL-6, and IL-8 in the supernatants were measured by ELISA. Values are the mean and SD from triplicate cultures. (d) Expression of inflammasome components in tophus with urate crystals excided from the left elbow. Expressions of cryopyrin (Cryopyrin), ASC (ASC), caspase-1 (Caspase-1), and IL-1β (IL-1β) were detected by immunohistochemistry with specific antibodies. Negative control (Control) and hematoxylin and eosin staining (H&E) are also shown. An asterisk indicates tophus deposit. Bar indicates 50 µm.
Interleukin: IL; MDP: muramyl dipeptide; LPS: lipopolysaccharide; tumor-necrosis factor: TNF; H&E: hematoxylin and eosin staining; ASC: apoptosis-associated speck-like protein containing a CARD.
After admission, medication of prednisolone at 20 mg/day and colchicine at 0.5 mg/day improved her fever, joint swelling and joint tenderness. Colchicine had to be stopped because of hair loss after six weeks of medication. We reduced the dose of prednisolone gradually, and then her arthritis flared up when the dose of prednisolone was 5 mg/day. Treatment of tocilizumab (8 mg/kg, every 4 weeks) was started 4 weeks after admission with two intraarticular injections of 20 mg of methylprednisolone in the 8 weeks after tocilizumab treatment. As a result, her arthritis gradually improved, subcutaneous nodules were reduced in size as determined by an X-ray image (Figure 1(a), After) and the patient’s laboratory data were also improved 24 weeks after starting the treatment of tocilizumab (Table 1) (Figure 1(b)). There was no fever or acute attack during the tocilizumab treatment (Figure 1(b)). Time course of medication and plotted body temperature, serum UA (mg/dL), serum CRP (mg/dL) and serum IL-6 (pg/mL) concentrations are indicated (Figure 1(b)).
Serum and synovial fluid cytokine concentrations
The analyses of patient materials were approved by the Human Research Ethical Committees of Ehime University and Dogo Spa Hospital. We measured the concentrations of IL-1β, IL-6, and TNF-α in the patient’s serum before and after tocilizumab treatment (Table 1), and synovial fluid before tocilizumab treatment by ELISA.
Cytokine secretion from peripheral mononuclear cells in culture supernatant
Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-gradient centrifugation (GE Healthcare Bio-Sciences AB, Piscataway, NJ). Synthetic N-acetylmuramyl-l-alanyl-d-isoglutamine, muramyl dipeptide (MDP) and lipopolysaccharide (LPS) from Escherichia coli O55:B5, cell culture tested, purified by trichloroacetic acid extraction and gel filtration chromatography, were purchased from Sigma-Aldrich (St Louis, MO). The cells were cultured in 24-well flat-bottom plates (BD Biosciences, San Jose, CA) with a final cell density of 1 × 106/mL in a volume of 1 mL of RPMI1640, including 10% fetal bovine serum (FBS) with MDP, LPS or left untreated for 8 h at 37°C in a humidified atmosphere with 5% CO2. Concentrations of IL-1β, IL-6, IL-8, and TNF-α in the culture supernatant were measured by ELISA using IL-1β-, IL-6-, IL-8-, and TNF-α-specific ELISA sets according to the manufacturer’s instructions (BD Biosciences). PBMCs isolated from the patient did not spontaneously secrete IL-1β, IL-6, IL-8 or TNF-α without any stimuli, and secreted these cytokines due to MDP (10 ng/mL or 1 µg/mL), LPS (0.1 ng/mL or 10 ng/mL) or a combination of MDP (10 ng/mL or 1 µg/mL) + LPS (0.1 ng/mL) in a dose-dependent manner (Figure 1(c)). The cytokine secretion patterns of PBMCs isolated from the patient were almost the same as those from healthy volunteers (Figure 1(c)).
Immunohistochemistry of inflammasome components in gouty nodule
The biopsy specimen, previously diagnosed by pathologists, embedded in a paraffin block, was subjected to immunohistochemical analysis. A 3-µm-thick serial sections of tissue were cut, deparaffinized in xylene, and hydrated in ethanol, and endogenous peroxidase activity was blocked by soaking in absolute methanol containing 0.3% H2O2 for 30 min. Before immunostaining, antigen retrieval was carried out by incubating tissue sections in a microwave in 10 mmol/L Tris-HCl buffer (pH 8.0) containing 1 mmol/L ethylenediaminetetraacetic acid (EDTA). Sections were blocked with 1% normal goat serum in 50 mmol/L Tris-buffered saline (TBS) (pH 7.6) and incubated with primary antibodies specific for cryopyrin (mouse mAb, clone: Nalpy3-b Alexis, San Diego, CA), ASC (mouse mAb, clone: 23-4, MBL, Nagoya, Japan), caspase-1 (rabbit pAb, No. #2225, Cell Signaling, Danvers, MA), and IL-1β (rabbit pAb, No. ab2105, abcam, Cambridge, MA) diluted 1:200, 1:100, 1:100 and 1:100, respectively, at 4°C overnight. After washing in TBS, slides were incubated with EnVision+ (DakoCytomation) for 60 min and 3,3-diaminobenzidine was used as the chromogen. These sections were counterstained with hematoxylin. In her excided tophus, expression signals of cryopyrin, ASC, caspase-1, and IL-1β were all detected in the monocytic cells and multinuclear giant cells around the MSU crystal foreign body granuloma (Figure 1(d), arrowheads).
Discussion
We have reported a Japanese case of tophaceous gout treated with the IL-6 antagonist tocilizumab. Tocilizumab treatment improved the clinical symptoms, especially arthritis, the subcutaneous nodule size as well as examination findings for inflammatory markers CRP and ferritin in her serum, the other biomarkers of creatinine and BUN, both of which represent renal function, and AST and ALT, both of which represent liver function (Table 1) (Figure 1(b)).
For standard treatments of chronic tophaceous gout, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, colchicine, and antihyperuricemic drugs were usually used. It was reported that MSU crystal was recognized by cryopyrin inflammasome in phagocytic cells, and IL-1β was released in stroma.3 Then, activated IL-1β drives inflammatory responses in synovial stroma, resulting in the production of inflammatory mediators such as IL-6, IL-8, and TNF-α.9 Thus, IL-1 blockade is thought to be the best therapy for a gout patient. However, an IL-1 receptor antagonist, anakinra, could not be used for gouty patients especially in Japan because it had not been approved.8 In addition, we sometimes encounter gout patients who are unable to undergo colchicine and/or NSAID treatments because of renal dysfunction.
Then, we decided to employ an alternative therapy, blockade of the IL-6 signaling pathway by tocilizumab, for our patient with tophaceous gout. Tocilizumab is a human IL-6 receptor antagonist, which can inhibit the IL-6 signaling pathway.10 MSU crystal increased IL-6 production from synoviocytes and monocytes.11 Actually, there are reported to be significantly higher proportions of the gout patients than healthy controls with high IL-6 levels in plasma.12,13 Consistent with this, serum IL-6 concentration was 371 pg/mL and her synovial fluid IL-6 concentration was 62,800 pg/mL before treatment with tocilizumab. After tocilizumab treatment for 24 weeks, the subcutaneous nodules decreased in size (Figure 1(a), Before and After), and her serum IL-6 concentration decreased to 78 pg/mL (Table 1) (Figure 1(b)). Associated with the improvement of clinical symptoms, serum CRP concentration improved from 29.8 mg/dL to 0.04 mg/dL after tocilizumab treatment for 24 weeks, and even high serum IL-6 concentration was observed during the treatment (Table 1) (Figure 1(b)).
We previously showed that continuous IL-1β secretion from a patient’s PBMCs may be a cause of the secretion of other inflammatory cytokines such as IL-6, IL-8, and IL-12p40 in Muckle–Wells syndrome (MWS), in which cryopyrin inflammasome of PBMCs was thought to be spontaneously activated.14 Then, we hypothesized that the cryopyrin inflammasome of PBMCs of a gout patient likely continuously secreted IL-1β and IL-6 under hyperuricemia as well as MWS. However, the secretion patterns of IL-1β, IL-6, IL-8, and TNF-α by the patient’s PBMCs in response to MDP (10 ng/mL or 1 µg/mL), LPS (0.1 ng/mL or 10 ng/mL) or a combination of MDP (10 ng/mL or 1 µg/mL) + LPS (0.1 ng/mL) were almost the same as those from healthy volunteers’ PBMCs (Figure 1(c)), suggesting that serum UA may not be able to activate the inflammasome of PBMCs, which does not seem to be a source of IL-6 and/or IL-1β, even under conditions of a high concentration of UA in a tophaceous gout patient. Consistent with this, it was also reported that mean levels of plasma IL-6 in patients with gout are normal.12
As such, where is the target of tocilizumab? We carried out immunohistochemical experiments in an excided tophus (Figure 1(d)). It is reported that MSU crystal activates cryopyrin inflammasome in phagocytic cells, which release processed IL-1β in stroma; then, IL-1β induces IL-6 production from stromal cells.9 Consistent with this, cryopyrin, ASC, caspase-1, and IL-1β were all expressed in the monocytic cells and multinuclear giant cells around the MSU crystal foreign body granuloma (Figure 1(d), arrowheads). We also obtained data of synovial fluid, which included a high concentration of IL-6. Thus, we speculate that focal stromal cells around MSU crystal deposition in synovium may be a target of tocilizumab. It also may be considered a possibility that the patient suffers from a comorbidity of gout and arthritis, which may be a target of tocilizumab, although further experiments should be carried out.
During preparation of this article, Pinto et al.15 reported that tocilizumab was effective in a patient with severe tophaceous gout resistant to treatment as well as our case of severe tophaceous gout. In addition, Mylona et al.16 reported that the production of IL-6 by MSU with stearic acid was in line with IL-1β production in PBMCs isolated from gout patients. The association between lipid metabolism and IL-6 production in gout patients also seems to be important for gout pathogenesis.
In conclusion, our case demonstrates that tocilizumab appeared to be effective for clinical symptoms of tophaceous gout, albeit with some difficulties, such as renal dysfunction and Japanese drug lag for blockade of the IL-1 receptor antagonist anakinra.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was supported by a Grant-in-Aid for Research on Measures for Intractable Diseases from the Ministry of Health, Labor and Welfare, Japan (11103549), and a Grant-in-Aid for Scientific Research on Innovative Areas “Homeostatic Inflammation” of the Ministry of Education, Culture, Sports, Science and Technology, Japan (24117710).
Research ethics and patient consent: The research was approved by the Human Research Ethical Committees of Ehime University and Dogo Spa Hospital (reference number 1301001), and written informed consent was obtained from the patient.
References
- 1. Mccarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med 1961; 54: 452–460. [DOI] [PubMed] [Google Scholar]
- 2. Bolzetta F, Veronese N, Manzato E, et al. Chronic gout in the elderly. Aging Clin Exp Res 2013; 25: 129–137. [DOI] [PubMed] [Google Scholar]
- 3. Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 4. Franchi L, McDonald C, Kanneganti TD, et al. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol 2006; 177: 3507–3513. [DOI] [PubMed] [Google Scholar]
- 5. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. So A, De Smedt T, Revaz S, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 2007; 9: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Misawa T, Takahama M, Kozaki T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 2013; 14: 454–460. [DOI] [PubMed] [Google Scholar]
- 8. Takeuchi T. Revolutionary change in rheumatoid arthritis management with biological therapy. Keio J Med 2011; 60: 75–81. [DOI] [PubMed] [Google Scholar]
- 9. Chen CJ, Shi Y, Hearn A, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 2006; 116: 2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 2005; 5: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 11. Guerne PA, Terkeltaub R, Zuraw B, et al. Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum 1989; 32: 1443–1452. [DOI] [PubMed] [Google Scholar]
- 12. Tsai PC, Chen CJ, Lai HM, et al. Analysis of polymorphisms in the promoter region and protein levels of interleukin-6 gene among gout patients. Clin Exp Rheumatol 2008; 26: 841–847. [PubMed] [Google Scholar]
- 13. Choe JY, Lee GH, Kim SK. Radiographic bone damage in chronic gout is negatively associated with the inflammatory cytokines soluble interleukin 6 receptor and osteoprotegerin. J Rheumatol 2011; 38: 485–491. [DOI] [PubMed] [Google Scholar]
- 14. Yamazaki T, Masumoto J, Agematsu K, et al. Anakinra improves sensory deafness in a Japanese patient with Muckle-Wells syndrome, possibly by inhibiting the cryopyrin inflammasome. Arthritis Rheum 2008; 58: 864–868. [DOI] [PubMed] [Google Scholar]
- 15. Pinto JL, Mora GE, Fernández-Avila DG, et al. Tocilizumab in a patient with tophaceous gout resistant to treatment. Rheumatol Clin 2013; 9: 178–180. [DOI] [PubMed] [Google Scholar]
- 16. Mylona EE, Mouktaroudi M, Crisan TO, et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res Ther 2012; 14: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]