Abstract
Objective:
Langerhans cell histiocytosis is an unusual disorder of unknown etiology with heterogeneous clinical behaviors and variable outcomes. It can involve one or more organs or systems, but to our best knowledge, parotid glands involvement in Langerhans cell histiocytosis is extremely rare in English literature.
Method:
We report a 13-month-old girl who presented with bilateral parotid swelling as presenting symptom. She was misdiagnosed for 4 months, but final diagnosis was multisystem Langerhans cell histiocytosis.
Result:
After being treated for 18 months, the patient acquired complete remission and attained similar growth status to other healthy children.
Conclusion:
Langerhans cell histiocytosis may involve any organ; in patients with parotid enlargement, Langerhans cell histiocytosis should be kept in mind in the differential diagnoses. We emphasize the importance of biopsy for histologic evaluation as soon as possible and even repeatedly if initial results are negative for Langerhans cell histiocytosis.
Keywords: Langerhans cell histiocytosis, parotid gland, pediatric, differential diagnosis
Introduction
Langerhans cells (LCs) have been first reported by Paul Langerhans in 1868, and Histiocyte Society formally coined Langerhans cell histiocytosis (LCH) in 1987. The characteristic of LCH is proliferation and accumulation of dendritic cells similar to epidermal LCs.1 Despite advances in understanding clinical course,2 the etiology and pathogenesis of LCH are still unclear. The incidence rate of LCH is estimated about 3–5 cases per million children aged under 15 years.3 LCH may involve multiple organs, including bone, skin, lymph node, lung, liver and pituitary. However, LCs rarely reside in parotid glands. Here, we report a LCH case of parotid involvement, accompanied by multiple organ involvement. The aim is to alert physicians to the heterogeneity of clinical manifestations for LCH and avoid misdiagnosis.
Case report
A 13-month-old girl presented with a 4-month history of bilateral parotid glands swelling, 2 weeks of intermittent fever and loss of appetite. Biopsy of cervical lymph node showed a large number of chronic inflammatory cells infiltration. She was diagnosed as recurrent purulent parotitis, but had no response to antibiotics. The patient was referred to our hospital. Further questioning about her medical history revealed that the patient never suffered from skin rashes, diarrhea, otorrhea, exophthalmos, polyuria or polydipsia. Physical examination showed jaundice of sclera and skin, without rashes. Both parotids (left measured 5 × 7 cm2, right measured 6 × 7 cm2) were tender, hard with obscure border. Cervical lymph nodes were tangible, just like soybean size. Bilateral breath sounds were coarse without rales. The liver edge was 6–7 cm below right costal margin. The spleen edge was 3 cm below left costal margin. A week later, scattered rashes spread to the trunk, and there was manifestation of light scaling, red or brownish papules and macules, occasionally coalesced into patches.
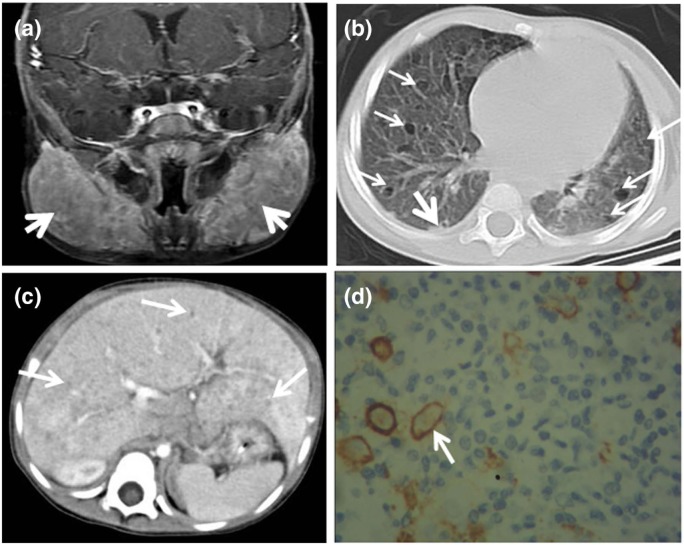
Laboratory data revealed that white blood cell count and platelet count were normal, and hemoglobin was 91 g/L. Liver function tests (LFTs) showed total serum bilirubin was 128.5 µmol/L (normal range: 3.42–20.52 µmol/L), conjugated bilirubin was 61.2 µmol/L (normal: <6.8 µmol/L), aspartate aminotransferase was 136.0 U/L (normal range: 8–38 U/L), alkaline phosphatase was 1025 U/L (normal range: 42–121 U/L) and other indexes were normal. Bone marrow aspiration was normal. The results from flow cytometry analysis gave no evidence of lymphoproliferative disease. Ultrasound showed bilateral parotids obviously enlarged with multiple cervical lymph nodes, splenomegaly and hepatomegaly with diffuse nodular-like lesions. These findings were confirmed by magnetic resonance imaging (MRI) and computerized tomography (CT). Head MRI indicated homogeneous enlargement of bilateral parotids (Figure 1(a)) and confluent lymph nodes. Imaging features on pulmonary high-resolution CT (Figure 1(b)) included combination of nodular or reticulonodular opacities with ill-defined margins and cysts with different thick walls, variable sizes and bizarre shapes. These lesions randomly distributed in both lung fields. Abdominal CT showed multiple round and oval hypodense nodules within liver (Figure 1(c)). Although bone scintigraphy showed an abnormality in the left temporal bone, head MRI images and X-ray of skeletal survey did not display typical osteolytic sign in skull or other bones.
Figure 1.
Imaging and histological characteristics of involved parotid gland, lung, liver and skin: (a) Contrast-enhanced MRI of head showed homogeneously enlarged parotid glands (arrow); (b) HRCT (lung window) revealed randomly distributed lesions in both lung fields, mainly included cysts (fine arrows) of variable size, shape and wall and nodules (coarse arrow); (c) contrast-enhanced CT scan indicated intrahepatic diffuse small nodules (arrows) and (d) CD1a antibody staining positive (arrow) in skin (magnification 400×).
MRI: magnetic resonance imaging; CT: computerized tomography; HRCT: high-resolution computed tomography.
Involved skin imprint cytology was obtained, but pathology report failed to find LCs. At that time, the patient’s general condition deteriorated with fluctuant fever, gradual jaundice, hepatosplenomegaly and somnolence. Asking consent from the parents, the dermatologist selected fresher abdominal rashes for punch biopsy. Meanwhile, the surgeon carried out ultrasound-guided needle biopsies of liver lesion and parotid. Punch biopsy of skin revealed LCs admixed with eosinophils and lymphocytes infiltration, and confirmatory staining with CD1a (Figure 1(d)) and Langerin was positive. However, liver biopsy only demonstrated hepatocellular cloudy swelling degeneration, normal structure in portal area, a few neutrophils and eosinophil infiltration, and immunochemical results were negative. Fine needle aspiration of the parotid found eosinophils, lymphocytes and histiocytes with occasional large nuclei and distinct nucleoli, and immunohistochemistry identified CD1a and S-100 positivity. All these findings were consistent with a diagnosis of LCH.
Finally, the patient was diagnosed as multisystem LCH with involvement of parotid, lymph node, skin, lung, liver, spleen and hematopoietic system.4 She was classified into “risk group” and enrolled into the Xinhua Children’s Hospital Refractory LCH protocol (see Supplemental Data). After initial 6-week therapy, there was resolution of her fever, parotid swelling, hepatosplenomegaly, rash, LFTs and anemia. Then, the child continued to accept maintenance treatment, the total course was 18 months. Now the patient has been followed up 36 months, without any evidence of local recurrence or disease progression, and has attained good physical and intellectual development equivalent to other healthy children with the same ages.
Discussion
LCH has been classified into histiocytic disorders with malignant clinical course and may range from isolated lesion to multifocal disease,5 but parotid involvement in LCH is rare. To the best of our knowledge, only 9 cases with LCH of parotid involvement previously described in English literature.6–13 The interval from onset of symptoms to pathological diagnosis was from 4 months to 4 years. It implies parotid LCH is easy to be misdiagnosed, if patients with parotid enlargement, LCH should be kept in mind in the differential diagnosis. Maybe parotitis more common in children and without other specific symptom, our case was initially misdiagnosed as recurrent suppurative parotitis. After 4 months, physical examination showed that the symptomatology was extensive. So, it is essential to perform a thorough workup to rule out other diseases. Parotid enlargement is either the most common performance of salivary gland lesions or local signs of systemic disease. The differential diagnoses of parotid enlargement are listed in Table 1. Even if rare, some tumor-like lesions (such as LCH) and malignances need to be distinguished in patients with parotid swelling. In addition to clinical and radiological features, the confirmed diagnosis of LCH is based on histological and immune-phenotypic examination. It is confirmed by positive staining with CD1a, S-100 or Langerin (CD207). Recent studies14–16 demonstrated the presence of BRAF mutations in 38%–57% of LCH cases, with the majority of cells co-expressing S-100 and CD1a harbored mutant BRAFV600E protein. It implies BRAFV600E mutations could be applied as a mutation-specific marker for routine screening in LCH patients. Unfortunately, in our case, this mutation was not detected. The dermatologist performed skin imprint cytology at first, but got negative result. However, skin punch biopsy found typical LCs which expressed positive CD1a and Langerin. This suggests that skin punch biopsy for the diagnosis of LCH is more valuable than that of skin imprint cytology, though the latter with less invasiveness. Although the liver function and imaging features were well matched with liver involvement, pathology supported without evidence. Jaffe17 thought confirmed diagnosis might be a challenge in liver, where Birbeck granules were not present and CD1a or Langerin maybe negative because LCH cells had regressed after having caused sclerosing cholangitis and cirrhosis. Therefore, the risk/benefit ratio of liver biopsy for suspected LCH cases should be carefully assessed.1
Table 1.
Differential diagnoses for parotid gland enlargement.
| Pathogeny | Disease | |
|---|---|---|
| Inflammatory (most common in children) | Acute inflammation | Mumps, lymphadenitis in parotid region, acute purulent parotitis and so on |
| Chronic inflammation | Chronic purulent parotitis, parotid tuberculosis and actinomycosis of the parotid gland | |
| Others | Parotid duct obstruction and oral disease | |
| Tumor and tumor-like lesion | Benign pathogeny | Parotid mixed tumor, adenolymphoma and myoepithelioma |
| Malignant | Primary malignant tumor (rhabdomyosarcoma, etc.) and metastatic tumor (neuroblastoma, leukemia, lymphoma, etc.) | |
| Tumor-like lesion | Langerhans cell histiocytosis, parotid cyst and parotid hemangioma | |
| Other causes | Benign parotid hypertrophy, drug-reactive parotid swelling and some syndromes | Sjögren syndrome, Wegener’s granulomatosis and uveoparotitis syndrome |
| Non-parotid disease | Benign masticatory muscle hypertrophy and infection of clearance under masticatory muscle |
Since LCH has unpredictable course, risk-tailored treatment is mandatory. Local control is recommended for isolated lesion, while systemic therapy is for multisystem involvement.18 Treatment options vary depending on the extent of the disease and the severity at onset. After being treated for 18 months and followed up for 36 months, our patient showed no disease evidence and attained similar growth status to other healthy children. The result indicates that our protocol is reasonable.
We emphasize the importance of recognizing the parotid involvement in LCH in the differential diagnosis. For suspected LCH cases, skin punch biopsy can give more accurate information than skin imprint cytology, and the indication of liver biopsy should be controlled strictly.
Acknowledgments
The authors are grateful to Dr Hui-min Li and Dr Wen-bin Guan for their excellent technical assistance with imaging and pathological diagnosis, respectively. The authors thank Dr Ching Lau, Texas Children’s Hospital, United States, for his critical review of the manuscript.
Footnotes
Consent: We have obtained the consent for publication from the patient’s kin and the ethics board.
Declaration of conflicting interests: None.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer 2013; 60(2): 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badalian-Very G, Vergilio JA, Degar BA, et al. Recent advances in the understanding of Langerhans cell histiocytosis. Br J Haematol 2012; 156(2): 163–172. [DOI] [PubMed] [Google Scholar]
- 3. Minkov M. Multisystem Langerhans cell histiocytosis in children: current treatment and future directions. Paediatr Drugs 2011; 13(2): 75–86. [DOI] [PubMed] [Google Scholar]
- 4. Donadieu J, Chalard F, Jeziorski E. Medical management of Langerhans cell histiocytosis from diagnosis to treatment. Expert Opin Pharmacother 2012; 13(9): 1309–1322. [DOI] [PubMed] [Google Scholar]
- 5. Kwon SH, Choi JW, Kim HJ, et al. Langerhans cell histiocytosis: a retrospective analysis in a Korean tertiary hospital from 2003 to 2012. J Dermatol 2013; 40(10): 824–828. [DOI] [PubMed] [Google Scholar]
- 6. Lieberman PH, Jones CR, Steinman RM, et al. Langerhans cell (eosinophilic) granulomatosis: a clinicopathologic study encompassing 50 years. Am J Surg Pathol 1996; 20(5): 519–552. [DOI] [PubMed] [Google Scholar]
- 7. Weinmann P, Crestani B, Tazi A, et al. 111In-pentetreotide scintigraphy in patients with Langerhans’ cell histiocytosis. J Nucl Med 2000; 41(11): 1808–1812. [PubMed] [Google Scholar]
- 8. Darvishian F, Hirawat S, Teichberg S, et al. Langerhans’ cell histiocytosis in the parotid gland. Ann Clin Lab Sci 2002; 32(2): 201–206. [PubMed] [Google Scholar]
- 9. Iqbal Y, Al-Shaalan M, Al-Alola S, et al. Langerhans cell histiocytosis presenting as a painless bilateral swelling of the parotid glands. J Pediatr Hematol Oncol 2004; 26(5): 276–278. [DOI] [PubMed] [Google Scholar]
- 10. Kojima M, Itoh H, Shimizu K, et al. Incidental Langerhans cell histiocytosis of the parotid gland resembling marginal zone B-cell lymphoma: a case report. J Oral Pathol Med 2006; 35(10): 630–632. [DOI] [PubMed] [Google Scholar]
- 11. Takahama A, Jr, Leon JE, de Almeida OP, et al. Nonlymphoid mesenchymal tumors of the parotid gland. Oral Oncol 2008; 44: 970–974. [DOI] [PubMed] [Google Scholar]
- 12. Shima H, Inokuchi M, Shimada H. A case of multisystem Langerhans cell histiocytosis with primary hypothyroidism followed by type 1 diabetes mellitus. Pediatr Blood Cancer 2009; 53: 232–234. [DOI] [PubMed] [Google Scholar]
- 13. Babacan T, Turkbeyler IH, Zengin O, et al. Adult Langerhans’ cell histiocytosis: a rare cause of parotid gland enlargement. J BUON 2013; 18(2): 546–547. [PubMed] [Google Scholar]
- 14. Sahm F, Capper D, Preusser M, et al. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood 2012; 120(12): e28–e34. [DOI] [PubMed] [Google Scholar]
- 15. Satoh T, Smith A, Sarde A, et al. B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS One 2012; 7(4): e33891 DOI: 10.1371/journal.pone.0033891. PMID: 22506009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010; 116(11): 1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaffe R. The diagnostic histopathology of Langerhans cell histiocytosis. In: Weitzman S, Egeler M. (eds) Histiocytic disorders of children and adults. Cambridge: Cambridge University Press, 2005, pp. 14–39. [Google Scholar]
- 18. Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood 2013; 121(25): 5006–5014. [DOI] [PubMed] [Google Scholar]