Abstract
Background
Antibiotic resistance due to the presence of extended-spectrum beta-lactamases (ESBLs) among Enterobacteriaceae is a worldwide problem. Data from Ghana regarding this resistance mechanism is limited. This study was designed to investigate the presence of TEM-type ESBL genes, their locations and their conjugabilities in clinical isolates of enterobacteria collected from the Korle-Bu Teaching Hospital in Ghana.
Methods
Study isolates were characterized with respect to ESBL phenotype, TEM-type ESBL gene detection, location of the ESBL gene(s) and conjugability of the ESBL phenotype using nalidixic acid-resistant Escherichia coli K-12 as recipient. Phenotyping was by Kirby Bauer disk diffusion using cefpodoxime, ceftazidime, cefotaxime and their combinations with clavulanate. Gene detections were by PCR using blaTEM primers.
Results
Overall, 37.96 % of 137 clinical isolates showed ESBL phenotype. The ESBLs occurred mostly in Klebsiella spp. (42.3 %) and then Escherichia coli (34.6 %). The TEM gene was detected in 48.1 % of ESBL-positive isolates and was determined to be plasmid-borne in 24 of 25 blaTEM detections. Overall, 62.7 % of TEM-producing isolates transferred the ESBL phenotype by conjugation.
Conclusions
The results highlight the presence of TEM-type ESBLs in the Korle-Bu Teaching Hospital and show considerable risk of environmental contamination through the urine of infected persons. An inhibition zone chart was generated which indicates the possible presence of complex beta-lactamase types. The data points to the fact that the ESBL-producing bacteria may disseminate this resistance mechanism via conjugation.
Keywords: Beta-lactamase, Inhibition zone chart, Conjugation, Reamplification
Background
The Enterobacteriaceae comprise a large family of clinically significant Gram-negative bacteria. They cause over 30 % of the morbidity and mortality associated with bacterial infections [1, 2] [CDC 2015, unpublished observation]. Resistance to β-lactam antimicrobials in Enterobacteriaceae has been due to largely the presence of β-lactamase enzymes [3, 4]. β-lactamase genes (bla) were originally found to be chromosomal [5]. Since the emergence of the first reports on plasmid-borne blaTEM-1 in the 1960s, many more which appear to be mutants of the classic TEM genes, as well as novel types including SHV, CTX-M and OXA, have been found [6–8]. β-lactamase-mediated resistance may develop in vivo during chemotherapy, lending support to the prevalent view that ESBL plasmids are conjugative, may be borne on transposons, and that the genes may have high mutation frequencies [9, 10]. Also of concern is the increasing dissemination of ESBLs across hitherto wildtype species [11].
ESBL-producing bacterial isolates have been reported across Africa, even in isolated remote communities [11–16]. Beyond detection, data on specific identification of ESBLs are needed for deciding local therapy options, control strategies and recognition of unusual cases. In Ghana, investigations into mechanisms of β-lactam resistance have often been limited to phenotypic characterizations. This study reports the presence of TEM-type β-lactamase genes, their location(s) and their transferability by conjugation in clinical enterobacteria isolates collected at the Korle-Bu Teaching Hospital (KBTH).
Methods
Study isolates
Bacteria were isolated from clinical specimen (sputum, blood, feces, urine, cerebrospinal fluid, high vaginal swabs, wound swabs, pus) submitted to the Microbiology Laboratory of Korle-Bu Teaching Hospital (KBTH). A total of 137 isolates were included in the study. The isolates comprised only those recovered as members of the family Enterobacteriaceae [17] and identified as being responsible for patients’ clinical conditions. Conclusive identification of isolates was done with API 20E rapid test strips (bioMerieux SA, Marcy l’Etoile, France). Isolates were stocked in Luria–Bertani (LB)-ampicillin (60 μg/ml) broth containing 15 % glycerol and kept at −20 °C. All stocks were routinely plated to check for purity. Four National Collection of Typed Cultures (NCTC) isolates were included in the study as controls; NCTC 10418 (β-lactamase negative), NCTC 13352 (TEM 10 positive), NCTC 13353 (CTX-M-15 positive), NCTC 165032 (SHV-3).
Detection of ESBL phenotype
Isolates were examined for ESBL phenotype by the Kirby-Bauer disk diffusion method of susceptibility testing and according to United Kingdom Health Protection Agency (HPA) guidelines (QSOP 51i2.2, 2008). Screening and confirmatory tests were done simultaneously on the same plate. Briefly, each test isolate was plated on Mueller–Hinton agar (Oxoid, UK). After incubating overnight at 37 °C, a 0.5 McFarland suspension in peptone broth (MAST, UK) of each was prepared. This suspension was then swabbed onto a cation-balanced Mueller–Hinton agar plate (MAST, UK) and left to dry completely. Antibiotic discs from a D52C ESBL detection kit (MAST, UK) were applied onto the plate and the setup was incubated at 37 °C for 18 h. Discs used were cefpodoxime (10 μg), ceftazidime (30 μg), cefotaxime (30 μg) and combination discs of cefotaxime/clavulanate (30/10 μg) and ceftazidime/clavulanate (30/10 μg). After incubation, inhibition zone diameters were measured and interpreted. A zone difference of ≥5 mm between the single and the combination disks for any of the antibiotics was regarded positive for ESBL production. Klebsiella pneumoniae ATCC 700603 was used as positive control for ESBL production.
Assessment of antibiotics for ESBL phenotyping
The diagnostic performance of each antibiotic used for ESBL phenotyping was assessed on the following criteria: sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Conjugation study
Only isolates confirmed as ESBL-producers were included in subsequent experiments. Conjugations were done using nalidixic acidRE. coli K-12 as the recipient strain. Three of the study isolates that had shown resistance to nalidixic acid were excluded from this stage of the study. Donor isolates were grown in LB-ampicillin (60 µg/ml) broth overnight. An aliquot of a pure stock of the recipient strain was also grown overnight in LB broth but with no antibiotic. For conjugation, aliquots of each donor and the recipient (1 ml each) were transferred into fresh LB broth and incubated for 2 h. Volumes of 500 µl of each donor culture were each taken and mixed with an equal volume of the recipient culture. The mixed cultures were incubated for 6 h at 37 °C. Selection for transconjugants was carried out on MacConkey agar (MAST, UK) supplemented with 32 µg/ml nalidixic acid and 100 µg/ml ampicillin [16]. Transconjugants were confirmed for ESBL production as previously described.
Plasmid DNA extraction
Plasmid DNA was extracted from both ESBL-producing donor isolates and transconjugants according to previously described methods [18, 19]. For electrophoresis, 10 μl of each extract was mixed with 2 μl of 6X gel loading dye and electrophoresed on 0.7 % agarose gel stained with ethidium bromide (1 μg/ml). Electrophoresis was carried out in 1X Tris–acetate-EDTA (TAE) buffer at 10 V/cm for 1 h and visualized under UV transillumination [20]. The plasmid DNA samples obtained were used in subsequent PCRs.
Total DNA extraction
Total DNA was also extracted from ESBL-producing donor isolates according to the method described by [21]. The DNA samples obtained by this procedure were used in subsequent PCR procedures.
PCR for ESBL Genes
PCR amplifications of blaTEM, blaSHV and blaCTX-M genes were performed in 25 µl reaction mixes containing 25 units/ml of Taq DNA polymerase, 200 µM each of dATP, dGTP, dTTP and dCTP, 0.2 µM of each primer, 1.5 mM MgCl2 and 5 µl of plasmid or total DNA template. Amplifications were carried out with the following thermal cycling profile: initial denaturation for 10 min at 94 °C followed by 35 cycles of amplification consisting of 30 s at 94 °C, 1 min at the appropriate annealing temperature for the specific primer and 1 min at 72 °C for primer extension, and then 10 min at 72 °C for the final extension with a soaking step at 4 °C.
In cases where reamplifications of the previous PCR product were necessary, the reagent concentrations were modified to use only half the primer concentration used in the previous reaction and 1.0 µl of the previous product as template. Primers used and their corresponding annealing temperatures are shown in Table 1. For electrophoresis, 10 µl of each PCR product were prepared and run as already described.
Table 1.
Sequences, annealing temperatures and expected product sizes of primer sequences targeting the specified ESBL genes
| Gene | Primer | Annealing temp. (°C) | Expected product size (bp) |
|---|---|---|---|
| bla TEM |
f: 5′-AAA CGC TGG TGA AAG TA-3′ r: 5′-AGC GAT CTG TCT AT-3′ |
45 | 720 |
| bla SHV |
f: 5′-ATG CGT TAT ATT CGC CTG TG-3′ r: 5′-TGC TTT GTT ATT CGG GCC AA-3′ |
60 | 726 |
| bla CTX-M |
f: 5′-GAC GAT GTC ACT GGC TGA GC-3′ r: 5′-AGC CGC CGA CGC TAA TAC A-3′ |
55 | 499 |
Results
A total of 137 clinical isolates belonging to E. coli, Klebsiella spp., Citrobacter spp., Enterobacter spp. and Proteus spp. were collected for this study. Table 2 shows the species distribution and clinical specimens from which isolates were recovered.
Table 2.
Distribution of study isolates according to clinical sources
| Isolate | Clinical source | Number of species | ||||||
|---|---|---|---|---|---|---|---|---|
| Urine | Blood | Sputum | CSF | HVS | Othera | Total | % | |
| Citrobacter spp. | 2 | 1 | 6 | 0 | 1 | 0 | 10 | 7.3 |
| Enterobacter spp. | 2 | 5 | 1 | 0 | 0 | 0 | 8 | 5.8 |
| Escherichia coli | 42 | 9 | 7 | 1 | 3 | 7 | 69 | 50.4 |
| Klebsiella spp. | 23 | 6 | 8 | 0 | 4 | 3 | 44 | 32.1 |
| Proteus spp. | 1 | 0 | 4 | 0 | 1 | 0 | 6 | 4.4 |
| Total | 70 (51.1 %) | 21 (15.3 %) | 26 (19.0 %) | 1 (0.7 %) | 9 (6.6 %) | 10 (7.3 %) | 137 | 100 |
Though only one instance of infection was observed from three CSF samples tested, it was separated due to the clinical significance of infection in the normally sterile cerebrospinal region
CSF cerebrospinal fluid, HVS high vaginal swab
aRefers to miscellaneous clinical sources (wound swabs, pus, aspirates, ear swabs)
Detection of ESBL-producing isolates
Figure 1 shows the setup for the disc diffusion assays for ESBL phenotype detection. Sensitivity values were 100 % for cefpodoxime (CPD), 100 % for cefotaxime (CTX) and 97.06 % for ceftazidime (CAZ). Cefpodoxime had highest specificity of 95.24 %, followed by CTX (94.64 %) and CAZ (92.98 %). Similarly, CPD recorded the highest PPD (92.98 %), then CTX (93.02 %) and CAZ (89.19 %). For NPV, CPD and CTX showed 100 %. Inhibition-zone size differences for Klebsiella spp. and E. coli isolates for all three antibiotics are presented in Fig. 2. Overall, 52 (37.96 %) of the 132 isolates included in this study expressed ESBLs (Table 3). The ESBL phenotype was most predominant in Enterobacter spp. (62.5 %) followed by Citrobacter spp. (60 %). Meanwhile, Klebsiella species comprised the majority of ESBL-producing isolates (42.3 %). Table 4 shows distribution of ESBL-producing isolates across clinical samples.
Fig. 1.
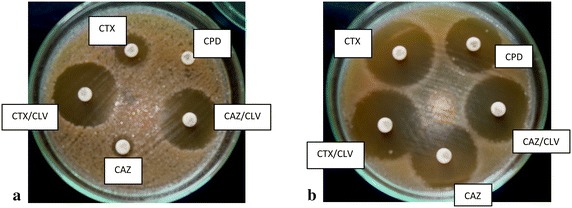
Appearance after over-night incubation for ESBL phenotyping of (a) an ESBL-positive strain and b an ESBL-negative strain. Plate a shows an example of what was taken as a positive ESBL test result. Plate b shows an example of a negative ESBL test result. In both cases, cefpodoxime was used only to screen and not in confirmation. Cefotaxime and ceftazidime were used in both screening and confirmation. CPD cefpodoxime 10 µg, CPD/CLV cefpodoxime 10 µg + clavulanate 10 µg, CTX cefotaxime 30 µg, CTX/CLV cefotaxime 30 µg + clavulanate 10 µg, CAZ ceftazidime 30 µg, CAZ/CLV ceftazidime 30 µg + clavulanate 10 µg
Fig. 2.
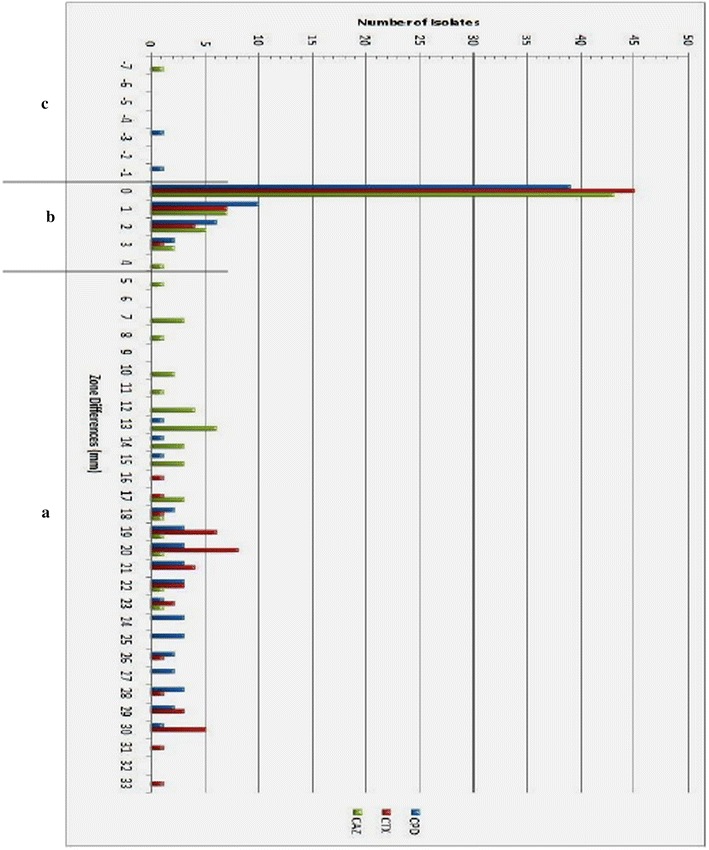
Inhibition zone chart for Klebsiella spp. and E. coli isolates. Zone differences observed between screening and confirmatory tests using each of the screening antibiotics alone and in its appropriate combination with clavulanate for confirmation. CPD cefpodoxime, CTX cefotaxime, CAZ ceftazidime. Region a zone differences for ESBL-positive phenotype. Region b zone-differences for ESBL-negative phenotype. Region (c) unusual zone differences
Table 3.
ESBL prevalence amongst study isolates
| Isolate | Not confirmed ESBL-positivea | ESBL prevalence | |
|---|---|---|---|
| Confirmed ESBL-positive | % of overall prevalence | ||
| Citrobacter spp. | 4 | 6 (60 %) | 11.5 |
| Enterobacter spp. | 3 | 5 (62.5 %) | 9.6 |
| Escherichia coli | 51 | 18 (26.1 %) | 34.6 |
| Klebsiella spp. | 22 | 22 (50.0 %) | 42.3 |
| Proteus spp. | 5 | 1 (16.7 %) | 1.9 |
| Total | 85 | 52 (37.9 %) | 100 |
ESBL prevalence was determined using MAST D52C combined discs by the Kirby-Bauer method of antibiotic susceptibility testing
aThe column for “not confirmed ESBL-positive” includes isolates that failed screening tests as well as those isolates that may have passed the screening test(s) but failed confirmatory tests
Table 4.
Distribution of ESBL-producing strains according to clinical sources
| Clinical source | Sample size | ESBL phenotype present | |
|---|---|---|---|
| Number | % of total number of ESBL-producers | ||
| Urine | 70 | 31 (44.3 %) | 59.6 |
| Blood | 21 | 9 (42.9 %) | 17.3 |
| CSF | 1 | 0 (0) | 0 |
| HVS | 9 | 3 (33.3 %) | 5.8 |
| Sputum | 26 | 5 (19.2 %) | 9.6 |
| Othera | 10 | 4 (40 %) | 7.7 |
| Total | 137 | 52 | 100 |
HVS high vaginal swab, CSF cerebrospinal fluid
aRefers to miscellaneous clinical sources (wound swabs, pus, aspirates, and ear swabs)
Conjugation studies
Results from the conjugation study indicated 64 % overall conjugability of ESBL genes amongst the ESBL-producing isolates (Table 5).
Table 5.
Conjugabilities of ESBL-producer phenotype from isolates confirmed for ESBL production
| Donor isolate | Total | Conjugation successful | Percent conjugability (%) |
|---|---|---|---|
| Klebsiella pneumoniae | 22 | 19 | 86.4 |
| Escherichia coli | 18 | 9 | 50.0 |
| Enterobacter spp. | 5 | 2 | 40.0 |
| Citrobacter spp. | 4 | 1 | 25.0 |
| Proteus spp. | 1 | 1 | 100 |
| Total | 50 | 32 | 64 |
ESBL-positive study isolates were conjugated to nalidixic acid-resistant E. coli K-12. Selection of transconjugants was done on MacConkey agar (MAST, UK) supplemented with ampicillin and nalidixic acid
ESBL genotyping
The TEM gene was found in 25 (19.6 %) of the 127 isolates with ESBL-producing phenotype. None of the ESBL-positive isolates had SHV- or CTX-M-type genes (Fig. 3). Of the 25 strains with TEM-type genes, 12 (48 %) transferred their ESBL phenotype in conjugation assays (Table 6). In 24 (0.96 %) of the 25 TEM-gene positive strains, the gene was detected in both plasmid and total-DNA extracts.
Fig. 3.
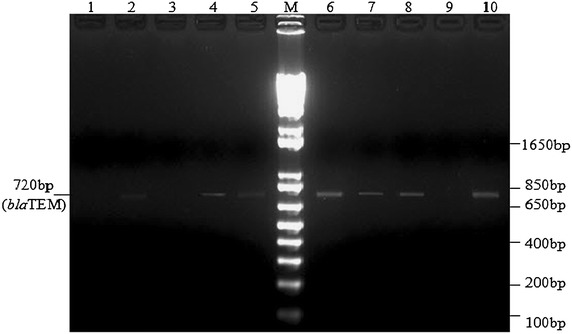
Bands observed after reamplification of previous PCR product with the TEM family-specific primers. PCR products were resolved on 2 % agarose gel stained with 1 µg/ml ethidium bromide at 100 V for 30 min. The gel was photographed under UV illumination. Lane M 1 kb plus DNA ladder, Lanes 2, 4, 5, 7, 8, 10 PCR product from showing amplified TEM gene with band position at 720 bp, Lane 6 TEM-3-producing E. coli NCTC 13351, Lanes 1, 3, 9 PCR product with no amplification
Table 6.
Numbers of detected conjugative TEM genes
| Isolate | TEM | Number that had transferred by conjugation |
|---|---|---|
| Citrobacter spp. | 0 | 0 |
| Enterobacter spp. | 1 | 0 |
| Escherichia coli | 11 | 3 |
| Klebsiella spp. | 12 | 8 |
| Proteus spp. | 1 | 1 |
The TEM primer produced PCR amplification product in DNA extracts from 25 of the ESBL-positive isolates. Verification of which of these genes had been transferred by conjugation was obtained by comparing this data to results of PCR on plasmid-DNA extracts from the transconjugants
Discussion
Over the last decade, many studies have demonstrated the presence of ESBL-mediated resistance in bacteria causing infections in patients [25–28]. Despite several reports of ESBL presence in Ghana, the characterization of ESBL genotypes in relation to chromosomal or plasmid locations—and their transferability by conjugation—are not described.
ESBLs in study isolates
The study revealed a moderate [37.96 % (n = 52/137)] prevalence of ESBL phenotype among the study isolates. In Table 3, the column for those that were not confirmed positive included all the bacteria that failed the screening tests as well as those that may have passed a screening test but failed all three confirmatory tests. The ESBL-producing isolates were found among every genus tested.
Two observations merit attention. First, the level of ESBLs is comparable to that reported by other ESBL-affected institutions in Ghana [29, 30] but higher than figures reported in many other reviews spanning across several regions [25–27]. In the African context however, the situation in KBTH appears to be rather moderate [13, 14, 30]. If current trends of lack of routine ESBL-monitoring and lack of ESBL-control strategies continue however, it can be expected that the prevalence will rapidly increase. This will lead to increased treatment expenses, longer hospital stays and possibly, mortality. Second, our results point to ESBL-producing isolates as being present among various members of the family Enterobacteriaceae and not just Escherichia coli and Klebsiella spp. In contrast to the attention paid mostly to ESBLs in Escherichia coli and Klebsiella spp. [25–28], the study showed that although a minority of the isolates were non-Escherichia coli and non-Klebsiella species, >40 % of these were ESBL-producers.
It is also noteworthy that ESBL detection was considerably high among Citrobacter freundii (>50 %) and Enterobacter cloacae (>60 %). Though only six Proteus spp. isolates were included in the study, their producer prevalence of 16.7 % cannot be over-looked. Therefore, the practice of not investigating ESBL presence in these pathogens may have an adverse impact on patients who are treated with extended-spectrum cephalosporins. With their hypermotility and ability to easily dominate biofilms, ESBL-producing Proteus spp. is a very uncomfortable prospect.
ESBL-producing strains appeared to be most common amongst isolates cultured from urine samples. Considering the fact that some bacteria will certainly be expelled in situ when urine is excreted, this has rather significant implications for risk of environmental contamination and cross-infections.
Performance of detection agents
Extent of reliability of phenotypic screening and confirmatory agents is needed to confidently rule out isolates that do not pass screening and/or confirmatory tests. Both cefpodoxime and cefotaxime had 100 % sensitivity scores and 100 % negative predictive value (NPV) scores. After comparing specificity and positive predictive value (PPV) scores however, it was evident that cefotaxime had a lesser likelihood of refusing a possible-positive than cefpodxime. It is generally accepted that cefpodoxime is the best single agent for ESBL detection [31]. From these results however, cefotaxime, with its slightly lower specificity score but higher positive predictive value score, performed just as well as, if not better than, cefpodoxime. Data of this nature are very important since for a given study involving large numbers of samples, one cannot screen all collected isolates by genotypic methods which are the gold standard in ESBL detection.
Unusual inhibition zone patterns should however be treated on a case-by-case basis.
Inferences from inhibition zones
Comparing the cefpodoxime zone size differences represented in Fig. 2 to data from the British society for antimicrobial chemotherapy [32], certain implications were realised. It was seen that the patterns of size differences represented in both figures were quite different. In the reference data, zone differences of the majority of ESBL-producer isolates fell between the ranges of 6–26 mm whereas from this study, zone differences fell between 13–30 mm with the majority ranging from 18 to 30 mm. The relatively higher zone sizes realized from this data may be indicative of differences in plasmid copy numbers and activity profiles of the enzymes as well as differences in permeability characteristics of the isolates from the two different localities.
Two Klebsiella isolates had cefpodoxime zone size differences that were in the negatives; −1 and −3 (Fig. 2). The lack of synergistic effect with the addition of clavulanate might suggest that they produce AmpC enzymes. However, they both tested screen-negative with each antibiotic used, uncharacteristic of AmpC-producing strains. Comparing to the reference data, those isolates were likely to produce K1 β-lactamases. The K1 enzymes are a family of β-lactamses, encoded by chromosomal genes, and which are presently thought to be unique to Klebsiella oxytoca. The two isolates however had corresponding ceftazidime zone-size differences of −7 and 1 mm. This implies that any K1 presence in them is unlikely since ceftazidime is notably susceptible to K1 enzymes. Also, the activity of K1 enzymes on ceftazidime is greatly diminished with the addition of clavulanate [33, 34]. Both isolates were amongst those most susceptible to the screening agents used, including ceftazidime, but were obviously not affected by clavulanate synergy. Currently, the only β-lactamase class that fits this profile of decreased affinity for β-lactam substrates and non-inhibition by clavulanate is the mutants of the inhibitor-resistant TEMs (IRTs) which belong to class A, subgroup 2br. The 2br subgroup contains the inhibitor-resistant TEM-type enzymes that evolved from the classical TEMs by acquiring the substitutions M69 V and N276D [29]. Derivatives of these enzymes are the complex TEM mutants (CTMs) that combine inhibitor-resistance with decreased affinity for cephalosporins [35].
A few isolates (4 for cefpodoxime, 3 for cefotaxime and 5 for ceftazidime) were screen-positive but failed the confirmatory tests, with 2 strains common to cefpodoxime and cefotaxime and 1 strain being common to all three antibiotics. This kind of phenotype, though typical of ampC-producers, may also be expressed by strains with CTMs, high levels of the TEM-1 enzyme and OXA-type ESBLs.
An interesting observation was one Klebsiella isolate which was clearly screen-positive with cefpodoxime, tested screen-negative for ceftazidime but was affected by clavulanate synergy with ceftazidime. Screening by ceftazidime alone would have led to the isolate being ruled as an ESBL non-producing strain. However, the observed synergy upon addition of clavulanate suggests, besides the possibility of AmpC-production, the presence of other β-lactamase types that are susceptible to the inhibitor.
It is seen from Fig. 2 that from 5 to 15 mm in region (a), inhibition of cefotaxime hydrolysis by clavulanate is not shown. This is a likely indication of the presence of CTX-M enzymes which preferentially hydrolyse cefotaxime relative to cefpodoxime and ceftazidime. Like all other ESBLs, CTX-M enzymes are susceptible to inhibition by clavulanate. The effect of clavulanate synergy with cefpodoxime and ceftazidime is seen but since CTX-M’s normally hydrolyse these with relatively lower turn-over rates, the zone size differences there are not as large as for cefotaxime which the enzymes would hydrolyse with greater efficiency in the absence of clavulanate.
Conjugation study
In the present study, about 63 % conjugability was observed, higher than the 38.7 % reported in Cameroon by [36]. In contrast to the report by [37] where E. coli showed higher ESBL conjugability than Klebsiella spp., Klebsiella had the highest rate of 86.4 % followed by E. coli’s 50 % (Table 5). This pattern of results may stem from the fact that Klebsiellae have been found to frequently harbor plasmids bearing blaSHV -type genes, and considering the usual sizes of blaTEM—(less than 80 kb) and blaSHV—(from about 80 kb to up to 350 kb in some Klebsiella isolates) bearing plasmids, the latter are probably more likely to be conjugable.
DNA extraction and PCR
All isolates positive for ESBL-production were selected for DNA extraction. Attempts at linearization of plasmids using PstI, HindIII and EcoRI restriction enzymes always failed. A plasmid which consistently appeared in the 22 kb position (data not shown) was found to be present in some of the plasmid extracts in which TEM genes were detected. Further resolution of the 22 kb band showed that it consisted of two clearly distinct bands placed closely together. All the plasmid extracts had the lower band. Plasmid preparations from a few of the transconjugants showed only the lower band, suggesting that the higher band may have been a non-conjugable plasmid species.
DNA extracted by a Promega Plus SV Miniprep DNA kit and [20] were used for PCR with blaTEM, blaSHV and blaCTX-M primers. Initial PCR rounds on control isolates showed amplification products for blaTEM but none for blaSHV and blaCTX-M positive controls (data not shown). Generally, the blaTEM products required re-amplification to be clearly visible (Fig. 3). A total DNA extract belonging to an Enterobacter spp. isolate produced amplification product but nothing was observed with its corresponding plasmid extract. This suggested that the detected gene may have been chromosomal. With the Klebsiella spp. and E. coli isolates that produced amplicons with both DNA extracts, there is at least a plasmid-borne blaTEM gene present even if there may be others that are chromosomal. Particularly with the Proteus spp. DNA sample, the detection made with the total-DNA extract was notably very faint relative to that from its plasmid extract. Due to the fact that any plasmid template present would be very dilute in the total-DNA preparation, this strongly suggested again that the gene detected by PCR may have been plasmid-borne in the Proteus isolate.
Plasmid mediation and conjugability
TEM-producing isolates that did not have the 22 kb plasmid included 2 E. coli, 1 Enterobacter and 1 Proteus isolate. This may have been a TEM-bearing plasmid since it was common to 84 % of the isolates in which TEM was detected. Judging from its size however, it was unlikely to be conjugative and this had been demonstrated by its absence in the plasmid extracts from the transconjugants. It was unlikely also to be mobilisable since it was not observed in any of the transconjugants tested. [38] has however previously reported the successful conjugation of a 10 kb ESBL-associated resistance determinant. The Proteus isolate had transferred its phenotype during the conjugation study (Table 6), suggesting that the gene it carried was on a mobilisable plasmid. The proportion of ESBL conjugability reported here also suggests that precautions should be taken to minimise the possibility of horizontal transfer of ESBL genes as well as clonal spread as reported by [39].
Conclusions
Detection level of ESBL-producing strains amongst the isolates studied was 37.96 % (n = 137). It is important that subsequent work carried out on ESBL-producing strains should take ampC β-lactamase detection as well as influence from other types of β-lactamases that may be present into account. This is because as more and more isolates begin to concomitantly produce these enzymes, interactions between them will make antibiograms of the isolates rather difficult to interpret. In the majority of cases, ESBL-producing bacteria in the hospital could transfer the phenotype by conjugation. Interpretative reading of the inhibition-zone chart suggests that other types of β-lactamases such as inhibitor-resistant TEMs (IRTs) and ampCs might have been present amongst the isolates. These need to be investigated.
Authors’ contributions
DO-M, NO-N and EYB carried out the laboratory work for the study. KT-D supervised the microbiological aspects of laboratory work. YDO and STS supervised the laboratory work in molecular biology. DO-M, EO-M and STS prepared the manuscript. EO-M and NO-N reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Virology Department of Noguchi Memorial Institiute for Medical Research and the technicians of the Microbiology Unit, Central Laboratory, Korle-Bu Teaching Hospital for the use of their facilities and for their assistance in various ways.
Competing interests
The authors declare that they have no competing interests.
Ethics
All ethical considerations and guidelines relating to the collection and use of bacterial isolates from clinical laboratory samples in Ghana were followed. The isolates were designated arbitrarily. The study was approved by the Ethical and Protocol Review Committee of University of Ghana Medical School, College of Health Sciences.
Abbreviations
- AmpC
ampicillin-hydrolyzing cephalosporinase
- bla
beta-lactamase gene
- ESBL
extended spectrum β-lactamase
- M
methionine
- V
valine
- N
asparagine
- D
aspartate
Contributor Information
Daniel Oduro-Mensah, Email: danomensah@gmail.com.
Noah Obeng-Nkrumah, Email: successfulnoahforchrist@yahoo.com.
Evelyn Yayra Bonney, Email: ebonney@noguchi.ug.edu.gh.
Ebenezer Oduro-Mensah, Email: ebomens78@yahoo.co.uk.
Kingsley Twum-Danso, Email: ktwumdanso@yahoo.com.
Yaa Difie Osei, Email: yaaosei@ug.edu.gh.
Sammy Tawiah Sackey, Email: sams@ug.edu.gh.
References
- 1.Kocsis B. Antibiotic resistance mechanisms in Enterobacteriaceae. In: Mendez-Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education. Badajoz: Formatex; 2013. pp. 251–257. [Google Scholar]
- 2.Rossolini GM, Mantengoli E, Docquier J-D, Musmanno RA, Coratza G. Epidemiology of infections caused by multiresistant Gram-negatives: ESBLs, MBLs, panresistant strains. New Microbiol. 2007;30:332–339. [PubMed] [Google Scholar]
- 3.Shaikh S, Fatima J, Shakil S, Mohd S, Rizvi D, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby GA, Munoz-Price LS. Mechanisms of disease: the new β-lactamases. N Engl J Med. 2005;352:380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 5.Araj GF, Samaha-Kfoury JN. Recent developments in β-Lactamases and extended-spectrum β-lactamases. BMJ. 2003;327:1209–1213. doi: 10.1136/bmj.327.7425.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya B, Beceiro A, Cabot G, Juan C, Zamorano L, Alberti S, Oliver A. Pan-beta-lactam resistance development in Pseudomonasaeruginosa clinical srains: molecular mechanisms, penicillin-binding protein profiles and binding affinities. Antimicrob Agents Chemother. 2012;56(9):4771–4778. doi: 10.1128/AAC.00680-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliot E, Brink AJ, van Greune J, Els Z, Woodford N, Turton J, Warner M, Livermore DM. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiellapeneumoniae with an extended-spectrum beta-lactamase. Clin Infect Dis. 2006;42(11):e95–e98. doi: 10.1086/503264. [DOI] [PubMed] [Google Scholar]
- 8.Bayer AS, Peters J, Parr TR, Chan L, Hancock RE. Role of beta-lactamase in in vivo development of ceftazidime resistance in experimental Pseudomonasaeruginosa endocarditis. Antimicrob Agents Cemother. 1987;31(2):253–258. doi: 10.1128/AAC.31.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K. New β-Lactamases in Gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis. 2001;32:1085–1089. doi: 10.1086/319610. [DOI] [PubMed] [Google Scholar]
- 10.Bradford P, Cherubin CE, Idemyor V. Multiply resistant Klebsiellapneumoniae from two Chicago hospitals: identification of the extended spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob Agents Chemother. 1994;38:761–766. doi: 10.1128/AAC.38.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obeng-Nkrumah N, Twum-Danso K, Krogfelt KA, Newman MJ. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg. 2013;89(5):960–964. doi: 10.4269/ajtmh.12-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fegloa P, Adu-Sarkodie Y, Jain R, Spurbeck RR, Springman AC, Englebergb NC, Newton DW, Xi C, Walk ST. Emergence of a Novel Extended-Spectrum-β-Lactamase (ESBL)-producing, fluoroquinolone-resistant clone of extraintestinal pathogenic Escherichia coli in Kumasi, Ghana. J Clin Microbiol. 2014;51(2):728–730. doi: 10.1128/JCM.03006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maningi NE, Ehlers MM, Hoosen AA, Makgothlo E, Omar S, Kock MM. Prevalence of ESBL and MBL antibiotic resistance genes in Klebsiellapneumoniae in pretoria academic hospital. Health sciences faculty day posters. 2008. http://hdl.handle.net/2263/8293. Accessed on 10 Nov 2015.
- 14.Iroha RI, Oji AE, Afiukwa TN, Nwuzo AC, Ejikeugwu PC. Extended spectrum β-lactamase (ESBL) mediated resistance to antibiotics among Klebsiellapneumoniae in Enugu metropolis. Macedonian J. Med. Sci. 2009;2(3):196–199. [Google Scholar]
- 15.Blomberg B, Jureen R, Manji KP, Tamim BS, Mwakagile DSM, Urassa WK, Fataki M, Msangi V, Tellevik MG, Maselle SY, Langeland N. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum β-lactamases in Dar es Salaam. Tanzania J Clin Microbiol. 2005;43:745–749. doi: 10.1128/JCM.43.2.745-749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kariuki S, Corkill JE, Revathi G, Musoke R, Hart CA. Molecular characterization of a novel plasmid-encoded cefotaximase (CTXM-12) found in clinical Klebsiellapneumoniae isolates from Kenya. Antimicrob Agents Chemother. 2001;45:2141–2143. doi: 10.1128/AAC.45.7.2141-2143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan ST, Steel KJ. Characteristics of gram negative bacteria. In: Barrow GI, Feltham RKA, editors. Cowan’s and Steel’s manual for identification of medical bacteria. London: Cambridge University Press; 1993. pp. 94–150. [Google Scholar]
- 18.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acid Res. 1979;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 21.Ruiz-Barba JL, Maldonado A, Jimenez-Diaz R. Small-scale total DNA extraction from bacteria and yeast for PCR applications. J Anal Biochem. 2005;347:333–335. doi: 10.1016/j.ab.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Bonomo RA, Paterson DL, Jacobs MR, Ewell AJ, Fishbain JT, Craft DW, Rather PN, Thomson JD, Sampath R, Eshoo MW, Massire C, Ecker DJ, Donskey CJ, Adams JM, Bajaksouzain S, Hulten EA, Hujer AM, Hujer KM. Multi-drug resistant Acinetobacter spp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center: analysis of antibiotic resistance genes. Antimicrob Agents Chemother. 2006;50(12):4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson ND, Hossain A, Pitout JDD. Phenotypic and molecular detection of CTX-M-β-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004;42(12):5715–5721. doi: 10.1128/JCM.42.12.5715-5721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonomo RA, Paterson DL, Yeiser B, Hujer KM, Hujer AM, Bonomo MD, International Klebsiella Study Group Extended-spectrum β-lactamases in Klebsiella pneumonia bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob Agents Chemother. 2003;47(11):3554–3560. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tansarli GS, Poulikakos P, Kapaskelis A, Falagas ME. Proportion of extended-spectrum β-lactamase (ESBL)-producing isolates among Enterobacteriaceae in Africa: evaluation of the evidence-systematic review. J Antimicrob Chemother. 2014;69:1177–1184. doi: 10.1093/jac/dkt500. [DOI] [PubMed] [Google Scholar]
- 26.Hoban DJ, Lascols C, Nicolle LE, Badal R, Bouchillon S, Hackel M, Hawser S. Antimicrobial susceptibility of enterobacteriaceae, including molecular characterization of extended-spectrum beta-lactamase-producing species, in urinary tract isolates from hospitalized patients in North America and Europe: results from the SMART study. Diagn Microbiol Infect Dis. 2012;74:62–67. doi: 10.1016/j.diagmicrobio.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M, Pathak S, Srivastava P. Prevalence and antibiogram of extended spectrum β-lactamase (ESBL) producing Gram negative bacilli and further molecular characterization of ESBL producing Escherichiacoli and Klebsiella spp. J Clin Diagn Res. 2013;7:2173–2177. doi: 10.7860/JCDR/2013/6460.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantón R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, Coque TM. Prevalence and spread of extended-spectrum beta-lactamase-producing enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;4(Suppl 1):144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 29.Hackman HK, Brown C, Twum-Danso K. Antibiotic Resistance Profile of Non-Extended Spectrum Beta-Lactamase-producing Escherichia coli and Klebsiella pneumoniae in Accra, Ghana. J Biol Agric Healthc. 2014;4:12–16. [Google Scholar]
- 30.Aibinu IE, Ogunsola FT, Ohaegbulam VC, Adenipekun EA, Odugbemi TO, Mee BJ. Extended-spectrum β-lactamase enzymes in clinical isolates of Enterobacter species from Lagos, Nigeria. J Clin Microbiol. 2003;41(5):2197–2198. doi: 10.1128/JCM.41.5.2197-2200.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacoby GA, Walsh KE, Walker VJ. Identification of extended-spectrum, ampC and carbapenem-hydrolyzing β-Lactamases in Escherichiacoli and Klebsiellapneumoniae by Disk Tests. J Clin Microbiol. 2006;44(6):1971–1976. doi: 10.1128/JCM.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livermore DM. Detection of ESBLs and AmpC. The british society for antimicrobial chemotherapy. 2008. www.bsac.org.uk/wp-content/uploads/…/Detecting_AmpC__ESBL_pruned.ppt. Accessed 28 June 2014.
- 33.Livermore DM, Hall LMC, Yuan M, Gheorghui R. Bases of variation in resistance to β-lactams in Klebsiellaoxytoca isolates hyperproducing K1 β-lactamase. J Antimicrob Chemother. 1997;40:533–541. doi: 10.1093/jac/40.3.335. [DOI] [PubMed] [Google Scholar]
- 34.Livermore DM. Determinants of the activity of β-lactamase inhibitor combinations. J Antimicrob Chemother. 1993;31(Suppl A):9–21. doi: 10.1093/jac/31.suppl_A.9. [DOI] [PubMed] [Google Scholar]
- 35.Robin F, Delmas J, Schweitzer C, Tournilhac O, Lesens O, Chanal C, Bonnet R. Evolution of TEM-type enzymes: biochemical and genetic characterization of two new complex mutant TEM enzymes, TEM-151 and TEM-152, from a single patient. Antimicrob Agents Chemother. 2007;51(4):1304–1309. doi: 10.1128/AAC.01058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangoue-Pieboji J, Bedenic B, Koulla-Shiro S, Randegger C, Adiogo D, Ngassam P, Ndumbe P, Hachler H. Extended-spectrum beta-lcatamase-producing Enterobacteriaceae in Yaounde. J Clin Microbiol. 2005;43(7):3273–3277. doi: 10.1128/JCM.43.7.3273-3277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao SPN, Rama PS, Gurushanthappa V, Manipura R, Srinivasan K. Extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoiae: a multi-centric study across Karnataka. J Lab Physicians. 2014;6(1):7–13. doi: 10.4103/0974-2727.129083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AitMhanda R, Soukrib A, Moustaouia N, Amarouchb H, Elmdaghria N, Sirotc D, Benbachira M. Plasmid-mediated TEM-3 extended-spectrum beta-lactamase production in Salmonella typhimurium in Casablanca. J Antimicrob Chemother. 2002;49(1):169–172. doi: 10.1093/jac/49.1.169. [DOI] [PubMed] [Google Scholar]
- 39.Agyekum A, Fajardo-Lubiána A, Ansong D, Partridge SR, Agbenyega T, Iredell JR. blaCTX-M-15 carried by IncF-type plasmids is the dominant ESBL gene in Escherichiacoli and Klebsiellapneumoniae at a hospital in Ghana. Diagn Microbiol Infect Dis. 2015 doi: 10.1016/j.diagmicrobio.2015.12.010. [DOI] [PubMed] [Google Scholar]


