Abstract
The recent discovery of hepatitis C virus (HCV)-related viruses in different animal species has raised new speculations regarding the origin of HCV and the possibility of a zoonotic source responsible for the endemic HCV transmission. As a consequence, these new findings prompt questions regarding the potential for cross-species transmissions of hepaciviruses. The closest relatives to HCV discovered to date are the non-primate hepaciviruses (NPHVs), which have been described to infect horses. To evaluate the risk of a potential zoonotic transmission, we analysed NPHV RNA and antibodies in humans with occupational exposure to horses in comparison with a low-risk group. Both groups were negative for NPHV RNA, even though low seroreactivities against various NPHV antigens could be detected irrespective of the group. In conclusion, we did not observe evidence of NPHV transmission between horses and humans.
Approximately 3 % of the world's population are chronically infected with hepatitis C virus (HCV) resulting in a high risk for liver diseases like fibrosis, cirrhosis and hepatocellular carcinoma (Alter, 2007). Since its discovery in the 1980s as a blood-transmitted non-A, non-B hepatitis, and identification as HCV in 1989, much progress has been made regarding cell culture models and antiviral treatment options (Pawlotsky, 2013; Steinmann & Pietschmann, 2013). Numerous RNA viruses spread efficiently between animals and humans by transmission across species barriers. Consequently, they are responsible for viral zoonotic infections, i.e. infections of animal hosts that are transmitted to humans by unintentional contact. Such cross-species transmissions of RNA viruses are accountable for some of the most dangerous infectious diseases, which threaten the health of many million humans. The origin of HCV remains unclear, and one could imagine a scenario in which HCV jumped from animals to humans, as seen for other viruses like human immunodeficiency virus, ebolavirus and coronaviruses (O'Shea et al., 2014; Scheel et al., 2015; Sharp et al., 2013).
Recently several new viruses belonging to the hepacivirus family have been discovered in different mammalian host species including dogs (Kapoor et al., 2011), horses (Burbelo et al., 2012), rodents (Drexler et al., 2013; Kapoor et al., 2013), bats (Drexler et al., 2013; Quan et al., 2013), non-human primates (Lauck et al., 2013) and rats (Firth et al., 2014). Among these viruses, the non-primate hepaciviruses (NPHVs), discovered first in dogs and subsequently in horses, exhibit the highest genetic similarity to HCV. Moreover, with regard to viral tropism and the course of infection, this virus also resembles HCV infection of humans (Pfaender et al., 2014, 2015). At the genomic level, HCV and NPHV share approximately 50 % nucleotide sequence divergence with a maximum amino acid identity in the non-structural proteins NS3 and NS5B (>55–65 %), whereas the glycoproteins show the lowest amino acid identity ( < 35–45 %) (Kapoor et al., 2011). This similarity is noteworthy, as HCV genotypes already differ from each other by 31–33 % at the nucleotide level, compared with 20–25 % between the individual subtypes (Simmonds et al., 2005). However, despite the sequence diversity of HCV as well as NPHV variants, the viruses share an identical architecture of collinear genes of similar or identical size in the large ORF (Kapoor et al., 2011; Pfaender et al., 2014). Given the close relationship between HCV and NPHV, one could imagine a possible risk of cross-species transmission from horses to humans. Such a scenario would be supported by the observation that the canine hepacivirus (CHV)/NPHV protease has been shown to cleave the mitochondrial antiviral-signalling protein and the TIR (Toll/IL-1 receptor) domain-containing adaptor protein (TRIF), and therefore, like HCV, successfully circumvents the immune system of the host even across species barriers (Li et al., 2005; Parera et al., 2012). We showed that the seroprevalence of NPHV in horses in northern Germany amounts to 30 % with 2–3 % also carrying viral RNA (Pfaender et al., 2015). In this study, we investigated the risk of a cross-species transmission from horses to humans by focusing on a high-risk group of humans that had occupational contact with horses in northern Germany, and compared this study group with humans who declared no contact with horses. To analyse the risk of a potential cross-species transmission of NPHV between horses and humans, human sera were collected and analysed for the presence of NPHV RNA and anti-NPHV antibodies using a luciferase immunoprecipitation system (LIPS) assay based on the detection of CHV/NPHV NS3 antibodies (Burbelo et al., 2012). Since in the absence of active viral replication no antibodies against the non-structural proteins will be generated, several variants of the more variable and exposed NPHV envelope proteins E1 and E2 (E1E2_H10, GenBank accession no. KP739811; E1E2_H12, GenBank accession no. KP739812; E1E2_H13, GenBank accession no. KP739813; and E1E2_H14, GenBank accession no. KP739814) were chosen for the serological assay and cloned into the pREN2 vector (Fig. 1a). As shown in the scheme, antigens were expressed upon transfection of Cos1 cells with the respective plasmids, harvested and co-incubated with 1 : 10-diluted sera. Antigen–antibody complexes were immunoprecipitated and the luciferase activity determined (Fig. 1b). To determine the sensitivity and specificity of the assay, a cut-off limit was calculated as the mean value of the samples containing only buffer A, the respective antigen and A/G beads (Pierce Biotechnology) plus 3 sd. Sequences of NPHV glycoproteins were aligned to equine hepaciviral E1E2 glycoproteins from GenBank, using clustal w implemented in mega6 (Tamura et al., 2013), involving 18 nucleotide sequences. Sequence analyses showed that the chosen E1E2 variants were variable and distinct from each other by an average of 12.5 % at the nucleotide level and 3.8 % at the amino acid level (Fig. 1c). Next, a total of 172 human serum samples were collected from volunteers with occupational horse contact after informed consent was obtained according to procedures approved by the ethical committee of the Hannover Medical School. For further characterization, all volunteers answered a questionnaire about their occupational and private contact with horses and their exposure to horse blood. The participants of this survey were employed at the respective horse clinic for a mean of 8 years (Fig. 2a) and had a mean contact with horses of 25 years (Fig. 2b). About 80 % of the volunteers also had regular private contact with horses (Fig. 2c) and 68 % declared to have a history of occupational injury with blood–blood contact involving horses (Fig. 2d). As a control cohort 159 volunteers were identified with no declared contact with horses, provided by the German Red Cross Blood Service NSTOB. All donors gave their written informed consent that their blood could be used for scientific purposes. To evaluate the serological assay for the detection of anti-NPHV antibodies, the different glycoprotein constructs were first tested against horse sera collected from the Clinic for Horses, University of Veterinary Medicine, Hannover, Foundation. The tested horse sera displayed a variable seroreactivity against the different constructs which may be attributed to the sequence variability of the glycoproteins (Fig. 3a). Negative-control sera did not react with the cloned glycoprotein variants (n = 3–8, Fig. 3a and data not shown). All human sera were tested for reactivity against different NPHV antigens using the LIPS assay. Interestingly, a low seroreactivity against different NPHV constructs could be detected in both study groups, irrespective of horse contact (Fig. 3b). Statistical analysis was carried out with the open source statistics software r (http://www.R-project.org/) and the library outliers. In order to account for assay-specific variations, the measurements were median normalised, i.e. the median of the values measured in the corresponding assay was subtracted from all values in this assay. A comparison of the distributions of the measurements for the group with horse contact to the control group was performed based on boxplots (see Fig. 3b) and on the Kolmogorov–Smirnov test and resulted in no statistically significant differences for the five measured NPHV antigens. Fisher's exact test was used to check whether there was a statistically significant difference in the number of persons with discordant values (discordant values were defined either as outliers based on Grubbs' test or as values more than 3 sd above the median, where a robust estimator for the sd based on the interquartile range was used). However, for both ways of counting discordant values, Fisher's exact test did not yield a statistically significant difference between the group with horse contact and the control group after Bonferroni–Holm correction for multiple testing. RNA was extracted from all human sera using a High Pure Viral RNA kit (Roche) according to the manufacturer's instructions. Extracted RNA was tested for the presence of NPHV RNA by quantitative real-time (RT)-PCR as described before (Pfaender et al., 2015). All human sera tested negative for the presence of NPHV RNA (data not shown). As the serological assay has been shown to cross-react with antibodies against HCV (Burbelo et al., 2012), all antibody-positive sera were additionally tested for the presence of HCV-specific antibodies; however, none were detected. In conclusion, as antibody-positive sera could be detected in both groups, our data suggest that there is no evidence of NPHV cross-species transmission in people with occupational horse contact compared with people with no horse contact. In this study, a high-risk group of humans with occupational exposure to horses was analysed for serological markers to analyse the potential for a cross-species transmission of the HCV-related non-primate hepacivirus between horses and humans. Since HCV is a blood-borne virus (Alter, 2007) it is possible that NPHV infection among horses occurs along the same route. As seen for another recently discovered horse virus, Theiler's disease-associated virus, which could be transmitted via inoculation of virus-containing serum products (Chandriani et al., 2013), a similar route of infection is likely for NPHV. Indeed, a recent study showed that NPHV could be transmitted experimentally between horses upon inoculation of NPHV-positive serum (Ramsay et al., 2015). It is likely that very close contact, if not a blood–blood contact, between an infected horse and a foreign species host is a prerequisite for NPHV to cross the species barrier. Therefore, serum samples of a high-risk group of people with occupational contact with horses and occasional blood-to-blood contact, e.g. by accidental needle-stick injuries or exposure due to lesions within the skin, were analysed for the presence of different anti-NPHV antibodies and compared with a low-risk group of people who were stated to have no contact with horses. Acutely HCV-infected individuals produce antibodies against epitopes within the structural as well as non-structural proteins (Heim & Thimme, 2014). However, in the case of accidental contact with NPHV virus, no antibodies against non-structural proteins can be generated as this protein is only expressed upon active viral RNA replication. Consequently, besides the conserved NS3 region several sequences from the envelope glycoprotein genes were chosen for the serological assay, since these regions are exposed at the surface of the virus particles and more variable. Sequence analyses of different NPHV glycoproteins showed a high variability between different isolates which is reflected in the different ability of anti-NPHV antibodies to bind to these antigens. We found that human samples were not positive for NPHV RNA using a real-time PCR assay with NPHV-specific primers and probe. This is in line with two previous studies that analysed NPHV RNA in randomly chosen humans (Levi et al., 2014; Lyons et al., 2014). Surprisingly some blood donors, irrespective of the high-risk or low-risk group, displayed weak reactivity against specific NPHV antigens. As NPHV antigens in the LIPS assay exhibit a cross-reactivity with anti-HCV antibodies (Burbelo et al., 2012), all positive samples from our study were tested for HCV seropositivity with no donor being found to be reactive. Since these antibodies were detected in both groups, it is unlikely that an NPHV infection was the cause for the positive test results. One could speculate that a previous infection with another not-yet-discovered hepacivirus occurred in these donors giving rise to these cross-binding antibodies.
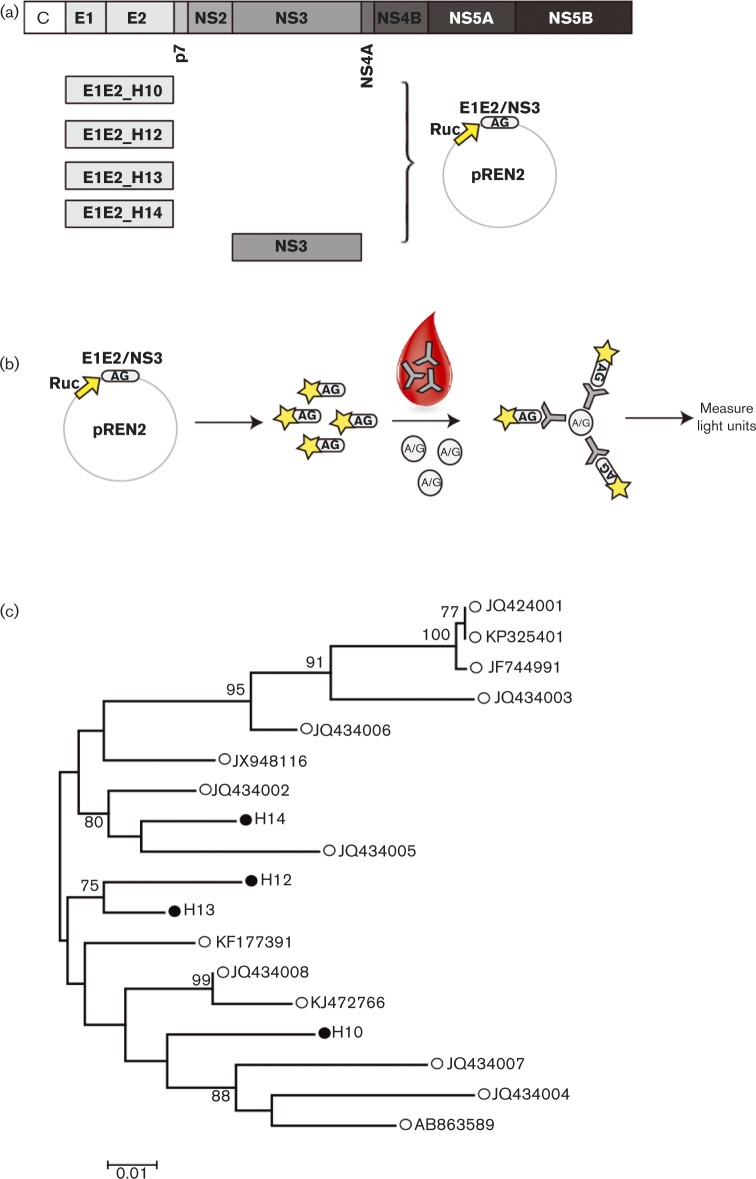
Fig. 1.
Experimental set-up of the luciferase immunoprecipitation (LIPS) assay. (a) Schematic representation of the NPHV genome. Four distinct NPHV E1E2 sequences and one CHV/NPHV NS3 sequence were cloned into the pREN2 plasmid generating an N-terminal fusion with Renilla luciferase (Ruc). (b) Cos1 cells were transfected with the constructs and the antigens (AG) expressed and purified. Purified antigens were incubated with sera, immunoprecipitated using A/G beads and the luciferase (Rluc) activity was determined. (c) Phylogenetic comparison of published NPHV E1E2 sequences (○) and four new NPHV E1E2 isolates (•). Molecular phylogenetic analysis was performed by using the maximum-likelihood method. Default parameters were used with a Hasegawa–Kishino–Yano substitution model and a gamma distribution of six discrete rate steps. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. There were a total of 526 codon positions included in the final dataset, and gaps were partially deleted with a 90 % cut-off. Initial trees for the heuristic search were obtained by applying the neighbour-joining method to a matrix of pairwise distances estimated using the maximum composite likelihood approach. Bootstrap values above 70 % are indicated next to the branches. Newly identified sequences were employed in the LIPS assay. Bar, nucleotide substitutions per site.
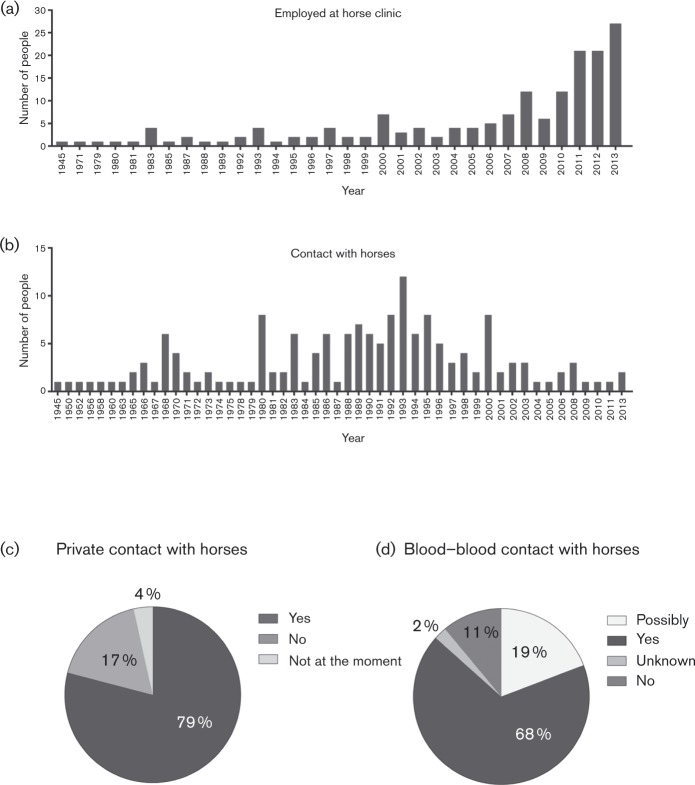
Fig. 2.
Questionnaire of people with occupational horse contact. A total of 172 volunteers with occupational horse contact answered a questionnaire regarding how long they had been employed at the horse clinics (a), how long they had contact with horses (b), whether they had private contact with horses (c) and if they ever had blood–blood contact with horses (d).
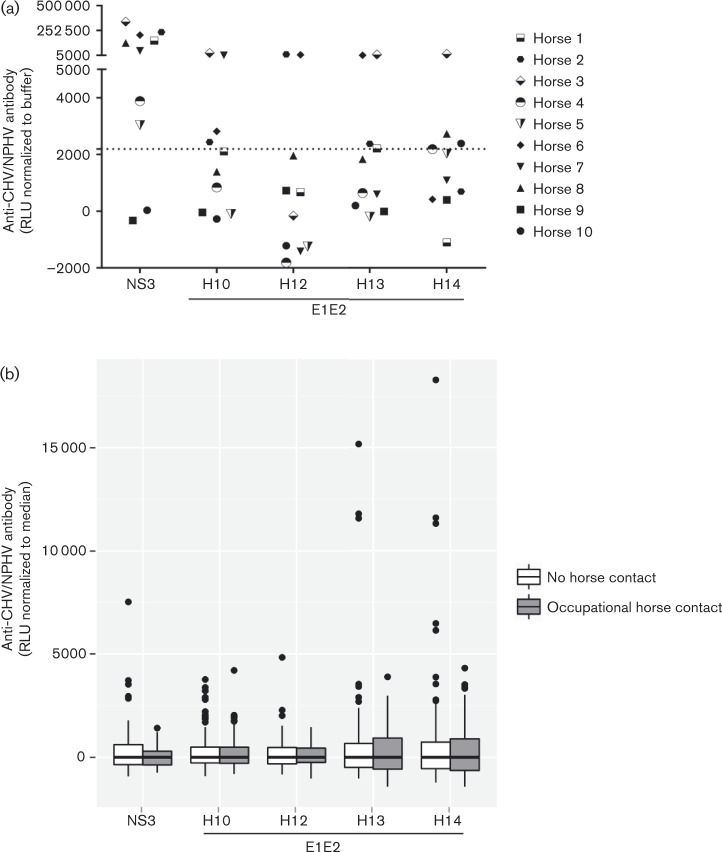
Fig. 3.
Evaluation of LIPS assay and analysis of human sera for the presence of anti-NPHV antibodies. (a) Ten horse sera were tested in triplicate for the presence of anti-NS3 or different anti-E1E2 antibodies using the LIPS assay. Rluc activities were measured and are depicted as relative light units (RLU) after subtraction of the buffer control (buffer A, the respective antigen and A/G beads). Depicted are the mean values of each individual horse. A cut-off limit was calculated as the mean value of the buffer control plus 3 sd. (b) Human sera of people with occupational horse contact (n = 172) or people with no contact with horses (n = 159) were screened for binding to NPHV NS3 or different NPHV E1E2 antigens (H10–H14) using the LIPS assay. Samples were screened in triplicate and luciferase activities were measured and are depicted as boxplots after normalisation (subtraction of the median) to account for assay-specific variations. The Kolmogorov–Smirnov test could not detect any significant differences between the horse contact and the control group. Fisher's exact test did not indicate a significant difference in the number of discordant values in the two groups where discordant values were either defined as differing by more than 3 sd from the median or based on Grubbs' test.
In conclusion, no potential markers suggesting a cross-species transmission of NPHV could be observed in a high-risk group with occupational exposure, indicating that a species jump from horses to humans is not likely to occur, even though both viruses share similar features.
Acknowledgements
We are grateful to all volunteers who contributed the serum samples, Annett Kessler and the German Red Cross blood service NSTOB for technical support in acquiring serum samples and all members of the Institute of Experimental Virology, Twincore, for helpful suggestions and discussions. S. P. was supported by a stipend from the international research training group 1273 (IRTG 1273) provided by the DFG. S. W. was supported by a stipend from the Helmholtz Centre for Infection Research and supported by Hannover Biomedical Research School (HBRS) and the Centre for Infection Biology (ZIB). E. S. was supported by the DFG (STE 1954/1-1) and intramural young investigator award of the Helmholtz Centre for Infection Research. Support for P. D. B. came from the Division of Intramural Research, NIH. T. P. was supported by a grant from the Helmholtz Association (SO-024) and by a grant from the European Research Council (ERC-2011-StG_281473-VIRAFRONT).
References
- Alter M.J. (2007). Epidemiology of hepatitis C virus infection World J Gastroenterol 13 2436–2441 10.3748/wjg.v13.i17.2436 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P.D., Dubovi E.J., Simmonds P., Medina J.L., Henriquez J.A., Mishra N., Wagner J., Tokarz R., Cullen J.M., other authors (2012). Serology-enabled discovery of genetically diverse hepaciviruses in a new host J Virol 86 6171–6178 10.1128/JVI.00250-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandriani S., Skewes-Cox P., Zhong W., Ganem D.E., Divers T.J., Van Blaricum A.J., Tennant B.C., Kistler A.L. (2013). Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis Proc Natl Acad Sci U S A 110 E1407–E1415 10.1073/pnas.1219217110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Müller M.A., Lukashev A.N., Gmyl A., Coutard B., Adam A., Ritz D., Leijten L.M., other authors (2013). Evidence for novel hepaciviruses in rodents PLoS Pathog 9 e1003438 10.1371/journal.ppat.1003438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth C., Bhat M., Firth M.A., Williams S.H., Frye M.J., Simmonds P., Conte J.M., Ng J., Garcia J., other authors (2014). Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City MBio 5 e01933–14 10.1128/mBio.01933-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M.H., Thimme R. (2014). Innate and adaptive immune responses in HCV infections J Hepatol 61 S14–S25 10.1016/j.jhep.2014.06.035 . [DOI] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Gerold G., Qaisar N., Jain K., Henriquez J.A., Firth C., Hirschberg D.L., Rice C.M., other authors (2011). Characterization of a canine homolog of hepatitis C virus Proc Natl Acad Sci U S A 108 11608–11613 10.1073/pnas.1101794108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Scheel T.K., Hjelle B., Cullen J.M., Burbelo P.D., Chauhan L.V., Duraisamy R., Sanchez Leon M., other authors (2013). Identification of rodent homologs of hepatitis C virus and pegiviruses MBio 4 e00216-13 10.1128/mBio.00216-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M., Sibley S.D., Lara J., Purdy M.A., Khudyakov Y., Hyeroba D., Tumukunde A., Weny G., Switzer W.M., other authors (2013). A novel hepacivirus with an unusually long and intrinsically disordered NS5A protein in a wild Old World primate J Virol 87 8971–8981 10.1128/JVI.00888-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi J.E., Cabral S.P., Nishiya A., Ferreira S., Romano C.M., Polite M.B., Pereira R.A., Mota M.A., Kutner J.M. (2014). Absence of nonprimate hepacivirus-related genomes in blood donors seroreactive for hepatitis C virus displaying indeterminate blot patterns J Viral Hepat 21 e164–e166 10.1111/jvh.12252 . [DOI] [PubMed] [Google Scholar]
- Li X.D., Sun L., Seth R.B., Pineda G., Chen Z.J. (2005). Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity Proc Natl Acad Sci U S A 102 17717–17722 10.1073/pnas.0508531102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S., Kapoor A., Schneider B.S., Wolfe N.D., Culshaw G., Corcoran B., Durham A.E., Burden F., McGorum B.C., Simmonds P. (2014). Viraemic frequencies and seroprevalence of non-primate hepacivirus and equine pegiviruses in horses and other mammalian species J Gen Virol 95 1701–1711 10.1099/vir.0.065094-0 . [DOI] [PubMed] [Google Scholar]
- O'Shea T.J., Cryan P.M., Cunningham A.A., Fooks A.R., Hayman D.T., Luis A.D., Peel A.J., Plowright R.K., Wood J.L. (2014). Bat flight and zoonotic viruses Emerg Infect Dis 20 741–745 10.3201/eid2005.130539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parera M., Martrus G., Franco S., Clotet B., Martinez M.A. (2012). Canine hepacivirus NS3 serine protease can cleave the human adaptor proteins MAVS and TRIF PLoS One 7 e42481 10.1371/journal.pone.0042481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlotsky J.M. (2013). Treatment of chronic hepatitis C: current and future Curr Top Microbiol Immunol 369 321–342 . [DOI] [PubMed] [Google Scholar]
- Pfaender S., Brown R.J.P., Pietschmann T., Steinmann E. (2014). Natural reservoirs for homologs of hepatitis C virus Emerg Microbes Infect 3 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender S., Cavalleri J.M., Walter S., Doerrbecker J., Campana B., Brown R.J., Burbelo P.D., Postel A., Hahn K., other authors (2015). Clinical course of infection and viral tissue tropism of hepatitis C virus-like non-primate hepaciviruses in horses Hepatology 61 447–459 . [DOI] [PubMed] [Google Scholar]
- Quan P.L., Firth C., Conte J.M., Williams S.H., Zambrana-Torrelio C.M., Anthony S.J., Ellison J.A., Gilbert A.T., Kuzmin I.V., other authors (2013). Bats are a major natural reservoir for hepaciviruses and pegiviruses Proc Natl Acad Sci U S A 110 8194–8199 10.1073/pnas.1303037110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay J.D., Evanoff R., Wilkinson T.E., Jr, Divers T.J., Knowles D.P., Mealey R.H. (2015). Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus Hepatology 61 1533–1546 10.1002/hep.27689 . [DOI] [PubMed] [Google Scholar]
- Scheel T.K., Simmonds P., Kapoor A. (2015). Surveying the global virome: identification and characterization of HCV-related animal hepaciviruses Antiviral Res 115 83–93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P.M., Rayner J.C., Hahn B.H. (2013). Evolution. Great apes and zoonoses Science 340 284–286 10.1126/science.1236958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Bukh J., Combet C., Deléage G., Enomoto N., Feinstone S., Halfon P., Inchauspé G., Kuiken C., other authors (2005). Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes Hepatology 42 962–973 10.1002/hep.20819 . [DOI] [PubMed] [Google Scholar]
- Steinmann E., Pietschmann T. (2013). Cell culture systems for hepatitis C virus Curr Top Microbiol Immunol 369 17–48 . [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0 Mol Biol Evol 30 2725–2729 10.1093/molbev/mst197 . [DOI] [PMC free article] [PubMed] [Google Scholar]