Abstract
Background. Neisseria meningitidis is a frequent colonizer of the human nasopharynx, with asymptomatic carriage providing the reservoir for invasive, disease-causing strains. Serogroup Y (MenY) strains are a major cause of meningococcal disease. High-resolution genetic analyses of carriage and disease isolates can establish epidemiological relationships and identify potential virulence factors.
Methods. Whole-genome sequence data were obtained for 99 MenY carriage isolates recovered in the United Kingdom during 1997–2010. Sequences were compared to those of 73 MenY invasive isolates recovered during 2010–2011, using a gene-by-gene approach.
Results. Comparisons across 1605 core genes resolved 91% of isolates into one of 8 clusters containing closely related disease and carriage isolates. Six clusters contained carried meningococci isolated during 1997–2001, suggesting temporal stability. One cluster of isolates, predominately sharing the designation Y: P1.5-1,10-1: F4-1: ST-1655 (cc23), was resolved into one subcluster with 86% carriage isolates and a second with 90% invasive isolates. These subclusters were defined by specific allelic differences in 5 core genes encoding glycerate kinase (glxK), valine-pyruvate transaminase (avtA), superoxide dismutase (sodB), and 2 hypothetical proteins.
Conclusions. High-resolution genetic analyses detected long-term temporal stability and temporally overlapping carriage and disease populations for MenY clones but also evidence of a disease-associated clone.
Keywords: Neisseria meningitidis, whole-genome sequencing, carriage, serogroup Y, epidemiology
Neisseria meningitidis is carried in the nasopharynx of 10%–30% of the population, with carriage rates influenced by setting and generally higher in young adults and close-contact populations [1, 2]. Occasionally, meningococci invade, resulting in meningococcemia and meningitis. Invasive meningococcal disease (IMD) results in substantial mortality and morbidity, despite effective antibiotic treatment [3].
A key virulence factor is the polysaccharide capsule, which allows the bacterium to resist complement-mediated lysis and opsonophagocytosis [4]. Twelve serogroups are recognized [5], but only 6—A, B, C, W, X, and Y—are responsible for the majority of disease worldwide [6]. DNA sequence–based approaches have been extensively applied to the analysis of the population structure of meningococci [7]. Multilocus sequence typing (MLST), using sequences of 7 representative housekeeping genes, has demonstrated a highly structured population, with most strains belonging to groups of closely related genotypes referred to as clonal complexes (CCs) [8]. Some of these clonal complexes correspond to hyperinvasive lineages, which are responsible for most cases of disease worldwide [9, 10]. These clonal complexes are often associated with specific combinations of antigenic proteins, such as porin A (PorA) and ferric enterobactin transport protein A (FetA), and serogroups [11, 12].
Much of the IMD in Europe and North America is caused by a limited range of serogroup/genotype combinations, such as N. meningitidis serogroup B (MenB) ST-41/44, ST-32, and ST-269 isolates and N. meningitidis serogroup C (MenC) isolates from ST-11 and ST-8 complexes [6, 13]; however, in recent decades, the incidence of IMD due to N. meningitidis serogroup Y (MenY) organisms, often belonging to CC23, has increased in several countries, including the United States, Sweden, and the United Kingdom [14–18]. In the United Kingdom, several carriage studies performed between 2008 and 2012 detected evidence of recent alterations in MenY carriage epidemiology in young adults [19–22]. For example, MenY meningococci were found in only 1%–2% of participants and constituted only approximately 10% of recovered isolates when carriage was assessed during 1997–1998 in first-year university students at the University of Nottingham, United Kingdom, and during 1999–2001 in >48 000 students aged 15–17 years throughout the United Kingdom [23, 24]. In contrast, during 2008–2009 and 2009–2010, significantly higher rates of overall carriage, principally resulting from the high prevalence of MenY strains, were detected in university students in Nottingham [19, 20]. These observations were supported by subsequent multisite studies undertaken to investigate carriage in United Kingdom school and university students [21, 22]. Identification of isolates in the 2008–2009 and 2009–2010 Nottingham carriage studies relied on polymerase chain reaction (PCR) amplification of capsule genes, and although some further typing information was generated for a subset of the 2008–2009 isolates [19], only limited information was available on the numbers and genetic background of the different MenY-associated CCs carried in 2009–2010.
High-resolution analyses of genome-wide genetic relationships can determine the prevalence of disease-causing isolates among collections of carriage isolates and can detect specific disease-associated loci. The PubMLST.org/neisseria database, which uses the Bacterial Isolate Genome Sequence database (BIGSdb) platform, is a scalable, open-source web-accessible database, to identify, index, and extract genetic variation data from whole-genome sequence (WGS) data [25]. This approach was used to resolve an outbreak of ST-11 disease [26], to investigate the evolution and global spread of the ET-5/ST-32 lineage [27], and to describe MenY disease isolates in Sweden [28]. Additionally, a genealogical analysis of 108 representative meningococcal genomes led to the proposal of a new lineage nomenclature reflecting the increased resolution of WGS typing, compared to MLST [29].
Here we investigated the population structure of MenY invasive and carriage isolates in the United Kingdom, using WGS data generated from 99 carriage isolates obtained from school or university students (typically 16–20 years old) between 1997 and 2010 and compared these genomic data with 73 publically available genomes from invasive MenY strains isolated in 2010–2011.
METHODS
Isolates
A total of 99 MenY isolates, all obtained from nasopharyngeal carriers in Nottingham (East Midlands), United Kingdom, were included in the WGS analysis (Supplementary Table 1). Of these, 77 were isolated from students attending the University of Nottingham in 2009 [20] and were chosen as follows: (1) 20 were obtained in September 2009 from first-year students, (2) 18 were obtained in September 2009 from second-year students, (3) 19 were obtained in December 2009 from first-year students, (4) and 20 were obtained in December 2009 from second-year students [20]. To provide context, 10 isolates were chosen randomly from a collection of MenY isolates from sixth-form school students in Nottingham in 1999–2001 [24], and 6 isolates were chosen from MenY carried isolates obtained from first-year students at the University of Nottingham during 1997–1998 [23]. All of these isolates were chosen as known MenY organisms, based on PCR or serological typing methods, without prior knowledge of their clonal complex. Six additional MenY carriage isolates were chosen as representative examples of the predominant MenY lineages circulating in a 2008–2009 cohort of first-year students at the University of Nottingham [19].
Genomic DNA Extraction, Illumina Sequencing, Assembly and Deposition
Meningococci were grown overnight on heated horse-blood (chocolate) agar (Oxoid) at 37°C in an atmosphere of air plus 5% CO2, and genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega). Genomic DNA was sequenced as described previously [29]. Short-read sequences were assembled using the VelvetOptimiser de novo short-read assembly program optimization script after which resultant contiguous sequences (contigs) were uploaded to the PubMLST.org/neisseria database. Sequence reads were deposited in the European Nucleotide Archive (Supplementary Table 1). Genome sequences of the 73 MenY disease isolates for the epidemiological year 2010–2011 in England, Wales, and Northern Ireland (Supplementary Table 2) were accessed via the Meningitis Research Foundation Meningococcus Genome Library database (http://pubmlst.org/perl/bigsdb/bigsdb.pl?db=pubmlst_neisseria_mrfgenomes; accessed September 2015).
Genomic Analyses
The genome assemblies deposited in the database are automatically curated and annotated for all loci currently defined in the database, thus identifying alleles with ≥98% sequence identity. Over 2600 loci were defined at the time of analysis. These have a “NEIS” prefix and are organized into schemes, which enables, for example, the rapid identification of isolate genogroup, clonal complex, and PorA and FetA antigen types. Further analysis was undertaken by means of the BIGSdb Genome Comparator tool implemented within the database, using the N. meningitidis cgMLST v1.0 core genome scheme (1605 loci) [29]. Output distance matrices (Nexus format) were used to generate NeighborNet graphs with SplitsTree4 (v4.13.1).
RESULTS
General Features of Sequenced MenY Carriage Genomes
After de novo assembly, the 100–base pair paired Illumina reads from the 99 MenY carriage isolates produced contiguous sequences between 2 018 731 base pairs and 2 214 168 base pairs in size, consistent with expectations for meningococcal genomes (Supplementary Table 1). Genome assemblies were automatically annotated in a gene-by-gene approach, using the BIGSdb platform and strain designation data extracted (Supplementary Table 1). Isolates from CC23 predominated (58%), followed by those from CC174 (18%), CC167 (11%), and CC22 (7%). The most-prevalent strain designations were Y: P1.5-1,10-1: F4-1: ST-1655 (CC23), Y: P1.5-1,2-2: F5-8: ST-23 (CC23), and Y: P1.21,16: F3-7: ST-1466 (CC174), which collectively accounted for 48% of the carriage isolates (Table 1). Of the 16 carriage strains isolated in 1997–2001, 11 shared identical strain designations with 2008–2010 carriage isolates, suggesting persistence of these strain designations over this 7–13-year period (Table 1).
Table 1.
Frequency of Strain Designations in the Serogroup Y Neisseria meningitidis Carriage and Invasive Collections
| Strain Designation | Carriage Group, No. |
Total Carriage, No. (n = 99) | Invasive 2010–2011, No. (n = 73) | Total Carriage and Invasive, No. (n = 172) | |
|---|---|---|---|---|---|
| 1997–2001 (n = 16) | 2008–2010 (n = 83) | ||||
| Y: P1.5-1,10-1: F4-1: ST-1655 (CC23) | 1 | 20 | 21 | 26 | 47 |
| Y: P1.5-1,2-2: F5-8: ST-23 (CC23) | 5 | 10 | 15 | 5 | 20 |
| Y: P1.21,16: F3-7: ST-1466 (CC174) | 0 | 12 | 12 | 5 | 17 |
| Y: P1.5-2,10-1: F4-1: ST-23 (CC23) | 1 | 4 | 5 | 4 | 9 |
| Y: P1.5-1,10-4: F4-1: ST-1655 (CC23) | 2 | 4 | 6 | 2 | 8 |
| Y: P1.5-1,10-4: F4-1: ST-6463 (CC23) | 0 | 6 | 6 | 2 | 8 |
| Y: P1.5-1,2-2: F5-1: ST-3651 (CC22) | 0 | 4 | 4 | 2 | 6 |
| Y: P1.5-1,10-10: F4-1: ST-1655 (CC23) | 0 | 2 | 2 | 4 | 6 |
| Y: P1.5-1,10-1: F1-3: ST-767 (CC167) | 2 | 3 | 5 | 0 | 5 |
| Y: P1.5-1,10-4: F4-1: ST-23 (CC23) | 0 | 0 | 0 | 4 | 4 |
| Y: P1.5-8,10-4: F5-2: ST-168 (CC167) | 0 | 1 | 1 | 2 | 3 |
| Y: P1.5-1,10-22: F5-1: ST-114 (CC22) | 0 | 2 | 2 | 0 | 2 |
| Y: P1.5-1,10-46: F3-9: ST-103 (CC103) | 0 | 2 | 2 | 0 | 2 |
| Y: P1.5-1,10-62: F1-3: ST-767 (CC167) | 2 | 0 | 2 | 0 | 2 |
| Y: P1.22,9: F3-7: ST-1466 (CC174) | 0 | 1 | 1 | 1 | 2 |
| Othera | 3 | 12 | 15 | 16 | 31 |
a Includes all strain designations occurring only once.
To investigate the occurrence of these carriage strain designations among invasive MenY isolates, identical typing information was extracted from the WGS data of 73 invasive United Kingdom MenY isolates recovered during 2010–2011 available via the MRF Meningococcus Genome Library database (Supplementary Table 2). Isolates from CC23 predominated (79%), followed by those from CC174 (10%), CC167 (5%), and CC22 (3%). The most prevalent strain designations among the invasive isolates matched those found in the carriage collection (Table 1). Ten designations were present in both carriage and invasive isolates: these designations accounted for 74% of carriage and 73% of invasive isolates, respectively (Table 1).
WGS Analysis of MenY Isolates Identified Clusters of Highly Related Isolates
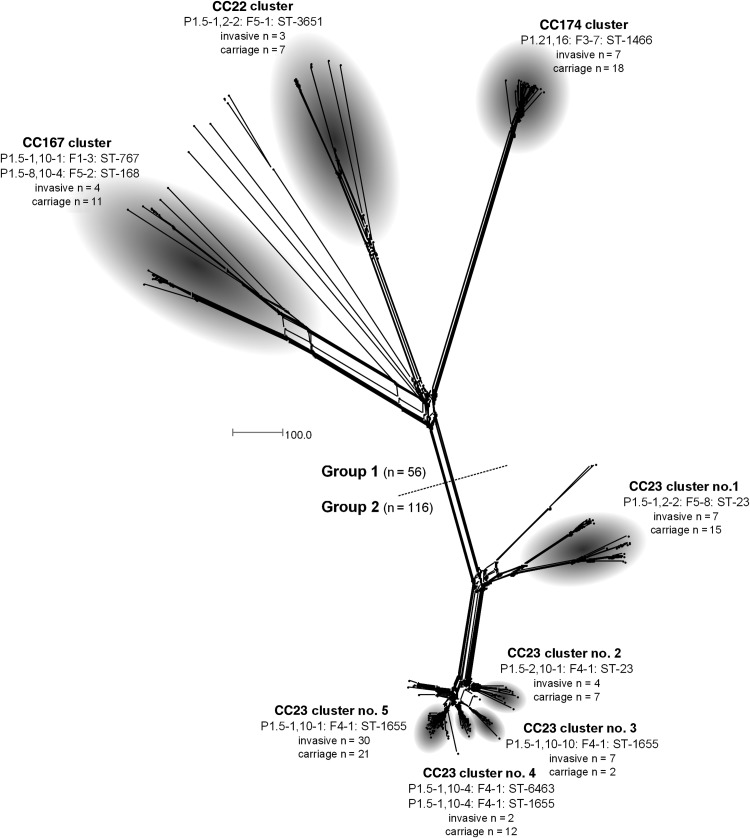
To allow higher-resolution genealogical analyses, comparison of all 172 MenY genomes was undertaken using the BIGSdb Genome Comparator tool, the principal output of which is a distance matrix based on the number of variable loci within those loci selected for analysis. These differences were then resolved into a network using standard algorithms [30]. Comparison of the genomes by using the core N. meningitidis cgMLST v1.0 scheme [29] identified 1157 loci that varied in at least 1 isolate and resolved isolates into 2 distinct groups comprising 56 and 116 isolates (Figure 1). Only 13 loci were found to be identical between these 2 groups: these included loci encoding ribosomal and hypothetical proteins. Within the 2 groups, distinct clusters of isolates containing multiple examples of both carriage and invasive isolates were evident. Group 1 comprised 3 clusters, containing isolates belonging to CC167, CC22, and CC174. Group 2 contained only CC23 meningococci, which formed 5 distinct clusters of carriage and invasive organisms (Figure 1). Overall, 91% of isolates (157 of 172) localized to one of these 8 clusters.
Figure 1.
NeighborNet graph comparison of 172 United Kingdom Neisseria meningitidis serogroup Y genome sequences analyzed by means of the BIGSdb Genome Comparator, using the Neisseria meningitidis cgMLST v1.0 scheme. A total of 91% of isolates analyzed localized to one of 8 clusters. Strain designation(s) represent the most frequently occurring designation(s) in each cluster. Unlabeled nodes represent unassigned invasive (n = 9) and carriage (n = 6) isolates. The scale bar denotes the number of allelic differences.
Relationships Between Invasive and Carriage MenY Isolates in Identified Clusters
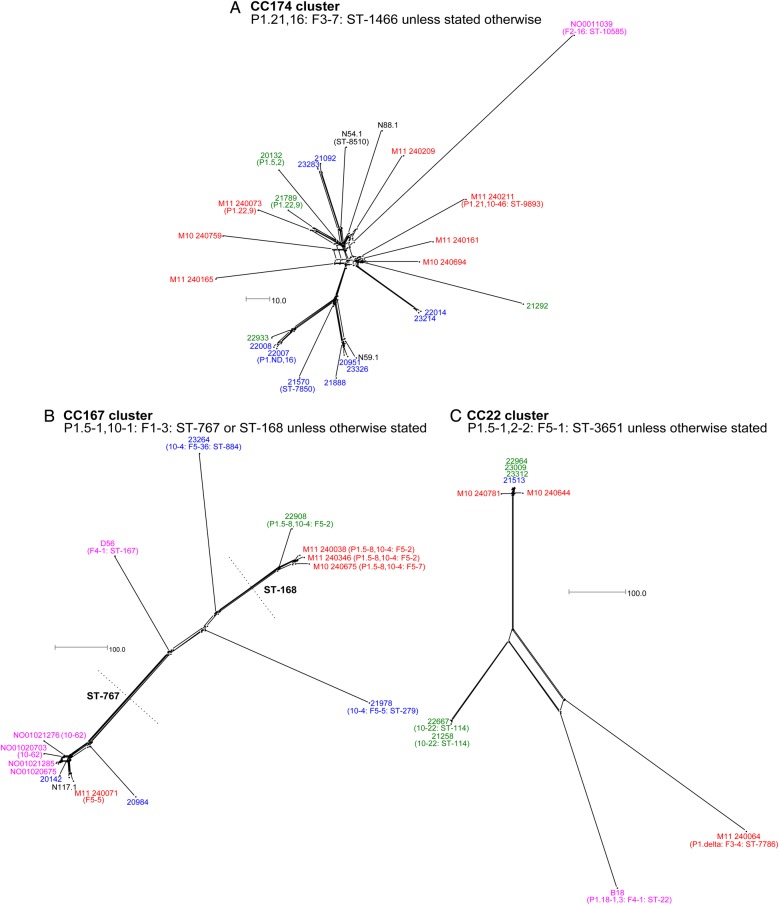
To visualize the relationships among closely related isolates, NeighborNet graphs were generated for each cluster, with color-coding of isolate names detailing provenance (Figures 2–4). Among the 25 isolates in the CC174 cluster (Figure 2A), evidence of extensive genetic similarities between carriage isolates was apparent with, for example, only 6 allelic differences distinguishing isolates 22014 and 23214. Highly related 2009–2010 carriage isolates were often isolated from students in the same year group suggestive of intra-year group transmission. This was also apparent in other clusters of isolates, such as CC22 (eg, isolates 22667 and 21258; 8 allelic differences; Figure 2C). Conversely, the CC22 cluster revealed highly related meningococci isolated from individuals in different year groups, suggestive of inter-year group transmission (eg, isolates 23009 and 21513; 3 allelic differences; Figure 2C).
Figure 2.
NeighborNet graphs comparing isolates in the CC174 (A), CC167 (B), and CC22 (C) clusters as defined in Figure 1. Sequences were analyzed by means of the BIGSdb Genome Comparator tool, using the Neisseria meningitidis cgMLST v1.0 scheme. Isolate names are color-coded as follows: 1997–2001 carriage isolates, fuchsia; 2008–2009 carriage isolates, black; 2009–2010 carriage isolates from first-year students, green; 2009–2010 carriage isolates from second-year students, blue; and invasive isolates from 2010 to 2011, red. The scale bar denotes the number of allelic differences. This figure is available in black and white in print and in color online.
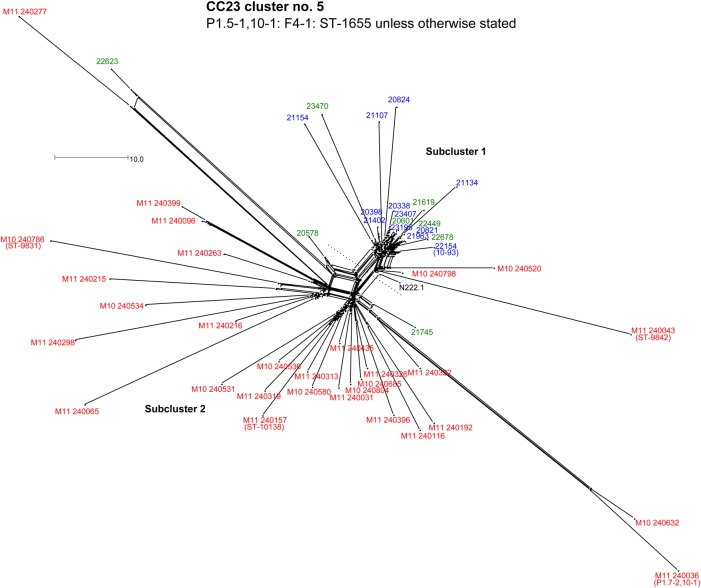
Figure 4.
NeighborNet graph comparison of isolates in the CC23 cluster 5 defined in Figure 1. Sequences were analyzed by means of the BIGSdb Genome Comparator tool, using the Neisseria meningitidis cgMLST v1.0 scheme. Isolate names are color-coded according to the scheme described in Figure 2 legend. The scale bar denotes the number of allelic differences. This figure is available in black and white in print and in color online.
The CC167 cluster (Figure 2B) and CC23 cluster 4 (Figure 3D) each resolved into distinct subclusters. The ST-767 CC167 subcluster (Figure 2B) contained carriage isolates from 2001, 2008, and 2009 and a 2011 invasive isolate (M11 240071), suggestive of a long-lived clone capable of causing disease. Only 27 allelic differences distinguished M11 240071 from N117.1; 62 differences distinguished the former from NO01020675, a carriage isolate obtained in 2001 (Figure 2B).
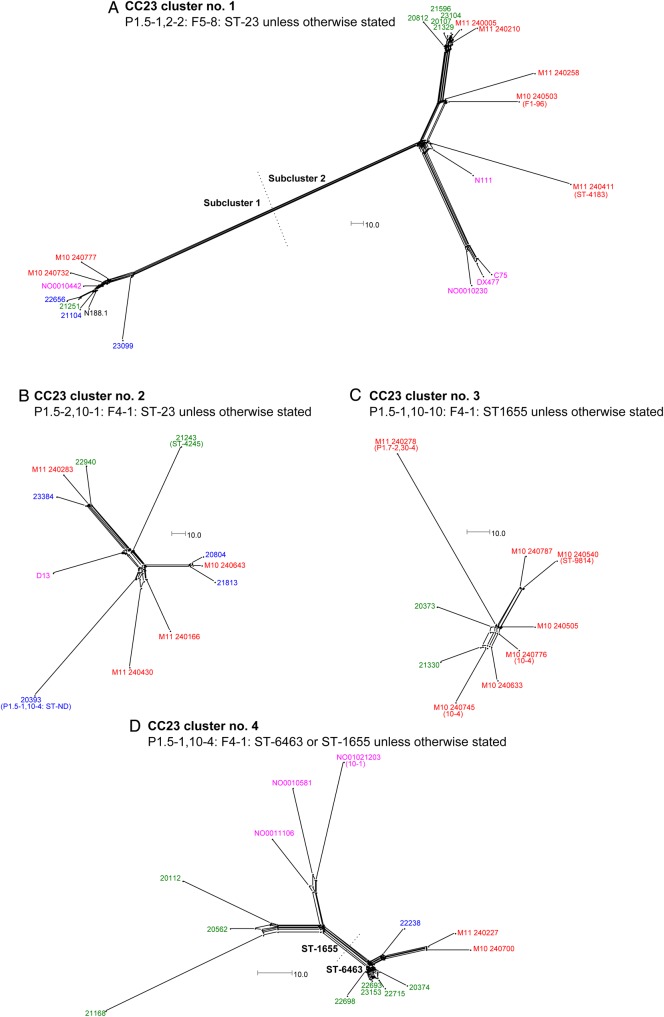
Figure 3.
NeighborNet graphs comparing isolates in the CC23 cluster numbers 1, 2, 3, and 4 (panels A–D, respectively) as defined in Figure 1. Sequences were analyzed by means of the BIGSdb Genome Comparator tool, using the Neisseria meningitidis cgMLST v1.0 scheme. Isolate names are color-coded according to the scheme described in the Figure 2 legend. The scale bar denotes the number of allelic differences. This figure is available in black and white in print and in color online.
In some cases, clusters containing isolates with identical designations could also be resolved into distinct subclusters on the basis of WGS analysis. Notably, CC23 cluster 1 could be resolved into 2 subclusters (Figure 3A). The first contained a carriage isolate from 2000 (NO0010442), 5 2008–2010 carriage isolates, and 2 2010–2011 invasive isolates. Since NO0010442 is only 34 allelic differences apart from 21251 (a 2009 carriage isolate) and 42 apart from the invasive isolate M10 240732, this subcluster represents another persistent clone, capable of causing disease.
WGS Analysis Resolves CC23 Cluster 5 Into Invasive and Carriage-Associated Subclusters
The CC23 cluster 5 contained the largest number of MenY isolates analyzed. Despite predominantly sharing a common strain designation, WGS-based analysis resolved meningococci in this cluster into 2 subclusters (Figure 4): subcluster 1 with 18 carriage isolates and 3 invasive isolates; and subcluster 2 with 3 carriage and 27 invasive meningococci. A total of 997 loci were identical among all CC23 cluster 5 isolates. These subclusters were defined by specific allelic differences in 5 core genes, encoding glycerate kinase (glxK), valine-pyruvate transaminase (avtA), superoxide dismutase (sodB), and 2 hypothetical proteins (Table 2).
Table 2.
Loci With Allelic Differences Between the 2 Subclusters of Clonal Complex 23 Cluster 5
| BIGSdb Neisseria Locus Identifier | Predicted Protein/Function (Gene) | Allele Number (%) |
Percentage Nucleotide Identity | Amino Acid Differences | |
|---|---|---|---|---|---|
| Subcluster 1 | Subcluster 2 | ||||
| NEIS0395 | Valine-pyruvate transaminase (avtA) | 112 (100) | 113 (96.7) | 99.9 | 1 |
| NEIS0825 | Superoxide dismutase (sodB) | 155 (100) | 22 (96.7) | 99.8 | 1 |
| NEIS0929 | Hypothetical protein | 42 (100) | 3 (100) | 99.6 | 0 |
| NEIS1199 | Glycerate kinase (glxK) | 47 (100) | 24 (100) | 99.9 | 1 |
| NEIS1568 | Hypothetical protein | 67 (100) | 68 (96.7) | 99.9 | 1 |
DISCUSSION
Nucleotide sequence-based methods involving small numbers of genes have been invaluable in characterizing the population structure and antigenic repertoires of meningococci [31]. The advent of WGS has greatly enhanced resolution and has begun to provide improved insights into the genetic relationships among bacterial isolates [32]. Since carriage is directly relevant to the epidemiology of IMD, we undertook to resolve the genealogical relationships between carriage and invasive isolates. We focused on MenY lineages because of recent observations of fluctuations in MenY disease and carriage levels in the United Kingdom. Although meningococci of this serogroup have been less prevalent globally as causes of disease than serogroups A, B, and C [33], the proportion of IMD attributable to MenY organisms, predominately those belonging to CC23, increased markedly, a trend first recognized in the mid-1990s in the United States [14, 34] and, more recently, in other countries, including the United Kingdom [17, 18] and Sweden [15, 35]. The higher MenY IMD case load in the United Kingdom was concomitant with a significant increase in MenY carriage, as first detected in studies of nasopharyngeal carriage in students at the University of Nottingham undertaken from 2008 to 2010 [19, 20].
The automated extraction of strain designation information from WGS data demonstrated the similarity of MenY isolates from carriage and invasive disease. This similarity was confirmed by the enhanced discrimination afforded by core genome analysis of the WGS data, which resolved most of the isolates into one of 8 defined clusters. While most isolates in a particular cluster shared the same strain designation (ie, ST, PorA, and FetA types), each cluster contained variants, demonstrating the enhanced discrimination afforded by WGS. A key finding was that every cluster contained both invasive and disease isolates, indicating that all MenY lineages have the ability to cause disease.
Bacterial populations are often viewed as unstable collections of rapidly evolving clones with frequent extinctions or replacement of older clones. Temporal shifts are potentially important components of IMD epidemiology. Thus, analysis of IMD cases indicated replacement of an early CC23 MenY lineage in the United States by an antigenically and genetically distinct late strain type [36, 37]. A parallel shift in carriage of these clones was assumed but not investigated. A significant finding from the present study was that 6 of 8 MenY clusters contained historical carriage isolates (ie, from 1997 to 2001). The stability of this association appears to be strong, as it was detected with only 16 historic genome sequences. Thus, these 6 MenY clusters are long-lived and have been present within the United Kingdom for a 7–13-year period. The uneven distribution (eg, CC167 and CC23 cluster 1) and apparent outlier position (eg, CC174 and CC22) of historical isolates in some clusters is suggestive of within-cluster evolution over time. The exception to this generalization was CC23 cluster 5, which was the largest cluster and yet contained no historic strain types, potentially suggesting the arrival of a non–United Kingdom-associated epidemic lineage or major alterations in the genetic structure of a long-lasting United Kingdom MenY clone. The presence of long-lasting clones indicates that the genetic structure of meningococcal clones is stable and that extinctions of clones are rare events. The presence of a long-lived host-adapted commensal population has importance, as introduction of the MenACWY vaccine into the main carrier population has the potential to radically perturb a long-lasting association with unknown consequences.
There was evidence for antigenic shifts among members of the CC23 isolates, which distributed into 5 clusters distinguished by PorA type but not ST type. Four of the clusters differed in sequence for VR2 of PorA, a major target of bactericidal antibodies, while the 2 clusters with identical PorA VR2 sequences had different PorA VR1 sequences, a variable target of bactericidal antibodies. The differences in the VR2 amino acid sequence are the increase in number of a 3–amino acid motif (NKQ), from 1 copy in P1.10-1 to 2 in P1.10-4 and 3 in P1.10-10—a rapid and minor change in protein structure. This was not a feature of all surface antigens, as there was limited variation in the FetA VR, with 4 CC23 clusters having the same FetA variant. Further analysis of WGS data may indicate other antigenic variants or allelic variants of other genes that correlate with this segregation of CC23 isolates; nevertheless, the PorA distribution suggests that minor differences in antigenicity may contribute to changes in population structure.
Geographic distribution of clones and potential sources of new clones was apparent from comparisons among WGS studies in different countries. Comparison of invasive CC23 isolates from Sweden, the United Kingdom, and the United States identified 3 principal CC23 sublineages (designated 23.1, 23.2, and 23.3) with overlapping but differentially prevalent repertoires in each country [28]. For example, the Swedish strain-type YI, which was largely responsible for the increase in Swedish MenY disease [16, 35], formed a cluster within the 23.1 sublineage but very rarely caused disease in the United Kingdom [28]. Using the overlap in MenY WGS data analyzed (ie, United Kingdom invasive CC23 strains isolated in 2010–2011 examined previously [28]), we further resolved the 23.1 sublineage into 4 subclusters (CC23 clusters 2–5) and found that CC23 cluster 1 corresponds to lineage 23.2. Cluster 5, which was responsible for most cases of United Kingdom IMD, was rarely observed in Swedish IMD isolates.
The resolution of CC23 cluster 5 into distinct carriage-associated and disease-associated subclusters, (1 and 2, respectively) was surprising, as this cluster contained the highest number of MenY disease and carriage isolates. A confounding factor is that the subcluster 1 carriage isolates were all isolated in 1 geographical location and, hence, may have a high level of one specific (highly transmissible) clone. Two of these isolates (20601 and 21619) were, however, isolated in the first week of term in September from first-year students who were presumed to have been colonized prior to arrival at the university. An alternative hypothesis is that the ability of subcluster 1 strains to cause disease is associated with rapid within-host evolution into a subcluster 2 phenotype; however, subclusters were defined by differences in loci encoding proteins with hypothetical or core enzymatic functions, not loci explicitly linked to adaptation to a systemic niche (eg, survival in blood). A further possibility is that subcluster 1 has recently evolved from subcluster 2 into a highly transmissible carriage strain with a consequent reduction in virulence. It is unlikely that any of the 5 differences in subcluster 1 and 2 defining core loci are directly responsible for differences in virulence, given the predicted functions of the 5 proteins, and that the allelic differences lead to, at most, only single amino acid changes, which are unlikely to be functionally significant. Instead, we hypothesize that differences that are outside the core alleles examined in this study but cosegregate with the 5 core loci differences are more likely to be responsible. A high-quality assembled CC23 genome is required to detect the effects on virulence mediated by noncore genes and to determine how the transition between these subclusters has occurred.
In summary, high-resolution genealogical relationships between MenY isolates highlighted the high degree of genetic similarity between carriage and invasive isolates and evidenced long-term stability of MenY clones. The detection and resolution of a highly prevalent United Kingdom clone (Y: P1.5-1,10-1: F4-1: ST-1655 CC23) into invasive- and carriage-associated subclusters exemplifies the improved precision of whole-genome analysis for separating apparently identical isolates.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. This publication made use of the Meningitis Research Foundation Meningococcus Genome Library (available at: http://www.meningitis.org/research/genome), developed by Public Health England, the Wellcome Trust Sanger Institute, and the University of Oxford as a collaboration and funded by Meningitis Research Foundation.
Financial support. This work was supported by the Wellcome Trust (grant 087622/Z/08/2 to M. C. J. M).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853–61. [DOI] [PubMed] [Google Scholar]
- 2.Soriano-Gabarró M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther 2011; 9:761–74. [DOI] [PubMed] [Google Scholar]
- 3.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007; 369:2196–210. [DOI] [PubMed] [Google Scholar]
- 4.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence 2014; 5:98–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison OB, Claus H, Jiang Y et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis 2013; 19:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:B51–63. [DOI] [PubMed] [Google Scholar]
- 7.Jolley K, Brehony C, Maiden M. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol Rev 2007; 31:89–96. [DOI] [PubMed] [Google Scholar]
- 8.Maiden MC, Bygraves JA, Feil E et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 1998; 95:3140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazdankhah SP, Kriz P, Tzanakaki G et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol 2004; 42:5146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caugant DA, Maiden MCJ. Meningococcal carriage and disease - population biology and evolution. Vaccine 2009; 27:B64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell JE, Jolley KA, Feavers IM, Maiden MC, Suker J. PorA variable regions of Neisseria meningitidis. Emerg Infect Dis 2004; 10:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect Immun 2004; 72:5955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotter C, Chandra M, Cano R et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev 2007; 31:27–36. [DOI] [PubMed] [Google Scholar]
- 14.Cohn AC, MacNeil JR, Harrison LH et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184–91. [DOI] [PubMed] [Google Scholar]
- 15.Bröker M, Bukovski S, Culic D et al. Meningococcal serogroup Y emergence in Europe. Hum Vaccin Immunother 2014; 10:1725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedberg ST, Toros B, Fredlund H, Olcen P, Molling P. Genetic characterisation of the emerging invasive Neisseria meningitidis serogroup Y in Sweden, 2000 to 2010. Euro Surveill 2011; 16:19885. [PubMed] [Google Scholar]
- 17.Ladhani SN, Lucidarme J, Newbold LS et al. Invasive meningococcal capsular group Y disease, England and Wales, 2007–2009. Emerg Infect Dis 2012; 18:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladhani SN, Flood JS, Ramsay ME et al. Invasive meningococcal disease in England and Wales: Implications for the introduction of new vaccines. Vaccine 2012; 30:3710–6. [DOI] [PubMed] [Google Scholar]
- 19.Bidmos FA, Neal KR, Oldfield NJ, Turner DPJ, Ala'Aldeen DAA, Bayliss CD. Rapid clonal expansion, persistence and clonal replacement of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol 2011; 49:506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ala'Aldeen DAA, Oldfield NJ, Bidmos FA et al. Carriage of meningococci by university students, United Kingdom. Emerg Infect Dis 2011; 19:1762–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeppesen CA, Snape MD, Robinson H et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect 2015; 71:43–52. [DOI] [PubMed] [Google Scholar]
- 22.Read RC, Baxter D, Chadwick DR et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet 2014; 384:2123–31. [DOI] [PubMed] [Google Scholar]
- 23.Ala'Aldeen DA, Neal KR, Ait-Tahar K et al. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol 2000; 38:2311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiden M, Ibarz-Pavón AB, Urwin R et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008; 197:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolley KA, Hill DM, Bratcher HB et al. Resolution of a meningococcal disease outbreak from whole-genome sequence data with rapid Web-based analysis methods. J Clin Microbiol 2012; 50:3046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison OB, Bray JE, Maiden MCJ, Caugant DA. Genomic analysis of the evolution and global spread of hyper-invasive meningococcal lineage 5. EBioMedicine 2015; 2:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Törös B, Hedberg ST, Unemo M et al. Genome-based characterization of emergent invasive Neisseria meningitidis serogroup Y in Sweden, 1995 to 2012. J Clin Microbiol 2015; 53:2154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratcher HB, Corton C, Jolley KA, Parkhill J, Maiden MC. A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics 2014; 15:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolley KA, Maiden MC. Automated extraction of typing information for bacterial pathogens from whole genome sequence data: Neisseria meningitidis as an exemplar. Euro Surveill 2013; 18:20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brehony C, Jolley KA, Maiden MC. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol Rev 2007; 31:15–26. [DOI] [PubMed] [Google Scholar]
- 32.Jolley KA, Maiden MC. Using multilocus sequence typing to study bacterial variation: prospects in the genomic era. Future Microbiol 2014; 9:623–30. [DOI] [PubMed] [Google Scholar]
- 33.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine 2009; 27:B71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baccarini C, Ternouth A, Wieffer H, Vyse A. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother 2013; 9:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Törös B, Thulin Hedberg S, Jacobsson S, Fredlund H, Olcén P, Mölling P. Surveillance of invasive Neisseria meningitidis with a serogroup Y update, Sweden 2010 to 2012. Euro Surveill 2014; 19:20940. [DOI] [PubMed] [Google Scholar]
- 36.Harrison LH, Jolley KA, Shutt KA et al. Antigenic shift and increased incidence of meningococcal disease. J Infect Dis 2006; 193:1266–74. [DOI] [PubMed] [Google Scholar]
- 37.Krauland MG, Dunning Hotopp JC, Riley DR et al. Whole genome sequencing to investigate the emergence of clonal complex 23 Neisseria meningitidis serogroup Y disease in the United States. PLoS One 2012; 7:e35699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.