Abstract
Borrelia burgdorferi harbors a limited set of transmembrane surface proteins, most of which constitute key targets of humoral immune responses. Here we show that BB0405, a conserved membrane-spanning protein of unknown function, fails to evoke detectable antibody responses despite its extracellular exposure. bb0405 is a member of an operon and ubiquitously expressed throughout the rodent-tick infection cycle. The gene product serves an essential function in vivo, as bb0405-deletion mutants are unable to transmit from ticks and establish infection in mammalian hosts. Despite the lack of BB0405-specific immunoglobulin M or immunoglobulin G antibodies during natural infection, mice immunized with a recombinant version of the protein elicited high-titer and remarkably long-lasting antibody responses, conferring significant host protection against tick-borne infection. Taken together, these studies highlight the essential role of an apparently immune-invisible borrelial transmembrane protein in facilitating infection and its usefulness as a target of protective host immunity blocking the transmission of B. burgdorferi.
Keywords: Borrelia burgdorferi, BB0405, pathogen persistence, transmission-blocking, vaccine
Lyme disease is a prevalent tick-borne infection in the United States and Europe [1–3]. Newly revised estimates from the Centers for Disease Control and Prevention suggest that there are >300 000 new cases per year in the United States. Although substantial progress has been made regarding proper diagnosis and treatment of Lyme disease [4], a vaccine to prevent human infection is currently unavailable [5]. The causative agent, Borrelia burgdorferi, survives in an enzootic life cycle consisting of arthropod vectors, Ixodes scapularis ticks, and various mammalian hosts, usually wild rodents [6]. Once transmitted from infected ticks to hosts, the spirochetes colonize target tissues—a process supported by the preferential expression of certain gene products that help them adapt to challenges specific to various microenvironments [7, 8]. As many of these gene products lack orthologs outside of the Borrelia clade [9–12], their biological significance is likely to be linked to specialized functions relevant to the intricate infection cycle of spirochetes [13].
In ticks, B. burgdorferi primarily resides within the lumen of the gut [8], and during the subsequent tick-engorgement process it undergoes replication, as well as genetic and antigenic alterations required for infection of the mammalian host [14]. Notably, these adaptive changes take place in the gut, where the spirochetes encounter host-derived molecules present in the blood meal, including ingested antibodies. Thus, in a limited number of cases, including after receipt of the former OspA-based Lyme vaccine [15, 16], host antibodies generated against specific borrelial antigens have been shown to inhibit microbial transmission from ticks to host [17]. Some of these gene products, such as BBA52 and OspC, assist spirochetes in migrating from ticks to mice and/or establishing host infection and may play a prominent role in pathogenesis [18–20]. However, given the remarkable genetic diversity of B. burgdorferi [21] and the ability of the spirochete to alter its surface proteome throughout the enzootic cycle, it is difficult to find single protective antigens. Thus, one goal of our ongoing studies is to identify additional stable and conserved borrelial antigens, particularly those that are expressed during tick feeding or mammalian infection and that serve essential roles in infectivity.
The enzootic cycle of Lyme disease spirochetes provides opportunities to target the bacteria either in the mammalian host or the arthropod vector. An advantage of targeting bacteria in the host is that a potential vaccine would only have to induce a memory B-cell response [22], with the infection serving as a proxy booster immunization to induce sufficient antibody levels for neutralization. On the other hand, a benefit of strategies aimed at neutralizing spirochetes in the arthropod vector is that the bacteria's antigenic profile has not been subjected to the evolutionary selective pressure of the mammalian host's immune response [23, 24]. In fact, Borrelia antigens expressed in the vector are mostly conserved [25], and antigenic variation mechanisms appear to be minimally used in the vector [26]. With this strategy, however, the host's antibodies should be maintained at high levels and stable (over a long duration) without requiring frequent booster immunizations. BB0405 was previously identified as one of the differentially expressed and surface-exposed spirochete antigens that are possible vaccine targets [27]. Here we show that BB0405 supports spirochete infection in mammals and that the antigen is a promising candidate for transmission-blocking vaccines against Lyme disease.
MATERIALS AND METHODS
B. burgdorferi, Mice, and Ticks
B. burgdorferi infectious isolate B31 A3 was used throughout the present study [19]. Spirochete cultures were grown in Barbour-Stoenner-Kelly H (BSK-H) medium with or without 350 µg/mL kanamycin. I. scapularis ticks were reared in the laboratory as described elsewhere [19]. Female C3H/HeN mice aged 4–6 weeks were purchased from the National Institutes of Health. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the University of Maryland, College Park.
Polymerase Chain Reaction (PCR) Analysis
The oligonucleotide sequences for each of the primers used in specific PCR reactions are listed in Supplementary Table 1. Total RNA was isolated using TRIzol reagent (Invitrogen) and reverse transcribed to complementary DNA (cDNA; AffinityScript, Stratagene/Invitrogen), and reverse transcription–PCR (RT-PCR) or quantitative RT-PCR (qRT-PCR) analyses were performed as described elsewhere [19, 28]. Expression of bb0405 was analyzed in various tissues of C3H/HeN mice (3 animals/group) 14 days after infection (105 spirochetes/mouse) or in naive or infected nymphal ticks that fed on infected mice or naive mice (20 ticks/mouse), respectively, as detailed elsewhere [19]. The levels of B. burgdorferi bb0405 transcript in tick and mouse samples were normalized against numbers of flaB transcripts. Cotranscription analyses of bb0404, bb0405, and bb0406 were performed with primers that span the genes, indicated in Supplementary Table 1.
Generation of Recombinant BB0405 and Antiserum and Localization of Native Protein
bb0405 was cloned into pGEX-6P-1 (GE Healthcare), using specific primers (Supplementary Table 1), and the recombinant protein without the N-terminal leader sequence was produced in Escherichia coli. Expression, purification, and enzymatic cleavage of the glutathione S-transferase (GST) fusion protein were performed as described previously [19, 28]. Polyclonal murine antisera against recombinant BB0405 (without the GST tag) were generated and assessed for titer and specificity, using an enzyme-linked immunosorbent assay (ELISA) and immunoblotting. Localization of native BB0405 in spirochetes was performed as described previously [19, 28].
ELISA and Immunoblotting
Plates were coated with recombinant BB0405 or BB0365 or 1 µg of spirochete lysate and blocked with 5% goat serum, followed by addition of either normal mouse serum or B. burgdorferi–infected serum (1:500). Serum samples were collected from B. burgdorferi–infected mice at various times following infection, with inoculation occurring via needle injection or by tick infestation, as detailed previously [28]. Signals were detected using secondary antibodies and peroxidase substrate (KPL). Immunoblotting was performed as described earlier [28], using murine sera or serum samples collected from 5 patients who tested positive for Lyme disease, as well from 5 healthy subjects, which were provided by Adriana Marques (National Institute of Allergy and Infectious Diseases).
Generation of bb0405-Deletion Mutant Isolates of B. burgdorferi
Genetic manipulation of spirochetes involved routine procedures performed as detailed previously [19, 28]. BB0405-deficient B. burgdorferi were generated by homologous recombination, replacing the 546–base pair internal fragment of bb0405 with a kanamycin resistance cassette, using primers as indicated in Supplementary Table 1. For genetic complementation via reinsertion of a wild-type copy of bb0405 in the chromosome of the mutant, a construct for allelic exchange was also generated, as detailed in other studies [19, 28]. However, our repeated attempts to complement bb0405-mutant isolates were unsuccessful; therefore, for phenotypic analysis, 2 isogenic and independent bb0405-mutant clones (designated as Mut1 and Mut2) were included in all studies. For in vitro growth analysis, spirochetes were diluted to a density of 105 cells/mL, grown until stationary phase (approximately 108 cells/mL), and counted by dark-field microscopy, using a Petroff-Hausser cell counter as detailed elsewhere [19, 28].
Phenotypic Analysis of bb0405-Mutant Isolates
To examine the phenotypes of bb0405 mutants in vivo, wild-type, Mut1, and Mut2 isolates were inoculated into separate groups of mice (3 animals/group; 105 spirochetes/mouse). Skin, joint, heart, and bladder samples were isolated 14–21 days of infection, and pathogen burdens were measured by qRT-PCR as detailed previously [19]. Skin and spleen samples were cultured in BSK-H medium for the presence of viable B. burgdorferi. For acquisition studies, mice that had been infected for 12 days were allowed to be parasitized by nymphs (30 ticks/group). Ticks were collected after 12 hours of feeding or after repletion. For transmission studies, nymphs were microinjected with 109 wild-type or bb0405-mutant clones of B. burgdorferi as detailed earlier [19]. The infected ticks were fed on naive mice (5 ticks/mouse; 3 mice/group). Engorged ticks were subjected to qRT-PCR analyses and confocal imaging as detailed earlier [19]. At day 10 following tick feeding, all mice were euthanized, and tissues were isolated and assessed for spirochete burden by qRT-PCR. Portions of skin and spleen were cultured in BSK-H medium.
Bactericidal Assay
BB0405 antibodies were tested for their bactericidal activities against B. burgdorferi by dark-field microscopy using a regrowth assay as described [28]. Briefly, spirochetes (107/mL) were incubated in BSK-H medium (Sigma-Aldrich) supplemented with BB0405 antibodies at 33°C. Normal mouse serum and mouse OspA antibodies (OspA) served as controls. At 48 hours following antiserum treatment, spirochetes were enumerated by dark-field microscopy as detailed previously [28].
Histopathologic Analysis
B. burgdorferi–infected mice underwent histologic evaluation for detection of arthritis as detailed previously. At least 3 ankle joints were collected from each group of mice (3 animals/group) infected with the different isolates and processed for hematoxylin and eosin staining, and signs of arthritis were evaluated as described elsewhere [29].
Immunization and Infection Studies
Groups of mice (3–5 animals/group) were immunized subcutaneously either with purified recombinant BB0405 (10 µg/mouse) or phosphate-buffered saline (PBS) mixed in Freund's complete adjuvant and then boosted with the antigen or PBS in incomplete Freund's adjuvant. Each animal received 3 immunizations, and serum samples were collected at weekly to monthly intervals. In some experiments, mice (3 animals/group) were infected with a mutant lacking OspA, OspB, and OspC as detailed elsewhere [30]. For protection studies, 10 days after the final boost, mice were challenged with an intradermal injection of B. burgdorferi (105 spirochetes/mouse), and tibiotarsal joints were examined for swelling and abnormal histologic findings as detailed elsewhere [30]. Mice were euthanized after 10 and 20 days of infection. Tissue samples were collected, and the B. burgdorferi burden was measured using qRT-PCR analysis of flaB messenger RNA. To assess protective efficacy of BB0405 immunization against tick-borne challenge, additional groups of mice were also immunized with recombinant BB0405 or PBS containing adjuvant, as described above. B. burgdorferi–infected nymphs were generated via an established microinjection procedure as described elsewhere [20]. Ten days after the final boost, mice were parasitized by B. burgdorferi–infected ticks (5 nymphs/mouse) and allowed to replete. Mice were euthanized 12 days following tick engorgement, tissue samples were collected, and the pathogen burden was determined by qRT-PCR analyses. To assess whether different mouse strains produce antibodies against BB0405, C3H, B6, and BALB/c mice (2–3 animals/group) were infected with B. burgdorferi (105 spirochetes/mouse), and the antibody response was analyzed following 2 weeks of infection by immunoblotting, using spirochete lysate and recombinant BB0405.
RESULTS
bb0405 Encodes a Surface-Exposed Outer Membrane Antigen and Is Ubiquitously Expressed In Vivo
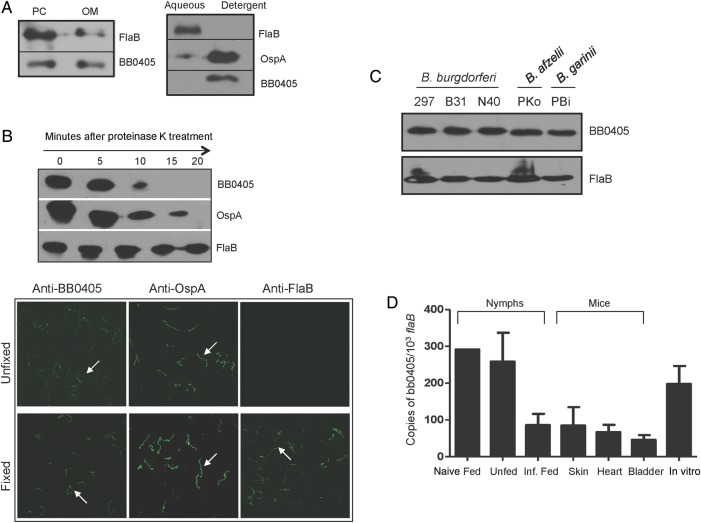
Previous studies suggested that bb0405 encodes a surface-exposed and transmembrane protein [27], and depending on the culture temperatures, the gene is transcribed at variable levels [31]. To study the potential role of BB0405 in microbial virulence and protective host immunity, we further assessed its cellular localization and antibody accessibility and analyzed its gene expression during infection. Results show that BB0405 is associated with the spirochete membrane (Figure 1A) and is exposed extracellularly, reflected by its rapid sensitivity to proteinase K treatment (Figure 1B). Also, antibodies generated against the B31 isolate recognized similar levels of BB0405 orthologs in other infectious isolates, suggesting its wide conservation across diverse B. burgdorferi sensu lato (Figure 1C). Detailed analyses of bb0405 expression in representative tick- and mammal-specific phases of B. burgdorferi infection indicated that the gene is consistently transcribed in vivo (Figure 1D).
Figure 1.
BB0405 is a conserved, surface-exposed outer membrane protein constitutively expressed during infection. A, BB0405 associated with isolated outer membrane or detergent-soluble membrane fractions of cultured spirochetes. Borrelia burgdorferi protoplasmic cylinder (PC) and outer membrane (OM) fractions were separated by sucrose density gradient centrifugation and immunoblotted with BB0405, FlaB, and OspA antisera (left panel). B. burgdorferi lysate was subjected to Triton X-114 partitioning (right panel) and immunoblotted with BB0405 antibodies or control antibodies. B, BB0405 is exposed on the surface of B. burgdorferi. Viable spirochetes were incubated with (+) or without (−) proteinase K for various times and processed for immunoblot analyses with antibodies against BB0405. OspA and FlaB antibodies served as surface and subsurface controls, respectively (upper panel). Similarly, in an immunofluorescence assay (lower panel), BB0405 antibodies recognize native protein on the surface of unfixed spirochetes. OspA and FlaB antibodies served as surface and subsurface controls, respectively. C, BB0405 is conserved in major infectious strains of B. burgdorferi sensu lato. Equal amounts of lysates prepared from Borrelia isolates were immunoblotted with anti-BB0405 antibodies against B. burgdorferi B31 isolate. D, bb0405 is induced during tick feeding. Gene expression was analyzed at representative stages of the experimental tick-mouse infection cycle. RNA was isolated from the skin, heart, and bladder tissues of mice (3 animals/group) 14 days after B. burgdorferi infection. Naive nymphs were allowed to feed on B. burgdorferi–infected mice (25 nymphs/mouse) and were collected after repletion. B. burgdorferi–infected nymphs were fed on naive mice and collected when fully engorged. Murine tissues (skin, heart, and bladder) were collected after 10 days of tick engorgement. Wild-type spirochetes were grown in Barbour-Stoenner-Kelly H medium. RNA from the mouse, tick, and culture samples were analyzed by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and presented as the number of copies of bb0405 transcripts per flaB transcripts. Abbreviations: B. afzelii, Borrelia afzelii; B. garinii, Borrelia garinii.
Analysis of the bb0405 Locus and Generation of BB0405-Deficient Spirochetes
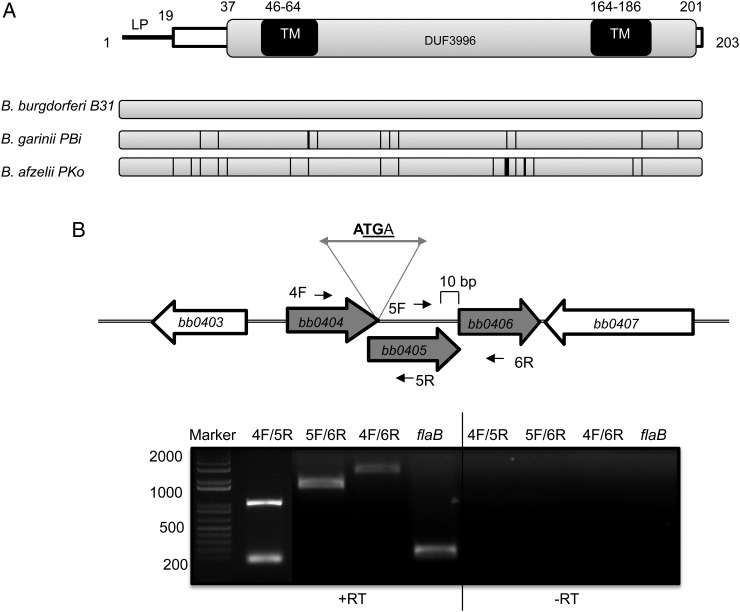
BB0405, which maintains high sequence conservation, possesses 2 recognizable transmembrane motifs and an 18-amino acid hydrophobic N-terminal leader sequence (Figure 2A). Analyses of the chromosomal locus revealed that the gene may be in an operon, which is further supported by RT-PCR–based detection of a single transcript from bb0404, bb0405, and bb0406, encoding 3 conserved hypothetical proteins of unknown biological significance (Figure 2B). To assess the role of BB0405 in B. burgdorferi survival and infectivity, we created targeted deletion mutants via homologous recombination (Supplementary Figure 1). Two clones (designated as Mut1 and Mut2) were selected for further analysis. Both lacked bb0405 RNA and protein expression without polar effects on transcription of surrounding members of the operon and retained a set of essential endogenous plasmids (Supplementary Figure 2), similar to wild-type spirochetes. Notably, compared with parental isolates, the bb0405 mutants displayed a significantly slower growth rate in vitro (Supplementary Figure 1). As multiple attempts to complement bb0405 mutants with a wild-type gene remained unsuccessful, to rule out anomalous effects of genetic manipulation, all subsequent phenotypic studies of mutants included simultaneous and independent analyses of clones Mut1 and Mut2.
Figure 2.
Organization of BB0405. A, Schematic representation of BB0405 protein. Bioinformatic analyses revealed 2 transmembrane motifs (TM) besides a putative signal peptidase I cleavable 18-amino acid hydrophobic sequence at its N-terminus (LP), as well as a functionally uncharacterized conserved domain (annotated as NCBI Conserved Protein Domain Family DUF3996; pfam13161). Lower panel is a schematic representation of BB0405 sequence conservation among representative Borrelia burgdorferi sensu lato species. Note a slightly greater diversification (indicated by bar thickness representing 1–4 amino acid differences) in Borrelia afzelii throughout the protein. B, bb0404, bb0405, and bb0406 are organized as a tricistronic operon. Upper panel shows organization of genes surrounding the bb0405 locus. Note overlapping (ATGA) stop and start codons for bb0404 and bb0405, respectively, and a small (10 base pairs) intergenic region between bb0405 and bb0406. Reverse transcription–polymerase chain reaction (RT-PCR) analysis confirming tricistronic organization of bb0404, bb0405, and bb0406 is shown in lower panel. RT-PCR was carried out using primers that span bb0404 and bb0405 and bb0405 and bb0406 as indicated. Absence of DNA contamination was ensured by RT-PCR reaction in the absence of reverse transcriptase (-RT).
BB0405-Deficient B. burgdorferi Are Not Infectious in Murine Hosts and Ticks
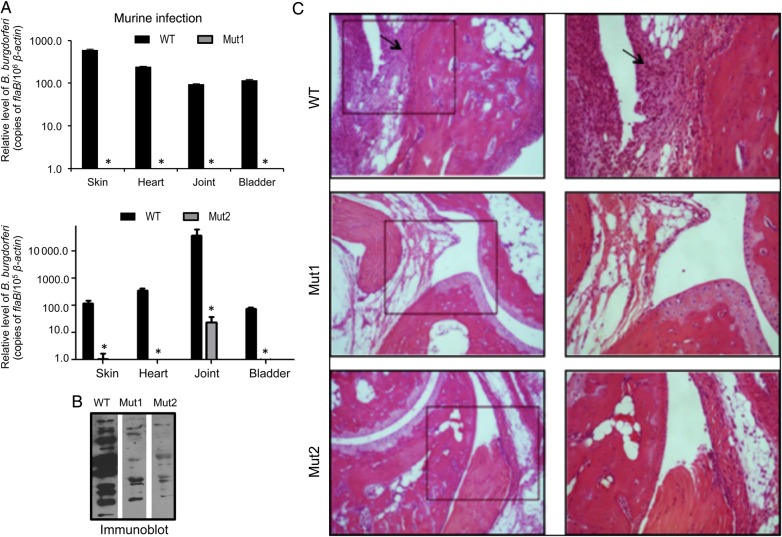
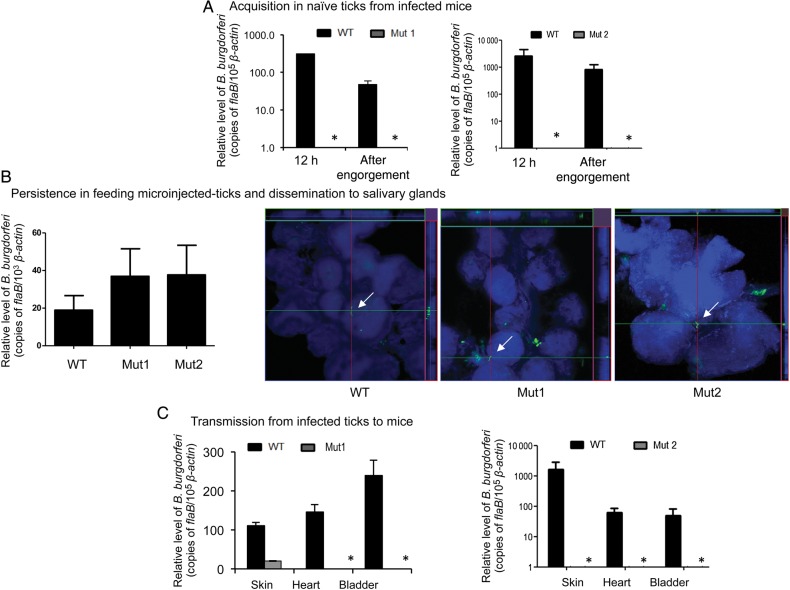
To determine whether the lack of BB0405 influences spirochete infectivity, C3H/HeN mice (3 animals/group) were inoculated intradermally with equal numbers of wild-type B. burgdorferi or one of 2 bb0405 mutant clones (105 spirochetes/mouse). Infection was assessed by qRT-PCR analyses of pathogen burden in skin, joint, heart, and bladder samples at day 20 of infection and by organ cultures. The qRT-PCR results indicated that, although wild-type spirochetes persisted in mice, bb0405 mutants were significantly reduced or undetectable (Figure 3A). Similarly, wild-type spirochetes were isolated by culture of infected spleen, whereas attempts to isolate either of the viable bb0405 mutant clones remained unsuccessful (data not shown). Mice infected with wild-type B. burgdorferi seroconverted (Figure 3B), which was reduced in mice infected with bb0405-mutant B. burgdorferi. Unlike with wild-type spirochetes, mice infected with bb0405 mutants displayed less obvious histopathological signs of arthritis (Figure 3C). We next studied the requirement of BB0405 in supporting the pathogen life cycle in ticks. Little to no bb0405 mutants were detected in feeding ticks analyzed after 12 hours of host attachment or in fully engorged nymphs, suggesting that bb0405 mutation impaired B. burgdorferi acquisition by ticks (Figure 4A). We also compared the ability of the bb0405 mutants to persist in ticks and transmit from infected ticks to naive mice. As ticks are unable to acquire bb0405 mutants by the natural engorgement process involving infected hosts (Figure 4A), separate groups of nymphs were microinjected with equal numbers of wild-type or mutant isolates. Injected ticks were allowed to parasitize naive C3H mice (3 animals/group) and were collected as fully engorged nymphs. Spirochete burdens in the ticks were assessed by qRT-PCR and confocal microscopic analyses, which indicated similar levels of wildtype and bb0405 mutants both in fed tick guts (Figure 4B) and within the salivary glands (Figure 4B), suggesting that BB0405 deficiency does not impair spirochete ability to persist in fed gut or migrate to tick salivary glands. However, 10 days after tick engorgement, little to no B. burgdorferi was detected in multiple tissues of mice parasitized by mutant-injected ticks, indicating that the mutants have a defect in their ability to infect mice via tick-borne infection (Figure 4C).
Figure 3.
bb0405 deletion impairs ability of Borrelia burgdorferi to persist in mice and cause disease. A, bb0405 deletion dramatically reduces the ability of B. burgdorferi to persist in mice. Animals (3 mice/group) were infected with wild type (WT; black bars) or either of the 2 bb0405 mutants Mut1 (gray bars; upper panel) or Mut2 (gray bars; lower panel). Pathogen levels were analyzed in skin, heart, joint, and bladder samples by measuring copies of B. burgdorferi flaB transcripts at 20 days of infection by quantitative polymerase chain reaction, and data were normalized against murine ß-actin levels. bb0405 mutants were either undetectable or persisted at levels significantly lower than those of corresponding WT pathogens (P < .05). B, bb0405 mutants are less infectious in mice, as assessed by serologic analysis. Serum samples from infected mice collected 14 days after infection were immunoblotted against B. burgdorferi lysate. C, Histopathological examination of the B. burgdorferi–infected murine joints. Mice were immunized with spirochetes, as detailed in Figure 3A, and joint samples were isolated after 14 days and subjected to histologic analysis. Left panel shows lower-resolution (original magnification ×10) images, and right panel reflects higher-resolution (original magnification ×20) images of selected areas from corresponding sections (box). Cellular infiltration is indicated by arrows.
Figure 4.
Deletion of bb0405 attenuates spirochete ability to transit between ticks and mice without affecting their ability to invade tick salivary glands. A, Borrelia burgdorferi burdens in fed ticks during pathogen acquisition. Naive Ixodes scapularis nymphs (30 ticks/group) were allowed to feed on mice 12 days after infection, and B. burgdorferi burdens in ticks were analyzed at the indicated intervals following feeding by measuring copies of B. burgdorferi flaB RNA and were normalized to amounts of tick β-actin messenger RNA. bb0405 mutants (Mut1, upper panel; Mut2, lower panel) were undetectable in ticks after feeding for 12 hours or after engorgement. B, bb0405 deletion does not impair B. burgdorferi persistence in feeding ticks or their ability to disseminate to salivary glands during transmission to murine hosts. Nymphal ticks were microinjected with wild-type (WT) or mutant spirochetes and placed on naive mice 48 hours after injection (5 ticks/mouse; 3 animals/group). Ticks were allowed to engorge, and pathogen levels were assessed by quantitative reverse transcription–polymerase chain reaction analysis of copies of flaB normalized against mouse β-actin levels (left panel) or by confocal microscopy of anti-B. burgdorferi antibodies within the infected salivary glands (right panels). Confocal orthogonal images revealing the distribution of spirochetes through the full thickness of salivary glands after 60 hours of feeding are shown. Spirochetes (arrows) were incubated with fluorescein isothiocyanate–labeled goat anti–B. burgdorferi antibodies (green), and glands were stained with DAPI (blue). C, bb0405 deletion affects B. burgdorferi transmission from infected ticks to murine hosts. Nymphal ticks were microinjected with WT or mutant spirochetes and were allowed to engorge on naive hosts. Transmission of pathogens was assessed by measuring copies of flaB normalized against mouse β-actin levels in indicated murine tissues 10 days after tick feeding. The bb0405 mutants (Mut1, left panel; Mut2, right panel) were undetectable (asterisks) in mice. This figure is available in black and white in print and in color online.
BB0405 Immunization Evokes Robust and Long-Lasting Humoral Responses Resulting in Protective Immunity Against Tick-Transmitted Infection
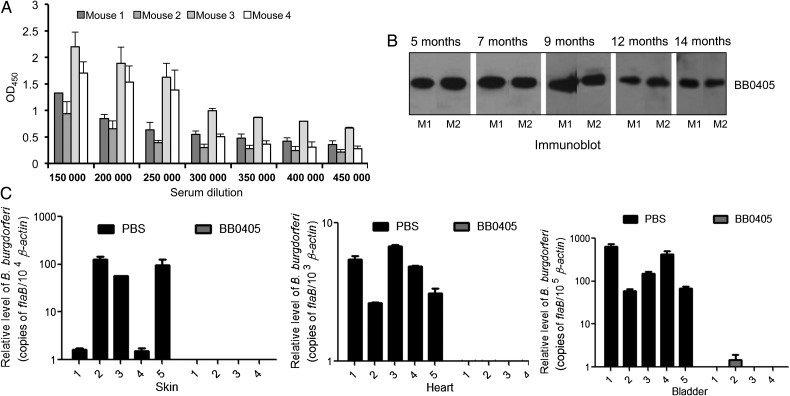
As BB0405 is a conserved antigen with antibody-accessible surface epitopes and is important for infection, we next assessed whether BB0405 immunization could elicit protective immunity in mice against tick-borne spirochete infection. To accomplish this, we produced recombinant BB0405 in E. coli and immunized groups of C3H mice (4–5 animals/group) with either purified protein or PBS (control) emulsified with the same volume of adjuvant. An ELISA and immunoblotting performed after final boosting indicated that all mice developed strong antibody titers (>1:450 000) that specifically recognized recombinant BB0405 (Figure 5A). The BB0405 antibodies in immunized rodents also lasted for a long time, as immunoblot analyses using equal amounts of recombinant BB0405 and sera collected at monthly intervals indicated strong responses even at the latest time point examined, 14 months after final boosting (Figure 5B).
Figure 5.
Active immunization of mice with recombinant BB0405 induces robust and long-lasting antibody responses and interferes with spirochete transmission from ticks. A, Enzyme-linked immunosorbent assay (ELISA) showing development of high-titer serum antibodies induced in recombinant BB0405-immunized mice. Groups of C3H mice (5 animals/group) were immunized with BB0405 or phosphate-buffered saline (PBS; control) mixed with an equal amount of adjuvant. Ten days after the final immunization, serum was collected and subjected to an ELISA. Wells were coated with recombinant BB0405 and probed with serially diluted normal mouse serum (NMS) or BB0405 antiserum. The data represent ODs for BB0405 titer after subtraction of corresponding NMS values. While 1 mouse generated low-titer antibodies (not shown), each of the other 4 mice developed very high-titer (>1:450 000) antisera. B, Immunoblot analysis showing long-lasting BB0405 antibodies in mice. Animals were immunized with recombinant protein as described in panel A, and serum samples were collected at weekly and monthly intervals until 14 months after immunization. While comparable levels of BB0405 antibody reactivity were recorded at all time points and in individual animals, a few representative times points for 2 mice (M1 and M2) are presented. C, Mice were immunized as described in panel A, and 10 days after the second boost, B. burgdorferi–infected nymphs were allowed to engorge on the naive immunized mice (5 ticks/mouse). Murine tissues were assessed for spirochete transmission 12 days after tick feeding. Total RNA was isolated, and flaB was measured using quantitative reverse transcription–polymerase chain reaction and normalized to the amount of mouse β-actin. The levels of B. burgdorferi in BB0405-immunized mice were either undetectable or significantly lower than in PBS-immunized animals (P < .05).
To assess vaccine potential of recombinant BB0405, mice (6 animals/group) were immunized with the antigen as detailed in Figure 5A, and 10 days following final immunization, they were infected with B. burgdorferi via syringe inoculation (105 cells/animal). Similar levels of pathogens were detected in all groups (Supplementary Figure 3), suggesting that BB0405 immunization does not affect pathogen burden in infected hosts. To assess whether BB0405 immunization blocks transmission of B. burgdorferi from the vector, mice were similarly immunized with BB0405 (at least 4 animals/group) and then challenged with B. burgdorferi–infected ticks (5 nymphs/mouse). While spirochete burden in repleted ticks was similar for all groups, a significant reduction was recorded in all tested tissues of mice immunized with BB0405, compared with the controls (Figure 5C), suggesting that BB0405 is a novel target for transmission-blocking Lyme disease vaccines.
BB0405 Antibodies Are Undetectable During Natural Infection and Lack Borreliacidal Activity In Vitro in the Absence of Complement
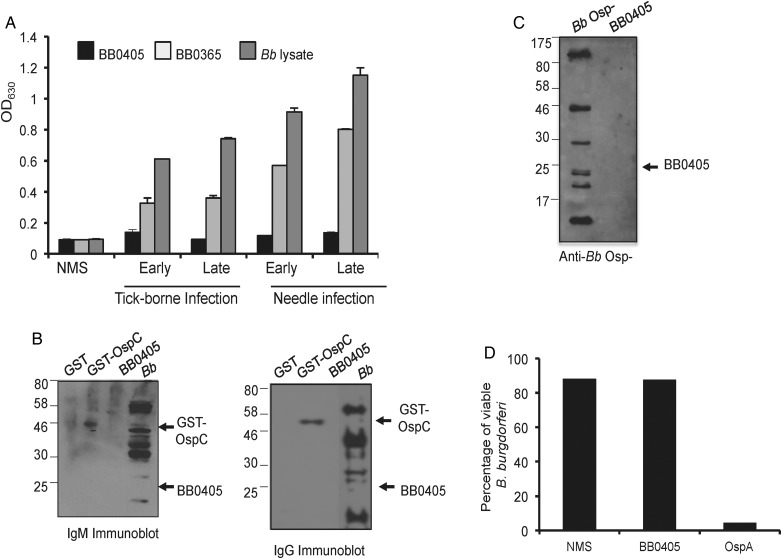
To further understand BB0405 function in borrelial infectivity and as a vaccine target, we studied whether mice develop BB0405-specific humoral immune responses during infection. Serum samples were collected from mice infected either with syringe-inoculated or tick-transmitted B. burgdorferi at various times representative of early or late infection, and antibody titers against control proteins, La7 [32] and borrelial lysate, were measured using an ELISA. While antibody responses against the control subsurface membrane protein La7 and B. burgdorferi were detected, we are unable to record a response against BB0405, irrespective of the infection route (syringe or ticks) or phase of infection (Figure 6A). A subsequent immunoblot analysis further confirmed that, while immunoglobulin M or immunoglobulin G responses against another recombinant borrelial protein, OspC, are detectable, BB0405 remained unrecognizable (Figure 6B). Parallel studies performed using additional mouse strains (B6 and BALB/c) show that neither strain developed antibodies against BB0405 during infection (data not shown). Additionally, immunoblot analysis using 5 serum samples collected from healthy individuals and 5 patients with diagnosed Lyme disease also suggested that BB0405-specific antibody responses were absent during human infection (data now shown). To assess whether surface production of a few known abundant Osps masks BB0405 epitopes and interferes with antibody development, we performed infection studies involving a borrelial mutant that we previously characterized as lacking abundant outer surface proteins OspA, OspB, and OspC [30]. Serum samples collected from groups of mice infected with a larger inoculum of Osp mutants (107 cells/animal) displayed antibody responses against a few antigens in borrelial lysate, but we were still unable to detect presence of anti-BB0405 antibodies (Figure 6C). Despite the ability of BB0405 antibodies to bind to the surface of cultured spirochetes (Figure 1D) and their reported borreliacidal activity in the presence of guinea pig complement [27], no such bactericidal activity was recorded when B. burgdorferi cells were exposed to antibodies against BB0405 in the absence of active complement, although antibodies directed against OspA readily killed the spirochetes (Figure 6D).
Figure 6.
BB0405 antibodies are undetectable during murine infection and lack in vitro bactericidal activities in the absence of active complement. A, BB0405-specific immunoglobulin G (IgG) responses are undetectable during spirochete infection of mice. Serum samples were collected from mice infected with Borrelia burgdorferi either by syringe inoculation (105 cells/mouse) or via tick challenge at early (10–14 days) and late (21–36 days) infection. Antigen-specific IgGs were detected using a standard enzyme-linked immunosorbent assay having wells coated with recombinant BB0405 or a subsurface membrane antigen, BB0365, or B. burgdorferi lysate. B, Immunoblot analysis demonstrating lack of anti-BB0405 immunoglobulin M (IgM) or IgG antibodies in infected hosts. Recombinant BB0405, control protein GST-OspC, and whole B. burgdorferi–cell lysate were probed with serum samples collected from mice infected with tick-transmitted pathogens after 20 days of infection. C, BB0405 antibodies are undetectable in mice infected with a high dose of mutants lacking abundant Osps. Mice were infected with mutants lacking OspA, OspB, and OspC (107 cells/animal), and serum samples were collected after 14 days of infection and used in an immunoblot analysis with recombinant BB0405 and mutant spirochete lysate. D, In vitro borreliacidal activities of antibodies generated against BB0405. B. burgdorferi was incubated with normal mouse serum or BB0405 or OspA antisera in the absence of active complement. The number of viable spirochetes was determined by dark-field microscopy. BB0405 antisera did not show a significant difference in borreliacidal activities as compared to that of normal serum (P > .05), while OspA antibodies significantly killed the spirochetes (P < .002).
DISCUSSION
The genome of the Lyme disease pathogen reflects notable structural and functional redundancy, as evidenced by the large number of paralogous genes, as well as experimental demonstrations that certain genes are nonessential for infectivity [6, 11]. Nevertheless, most of the spirochete membrane proteins that are differentially produced at a given time and in a specific tissue support bacterial persistence [8]. Our study suggested that, although bb0405 is downregulated in feeding ticks during borrelial transmission and/or within a mammalian host, the gene product is indispensable for infectivity. bb0405 was first identified as a temperature-induced gene [31] that can also be upregulated in mammalian host-adapted spirochetes [27]. This protein represents one of the few examples of spirochete integral outer membrane proteins [33], along with P13, P66, BesC, BamA, and Lmp1 [34], that are predominantly encoded by chromosomal genes [34] serving vital functions in borrelial infectivity [35–37]. The gene product might support replicative growth of spirochetes, as we observed impaired survival of bb0405 mutants, particularly in mid-log phase of culture. Although BB0405 antibodies are unable to exert a microbicidal effect in the absence of complement, their borreliacidal properties in the presence of guinea pig complement, as well as growth-inhibiting effects, were previously reported [27]. Additionally, as described for other borrelial transmembrane proteins, such as P66 [35], BB0405 could have additional or alternate functions, including roles in host-pathogen interaction and B. burgdorferi dissemination through the host.
Being an extracellular pathogen, B. burgdorferi is subjected to the humoral immune response. However, despite detectable expression of the gene during spirochete infection of mice, BB0405 remained invisible to host humoral immune responses. This is remarkable, as a recombinant version of the protein antigen is highly immunogenic and also resulted in long-lasting antibody development. Thus, unlike in cultured spirochetes, BB0405 may be hidden under the microbial surface during mammalian infection or become masked by abundant neighboring outer membrane proteins, as previously described for P66 [38]. This also explains why active immunization of mice with BB0405, despite providing high and specific antibody responses, resulted in nonprotective host immunity against syringe-inoculated spirochetes. On the other hand, notwithstanding a redundant role of BB0405 in spirochete persistence in feeding ticks, immunization of hosts conferred protection against tick-transmitted infection. It is likely that BB0405 antibodies enter the feeding tick gut with the ingested blood meal. Relative abundance or topological distribution of the antigen on the spirochete surface possibly facilitates antibody binding, blocking of BB0405 function, or interference with other antigens via steric hindrance or another unknown mechanism that ultimately obstructs pathogen transmission to the hosts. Because of the apparent immune invisibility, BB0405 is unlikely to produce an anamnestic response in vaccinated hosts during subsequent spirochete infection. However, such a secondary immune response is possibly of lesser importance for transmission-blocking vaccine candidates like BB0405, as high-titer antibodies must block pathogen dissemination to the tick bite site, preventing early stages of the infection. Identification of conserved and ubiquitously expressed borrelial proteins like BB0405 that are not only critical for microbial virulence but also represent preventive targets against spirochete infection is important for development of second-generation Lyme disease vaccines.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank John Anderson and Adriana Marques for their assistance with this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (award R01AI080615 to U. P.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol 2008; 53:323–43. [DOI] [PubMed] [Google Scholar]
- 2.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am 2015; 29:187–210. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest 2004; 113:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379:461–73. [DOI] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 2012; 10:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Silva AM, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest 1997; 99:377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kung F, Anguita J, Pal U. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol 2013; 8:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casjens S, Palmer N, van Vugt R et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 2000; 35:490–516. [DOI] [PubMed] [Google Scholar]
- 10.Casjens SR, Mongodin EF, Qiu WG et al. Whole-genome sequences of two Borrelia afzelii and two Borrelia garinii Lyme disease agent isolates. J Bacteriol 2011; 193:6995–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser CM, Casjens S, Huang WM et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997; 390:580–6. [DOI] [PubMed] [Google Scholar]
- 12.Schutzer SE, Fraser-Liggett CM, Casjens SR et al. Whole-genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol 2011; 193:1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 2005; 3:129–43. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A 2001; 98:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 1990; 250:553–6. [DOI] [PubMed] [Google Scholar]
- 16.Steere AC, Sikand VK, Meurice F et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med 1998; 339:209–15. [DOI] [PubMed] [Google Scholar]
- 17.de Silva AM, Telford SR, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med 1996; 183:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm D, Tilly K, Byram R et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 2004; 101:3142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis 2010; 201:1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal U, Yang X, Chen M et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest 2004; 113:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet 2012; 46:515–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity 2010; 33:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sironi M, Cagliani R, Forni D, Clerici M. Evolutionary insights into host-pathogen interactions from mammalian sequence data. Nat Rev Genet 2015; 16:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi P, Wang N. Host immune responses accelerate pathogen evolution. The ISME journal 2014; 8:727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indest KJ, Howell JK, Jacobs MB, Scholl-Meeker D, Norris SJ, Philipp MT. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect Immun 2001; 69:7083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi J, Schneider B, Messer WB, Piesman J, de Silva AM. Genetic variation at the vlsE locus of Borrelia burgdorferi within ticks and mice over the course of a single transmission cycle. J Bacteriol 2003; 185:4432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks CS, Vuppala SR, Jett AM, Akins DR. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun 2006; 74:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, Pal U. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun 2010; 78:4477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Qin J, Promnares K, Kariu T, Anderson JF, Pal U. Novel microbial virulence factor triggers murine Lyme arthritis. J Infect Dis 2013; 207:907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Promnares K, Qin J et al. Characterization of multiprotein complexes of the Borrelia burgdorferi outer membrane vesicles. J Proteome Res 2011; 10:4556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojaimi C, Brooks C, Casjens S et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun 2003; 71:1689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Hegde S, Shroder DY et al. The lipoprotein La7 contributes to Borrelia burgdorferi persistence in ticks and their transmission to naive hosts. Microbes Infect 2013; 15:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostberg Y, Pinne M, Benz R, Rosa P, Bergstrom S. Elimination of channel-forming activity by insertional inactivation of the p13 gene in Borrelia burgdorferi. J Bacteriol 2002; 184:6811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenedy MR, Lenhart TR, Akins DR. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol 2012; 66:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coburn J, Leong J, Chaconas G. Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol 2013; 21:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koebnik R, Locher KP, Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 2000; 37:239–53. [DOI] [PubMed] [Google Scholar]
- 37.Schulz GE. The structure of bacterial outer membrane proteins. Biochim Biophys Acta 2002; 1565:308–17. [DOI] [PubMed] [Google Scholar]
- 38.Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun 1999; 67:2874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.