Abstract
Canine leishmaniasis (CanL) is a chronic fatal disease of dogs and a major source of human infection through propagation of parasites in vectors. Here, we infected 8 beagles through multiple experimental vector transmissions with Leishmania infantum–infected Lutzomyia longipalpis. CanL clinical signs varied, although live parasites were recovered from all dog spleens. Splenic parasite burdens correlated positively with Leishmania-specific interleukin 10 levels, negatively with Leishmania-specific interferon γ and interleukin 2 levels, and negatively with Leishmania skin test reactivity. A key finding was parasite persistence for 6 months in lesions observed at the bite sites in all dogs. These recrudesced following a second transmission performed at a distal site. Notably, sand flies efficiently acquired parasites after feeding on lesions at the primary bite site. In this study, controlled vector transmissions identify a potentially unappreciated role for skin at infectious bite sites in dogs with CanL, providing a new perspective regarding the mechanism of Leishmania transmissibility to vector sand flies.
Keywords: canine leishmaniasis, Leishmania infantum, Lutzomyia longipalpis, dogs, reservoir, vector transmission, skin, bite site, infectivity, parasite-pickup
Canine leishmaniasis (CanL) in dogs is a fatal chronic disease caused by Leishmania infantum [1]. CanL is also a public health concern, since infected dogs represent the most significant risk factor for acquisition of zoonotic visceral leishmaniasis (ZVL) in humans [2].
In long-term prospective studies in areas of endemicity, a limited percentage of infected dogs become sick, the rest remaining in apparent good health for many years [3–7]. Various infection models of CanL produced a variable proportion of clinical signs in dogs [2, 8–16]. Despite a parasite inoculum well in excess of what is injected by a sand fly [17–19], most studies did not present clear evidence that dogs became sick due to CanL.
In naturally infected dogs, the major clinical signs of CanL include anorexia, lymphadenopathy, and hepatosplenomegaly [6], and renal disease is generally the main cause of death [20–22]. Another clinical feature of CanL is the manifestation of skin lesions in a proportion of clinically patent dogs [22]. Infected dogs that develop a Leishmania-specific cellular immune response characterized by the production of interferon γ (IFN-γ) and tumor necrosis factor α and a positive leishmanin test result show low parasite loads and control the disease [2, 23], while those with increased levels of interleukin 10 (IL-10), transforming growth factor β, and antibodies to Leishmania tend to have high parasite loads [2].
The relative contribution of symptomatic and asymptomatic dogs in propagating L. infantum in vector populations, the backbone of maintaining ZVL foci, remains unclear. Skin lesions observed in symptomatic dogs have been associated with the ability to transmit L. infantum back to the sand fly population [22, 24, 25]. However, the significance of intact skin in transmission to sand flies is less clear. By use of xenodiagnoses, some studies concluded that only symptomatic dogs act as reservoirs [24, 26, 27] and that only a small proportion of dogs with high skin parasite loads, correlated to more-severe disease, are infectious to sand flies [5, 28]. In contrast, other studies showed that both symptomatic and asymptomatic dogs were infectious to sand flies [7, 29–32]. In one study, L. infantum was isolated from intact skin of 78.7% of seropositive dogs, of which only 21.9% were classified as symptomatic [30]. By use of xenodiagnoses, another study detected parasites by polymerase chain reaction analysis in sand flies fed on asymptomatic dogs [32]. Unfortunately, CanL studies suffer from a lack of consistency in the definition of asymptomatic dogs, which may have factored in the contradictive conclusions reported above. Since examination of the bite sites in naturally infected dogs is not possible, skin at the site of infected bites has never been considered as a source of parasites or as a contributor to the cycle of parasite transmission between dog and sand fly.
Here, we report that experimental vector transmission of CanL by Lutzomyia longipalpis, the natural vector of L. infantum, reveals long-term persistence of parasites at bite sites in the skin of infected dogs, irrespective of their disease status. This study puts forward a novel potential mechanism by which dogs propagate Leishmania in vector populations and reveals the refined efficacy of infected dogs as disease reservoirs for L. infantum, the etiological agent of human ZVL.
MATERIALS AND METHODS
Animals and Parasites
Dogs (Marshall Farms, New York) were housed in 2 groups: dogs 1592, 1516, 2912, and 1702 were in one group, and dogs 2474, 5635, 1605, and 3080 were in the other group. The dogs were genotyped on the basis of single-nucleotide polymorphism markers within the Slc11a1 gene [33]; dog 1516 and dog 2912 were found to be heterozygous. Animal experimental procedures were approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee under animal protocol LMVR 7E. L. infantum (MCAN/IT/11/10545) parasites were recently isolated from an area of endemicity in Naples, Italy. Amastigotes or early culture passages (P0–P4) were used for sand fly infections.
Sand Fly Infection
L. longipalpis females were infected by artificial feeding on defibrinated rabbit blood containing 5 million parasites and 35 µL of penicillin/streptomycin (10 000 units of penicillin/10 mg streptomycin, Gibco, Grand Island, New York) per milliliter of blood as previously described [34].
Vector Transmission
Individual midguts of infected sand flies were macerated manually in 50 µL of phosphate-buffered saline (PBS) with a pestle (Kimble Chase, Vineland, New Jersey) to determine the parasite load and percentage of metacyclics per midgut, using a hemocytometer (Supplementary Figure 1A). Metacyclics were distinguished by morphology and motility. Each dog was exposed to 20 infected sand flies, using custom-made feeders (Supplementary Figure 5) applied to the neck or belly in the dark for 2 hours. Thereafter, the number of blood-fed flies and their infection status were determined for each dog (Supplementary Figure 1B). Flies were assigned a transmissible-infection status when numerous active metacyclic promastigotes were present (Supplementary Video 1).
Serodiagnostic Indirect Fluorescence Antibody Testing (IFAT) and Enzyme-Linked Immunosorbent Assay (ELISA)
For IFAT, detection of anti-Leishmania immunoglobulin G (IgG) antibodies was performed using L. infantum promastigotes (World Health Organization reference strain MHOM/TN/1980/IPT-1) as antigen and following the protocol recommended for canine leishmaniasis by the Office International des Epizooties [35, 36]. For ELISA, 100 µL of L. longipalpis salivary gland homogenate diluted to 1 pair/mL or 100 µL of Leishmania antigen (SLA, 10 µg/mL) were used to coat plates. Serum was used at a ratio of 1:50, alkaline phosphatase-conjugated anti-dog IgG (dilution, 1:5000; Jackson ImmunoResearch, West Grove, Pennsylvania) was used as a secondary antibody, and the reaction was developed using p-nitrophenyl phosphate liquid substrate system (Promega, Madison, Wisconsin). Absorbance was measured at 405 nm.
Blood Collection and Splenic and Bone Marrow Aspiration
Blood and spleen and bone marrow aspirates were collected every 2–3 months from all dogs.
Clinical Evaluation and Blood Chemistry
All dogs were clinically examined every 2 weeks for up to 22 months for the presence and severity of clinical signs associated with CanL. Blood and urine specimens were collected monthly starting at month 6, for analysis of the whole-blood chemistry panel and determination of the urine protein to creatinine ratio (ANTECH Diagnostic Laboratories, Rockville, Maryland).
Parasite Detection
Skin lesion specimens and spleen and bone marrow aspirates were cultured in biphasic medium and checked weekly under light microscopy for 1 month. The parasite load was determined by a limiting dilution assay as previously described [37, 38]. After necropsy, the submandibular, prescapular, and popliteal lymph nodes and a pool of 3 sections from each spleen (from medial and lateral parts) per dogs were macerated aseptically through 70-µm cell strainers in complete Schneider's for parasite load assessment.
Cytokine Detection
Peripheral blood mononuclear cells (PBMCs) were isolated as previously described [39]. One million cells per well were cultured for 6 days in complete Roswell Park Memorial Institute medium alone, with SLA (50 µg/mL) at day 0 or with ConA (2.5 µg/mL) at day 3. Cytokine production levels were measured using a Multiplex Luminex assay (Procarta Canine Cytokine Assay kit, Affymetrix, Santa Clara, California) in supernatants collected at day 6, and results were read on the Luminex instrument.
Xenodiagnosis
Dogs were exposed to 20 uninfected sand flies per site for 1 hour in the dark. Sand flies were placed against normal dog skin, the original site of transmission, and at observed skin lesions. Two days after exposure, blood-fed flies were dissected and examined by microscopy for the presence of live Leishmania parasites.
Leishmanin Test
SLA was diluted in PBS to a final concentration of 2.5 mg/mL. Each dog was intradermally injected with 100 µL of SLA (250 µg) in the abdomen. Forty-eight hours after injection, the mean of 2 perpendicular diameters of the induration area was calculated. Skin indurations of >5 mm in diameter were considered positive [40].
Histopathologic Analysis
At necropsy, spleen tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin. Slides were examined blindly.
Statistical Analysis
Correlation graphs are presented with a linear regression slope, and dotted lines illustrate the 95% confidence interval. Statistical differences between groups were tested by 1-way analysis of variance, followed by the Fisher least significant difference post hoc test. Correlations were tested by the Spearman test; the distribution normality of samples was determined by the Shapiro–Wilk test. We considered P values of <.05 as statistically significant. All statistical analyses were performed using GraphPad Prism software (La Jolla, California).
RESULTS
Clinicopathological Manifestations After Controlled Vector Transmission of L. infantum to Beagles Revealed Parasite Persistence at Infected Bite Sites in the Skin
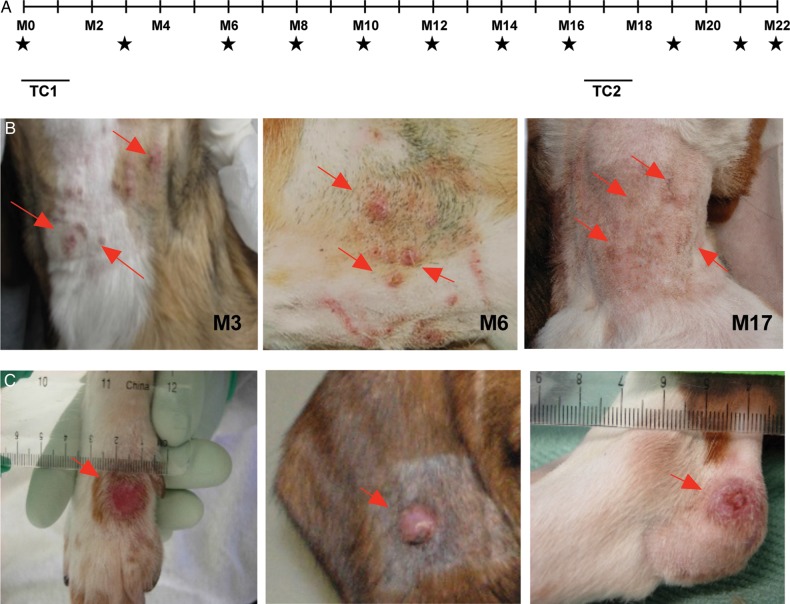
L. longipalpis was infected with L. infantum and maintained until a good proportion of sand flies held transmissible infections (Supplementary Figure 1A and 1B and Supplementary Video 1). Eight naive beagles were infected via vector bites occurring during 2 transmission clusters, transmission cluster 1 (TC1), on the ventral neck at month 0; and transmission cluster 2 (TC2), on the lower abdomen at month 17, to mimic transmission recurrence during a consecutive sand fly season (Figure 1A). The percentage of blood-fed flies harboring transmissible infections, used as an indicator of transmission success, was comparable among dogs (Supplementary Figure 1C). Animals underwent a thorough monthly clinical examination until month 22, the study end point. During the follow-up period, serum specimens, blood specimens, and spleen and bone marrow aspirates were collected bimonthly (Figure 1A).
Figure 1.
Eight beagles were subjected to 2 transmission clusters composed of 9 and 7 vector transmissions. A, Timeline of transmissions and follow-up. B, Evolution of lesions at bite sites in representative dog 1605 at month 3 M3, M12, and M17 after vector transmission. C, Lesions distal to the bite site in representative dogs 3080, 2912, and 1516. Stars denotes times of xenodiagnoses, spleen and bone marrow aspiration, and blood collection (plasma and peripheral blood mononuclear cells). Arrows point to lesions. Abbreviations: TC1, transmission cluster 1; TC2, transmission cluster 2.
The first clinical sign observed in all dogs was the presence of nodular or ulcerative skin lesions (Figure 1B, Supplementary Figure 2, and Table 1), located at the original site of vector transmission (ventral neck) and accompanied by enlarged submandibular lymph nodes. Importantly, skin lesions from all dogs tested positive for Leishmania in culture until month 6 after transmission (Table 1). Thereafter, Leishmania isolation fluctuated over time, with the majority of lesion aspirates being positive at any time during the study (Table 1). In some dogs, the lesion at the original site of transmission persisted and was long-lasting (Table 1 and Supplementary Figure 2). Importantly, after TC2, we observed a reactivation of primary skin lesions in 7 of 8 dogs and an increased percentage of cultures positive for Leishmania (Table 1), although no visible lesions were seen at sites of TC2. Prior to TC2, 5 dogs also developed skin lesions in locations distal to the original bite site (Figure 1C and Table 2). Overall, distal lesions displayed a lower rate of Leishmania culture positivity as compared to the lesions at the original site of sand fly bites (Tables 1 and 2). However, the rate of culture positivity among distal lesions also increased after TC2. Compared with only 0–2 distal lesions per dog, dog 3080 presented the most-severe clinical signs, with lesions at multiple distal sites that were 100% culture positive throughout the study (Supplementary Figure 3).
Table 1.
Prevalence of Leishmania Skin Lesions at the Site of Transmission Cluster 1 (Ventral Neck)
| Dog ID | Mo 3 | Mo 6 | Mo 8 | Mo 10 | Mo 12 | Mo 14 | Mo 16 | Mo 19 | Mo 21 |
|---|---|---|---|---|---|---|---|---|---|
| 1592 | |||||||||
| Lesion | + | + | − | − | + | + | − | − | + |
| Culture | + | + | NA | NA | − | − | NA | NA | − |
| 1516 | |||||||||
| Lesion | + | + | − | − | − | − | − | − | − |
| Culture | + | + | NA | NA | NA | NA | NA | NA | NA |
| 2474 | |||||||||
| Lesion | + | + | + | − | − | − | + | + | + |
| Culture | + | + | + | NA | NA | NA | − | + | − |
| 5635 | |||||||||
| Lesion | + | + | − | − | − | − | − | + | + |
| Culture | + | + | NA | NA | NA | NA | NA | − | − |
| 2912 | |||||||||
| Lesion | + | + | + | + | + | + | + | − | + |
| Culture | + | + | + | + | − | + | − | NA | + |
| 1702 | |||||||||
| Lesion | + | + | − | + | + | + | − | + | + |
| Culture | + | + | NA | + | + | + | NA | + | + |
| 1605 | |||||||||
| Lesion | + | + | − | − | + | + | + | + | + |
| Culture | + | + | NA | NA | + | − | − | + | + |
| 3080 | |||||||||
| Lesion | + | + | + | + | + | − | + | + | + |
| Culture | + | + | + | + | + | NA | − | + | + |
| Overall positivity, % | |||||||||
| Lesion | 100 | 100 | 37.5 | 37.5 | 62.5 | 50 | 50 | 62.5 | 87.5 |
| Culture | 100 | 100 | 100 | 100 | 60 | 50 | 0 | 80 | 57 |
Transmission cluster 2 (abdomen) began at mo 17.
Abbreviations: −, negative; +, positive; NA, not applicable.
Table 2.
Prevalence of Leishmania-Positive Skin Lesions Distal From the Transmission Sites
| Dog ID | Mo 3 | Mo 6 | Mo 8 | Mo 10 | Mo 12 | Mo 14 | Mo 16 | Mo 19 | Mo 21 |
|---|---|---|---|---|---|---|---|---|---|
| 1592 | |||||||||
| Lesion | + | + | − | − | − | − | − | − | − |
| Culture | − | − | NA | NA | NA | NA | NA | NA | NA |
| 1516 | |||||||||
| Lesion | − | − | − | + | + | − | + | + | + |
| Culture | NA | NA | NA | − | − | NA | − | − | − |
| 2474 | |||||||||
| Lesion | − | − | − | − | − | − | − | − | − |
| Culture | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5635 | |||||||||
| Lesion | − | − | − | − | − | − | − | − | − |
| Culture | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2912 | |||||||||
| Lesion | − | − | + | + | − | + | + | + | + |
| Culture | NA | NA | + | − | NA | − | − | + | + |
| 1702 | |||||||||
| Lesion | − | − | + | + | + | + | + | + | + |
| Culture | NA | NA | NA | + | − | − | + | + | − |
| 1605 | |||||||||
| Lesion | − | − | − | − | − | − | − | + | + |
| Culture | NA | NA | NA | NA | NA | NA | NA | − | + |
| 3080 | |||||||||
| Lesion | + | + | + | + | + | + | + | + | + |
| Culture | + | + | + | + | + | + | + | + | + |
| Overall positivity, % | |||||||||
| Lesion | 25 | 25 | 37.5 | 50 | 37.5 | 66 | 50 | 62.5 | 62.5 |
| Culture | 50 | 50 | 66.6 | 50 | 33 | 33 | 50 | 60 | 60 |
Transmission cluster 2 (abdomen) began at mo 17.
Abbreviations: −, negative; +, positive; NA, not applicable.
We also addressed the transmissibility of Leishmania in normal skin (located away from the sites of vector transmission) to sand flies. Each dog was exposed to uninfected sand flies every 2 months throughout the study period (Supplementary Table 1). Xenodiagnosis from normal skin was negative in all dogs, apart from dog 3080, which became positive from month 14 until the study end point, at month 22. The rate of sand fly positivity in normal skin in dog 3080 was low at month 14, with 4.7% efficiency, but increased to 10% and 14% at month 16 and month 19, respectively (Supplementary Tables 1 and 2). In contrast, when uninfected sand flies were exposed to 2 dogs with lesions at month 12, sand flies picked-up parasites successfully and efficiently from the original site of transmission, similar to distal lesions at month 12 and month 14 after transmission (Supplementary Table 2), demonstrating the accessibility of parasites from lesions at bite sites to sand flies.
No significant weight loss was observed in any of the infected dogs throughout the study period (Supplementary Figure 4A). Complete blood count and blood chemistry evaluations were normal apart from the parameters displayed in Supplementary Figure 4B. Values for dog 3080 fell outside the normal range for the total protein level, the albumin to globulin ratio, the aspartate aminotransferase (AST) level, the ratio of blood urea nitrogen (BUN) level to creatinine level, and the ratio of urine protein level to urine creatinine level (Supplementary Figure 4B). The ratio of BUN to creatinine levels and the ratio of urine protein to urine creatinine levels exceeded the upper limit of the normal range for dogs 1592, 1516, and 2474 and dogs 1605, 1702, and 2912, respectively (Supplementary Figure 4B).
Isolation of Live L. infantum From Splenic Aspirates From Beagles After Controlled Vector Transmission
Dogs 1702, 1605, and 3080 had positive spleen aspirates continuously starting at month 12, month 19, and month 6, respectively (Table 3). Spleen aspirates from 2 animals (dogs 5635 and 2912) were culture positive at 2 consecutive time points (month 6 and month 8; Table 3). By month 16, live parasites were also recovered from dogs 1516 and 2474 at least once. Importantly, after necropsy, performed at month 22, live parasites were detected in spleen biopsy specimens of all 8 dogs, demonstrating a 100% parasite visceralization (Table 3).
Table 3.
Parasite Detection in Culture of Spleen Aspirates Following Experimental Vector Transmission to Eight Dogs
| Dog ID | Mo 0 | Mo 3 | Mo 6 | Mo 8 | Mo 10 | Mo 12 | Mo 14 | Mo 16 | Mo 19 | Mo 21 | Mo 22a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1592 | − | − | − | − | − | − | − | − | − | − | + |
| 1516 | − | − | − | − | − | − | − | + | − | − | + |
| 2474 | − | − | − | − | − | − | + | − | − | − | + |
| 5635 | − | − | + | + | − | − | − | − | + | − | + |
| 2912 | − | − | + | + | − | − | + | − | − | + | + |
| 1702 | − | − | − | − | − | + | + | + | + | + | + |
| 1605 | − | − | − | − | − | − | − | − | + | + | + |
| 3080 | − | − | + | + | + | + | + | + | + | + | + |
| Positivity, % | 0 | 0 | 37.5 | 37.5 | 12.5 | 25 | 50 | 37.5 | 50 | 50 | 100 |
Transmission cluster 2 (abdomen) began at mo 17.
Abbreviations: −, negative; +, positive.
a Parasites at mo 22 were grown from spleen tissue after necropsy.
Pathology and Leishmanin Test Reactions 22 Months After Controlled Vector Transmission of L. infantum to Beagles
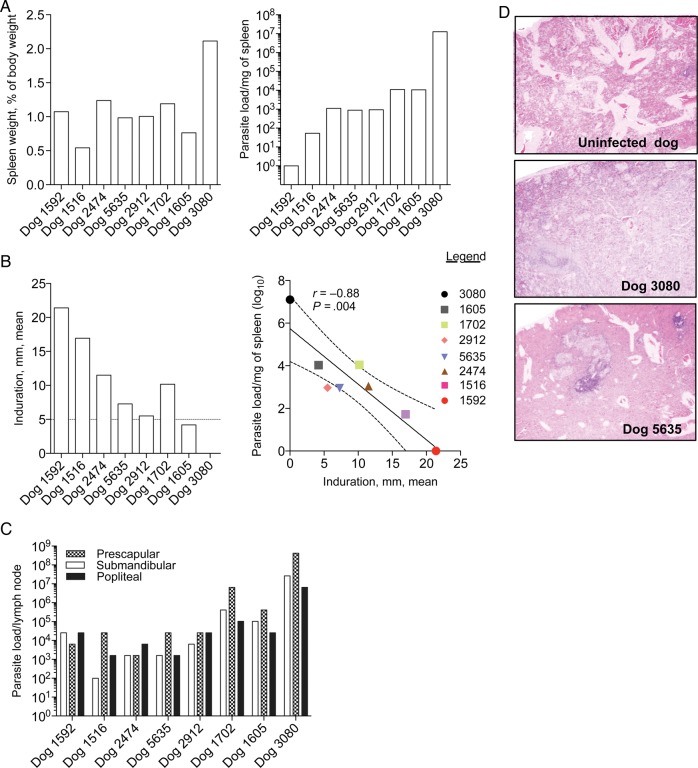
After necropsy (at month 22), dog 3080 showed the highest spleen to body weight ratio and splenic parasite load, while dogs 1952 and 1516 had low spleen to body weight ratios and low splenic parasite loads (Figure 2A). Dogs 1605 and 1702 had the highest splenic parasite loads, after dog 3080 (Figure 2A). Prior to euthanasia, a leishmanin test was performed as a surrogate of cellular immunity to Leishmania. Forty-eight hours after intradermal injection of Leishmania antigen, dogs 1592 and 1516 displayed the highest mean induration size (21.4 mm and 16.9 mm, respectively), while dogs 3080 and 1605 had negative reactions, showing no detectable or a minor induration (size, 4.2 mm), respectively (Figure 2B). Dogs 2474, 1702, 5635, and 2912 presented positive reactions, with mean induration sizes of 11.5 mm, 10.1 mm, 7.2 mm, and 5.5 mm, respectively (Figure 2B). Of note, the mean induration size yielded by the leishmanin test correlated negatively with the splenic parasite load (r = −0.88; P = .004; Figure 2B). The submandibular, prescapular, and popliteal lymph nodes were Leishmania positive at month 22, with dogs 3080, 1605, and 1702 showing a considerably higher number of parasites in all 3 lymph nodes, compared with the rest of the dogs (Figure 2C). Upon detailed examination, the spleens often contained multifocal to coalescing pale tan nodules throughout, ranging from 2 mm to 1 cm in diameter, and tended to be larger and more numerous in dogs 3080, 1605, and 1702 (data not shown). Histologically, changes in the spleen of infected animals ranged from some vascular congestion (in dog 1592) to severe histiocytosis with multiple small granulomas (in dog 3080). Granulomas were characterized by a central core of macrophages admixed with neutrophils and few multinucleated giant cells surrounded by a thin rim of lymphocytes. Histopathological findings of the spleen for 2 representative dogs, dog 3080 and dog 5635, are shown with those for an uninfected dog in Figure 2D.
Figure 2.
Pathology and leishmanin test reactions 22 months after experimental vector transmission to beagles. A, Spleen weight as a percentage of body weight (left) and parasite load in homogenized spleen tissue (right). B, The left panel shows leishmanin test indurations measured 48 hours after intradermal injection of soluble Leishmania antigen prior to necropsy. The mean of 2 perpendicular measurements delimiting the induration area is given. A mean induration of ≥5 mm (dotted line) was considered positive. The right panel shows a negative correlation between splenic parasite load and leishmanin test positivity. The solid line represents the mean. C, Parasite loads per lymph node for submandibular, prescapular, and popliteal lymph nodes. Parasite loads were determined by a limiting dilution assay. D, Hematoxylin and eosin–stained sections of the most affected part of the spleen in representative dogs 3080 and 5635. A section from the spleen of an uninfected dog was used as control. Images were taken at the magnification ×40.
Progression of Humoral and Cellular Anti-Leishmania Immunity Over 22 Months After Controlled Vector Transmission to Beagles
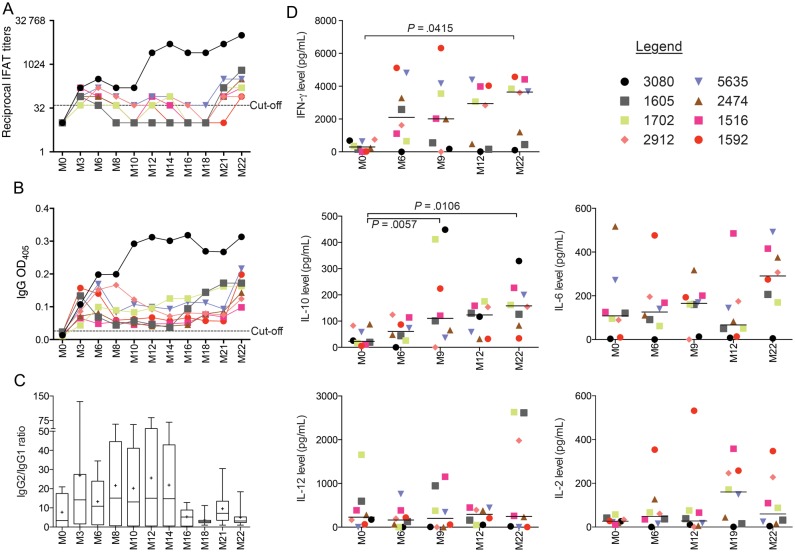
Three months following TC1, 75% (6 of 8 dogs) and 100% (8 of 8) developed specific anti-Leishmania antibodies, according to IFAT and ELISA, respectively (Figure 3A and 3B). After this initial spike, IFAT and ELISA titers decreased in all dogs but dog 3080. Most dogs maintained low titers over time, spiking again at month 22, about 3 months following TC2 (Figure 3A and 3B). In contrast, IFAT and ELISA titers for dog 3080 rose steadily over time and were considerably higher than those for the other dogs. IgG2 antibody titers predominated during the first year following transmission, after which the IgG2 to IgG1 ratio dropped sharply, from a mean of 25.59 at month 12 to a mean of 5.05 at month 22 (Figure 3C).
Figure 3.
Development of Leishmania-specific immunity in beagles after experimental vector transmission. A, Eight beagles were followed serologically for 22 months by indirect fluorescence antibody testing (IFAT; A) and enzyme-linked immunosorbent assay (ELISA; B). Dotted lines indicate IFAT and ELISA cutoffs. A titer of 1:40 was used as a cutoff for the IFAT-positive response. The mean values ( + 2 SDs) for sera from 6 uninfected dogs were used to calculate the ELISA cutoff. C, Box plot of the ratio of immunoglobulin G2 (IgG2) to IgG1 for the 8 infected dogs. The plus signs denotes the means, the solid lines denote the medians, and the bars represent the 5th–95th percentiles. D, Dog peripheral blood mononuclear cells were stimulated for 6 days with soluble Leishmania antigen or left unstimulated. Supernatants were used to measure cytokine levels for interferon γ (IFN-γ), interleukin 10 (IL-10), interleukin 6 (IL-6), interleukin 12 (IL-12), and interleukin 2 (IL-2), using a 5-plex Luminex assay. Lines represent the mean. Symbols identify each dog. Abbreviation: M, month.
The levels of IFN-γ, IL-10, IL-12, IL-6, and IL-2 cytokines were investigated in PBMCs stimulated with Leishmania antigen before (at month 0) and at month 6, month 9, month 12, month 19, and month 22 after TC1 (Figure 3D). Generally, IFN-γ production increased in most dogs over time and was significantly higher at month 22 after TC1, compared with that at month 0 (P = .0415; Figure 3D). Interestingly, we observed 2 distinct clusters of dogs consisting of high (dogs 1592, 1516, 1702, 2912, and 5635) and low (dogs 3080, 1605, and 2474) IFN-γ producers (Figure 3D). IL-10 production also increased over time and was significantly different at month 9 (P = .0057) and month 22 (P = .0106), compared with month 0 (Figure 3D). There was no statistically significant increase in IL-2, IL-6, and IL-12 production.
IFN-γ Is a Strong Correlate of Protective Anti-Leishmania Immunity in Beagles Infected via Controlled Vector Transmission
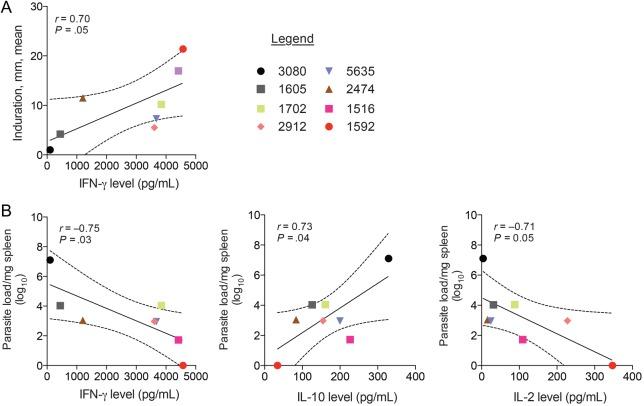
At month 22, IFN-γ was the only cytokine of 5 tested to show a significant positive correlation with mean indurations resulting from the leishmanin test (r = 0.70; P = .05; Figure 4A). Additionally, IFN-γ levels (r = −0.75; P = .03) and IL-2 levels (r = −0.71; P = .05) were negatively correlated with splenic parasite loads, while IL-10 levels (r = 0.73; P = .04) were positively correlated to the number of parasites in the spleen (Figure 4B). IL-12 and IL-6 levels did not correlate with either leishmanin test indurations or splenic parasite loads.
Figure 4.
Correlates of immunity to Leishmania infantum in beagles after experimental vector transmission. A, Leishmania skin test induration correlates positively to levels of interferon γ (IFN-γ). B, Correlation of splenic parasite load with IFN-γ, interleukin 10 (IL-10), and interleukin 2 (IL-2) levels. Symbols identify each dog. Solid lines in scatterplots represent mean values.
DISCUSSION
CanL caused by L. infantum has a complex etiology that evolves into a spectrum of clinical manifestations in dogs [1, 22]. Additionally, CanL is chronic, and most dogs exhibit clinical signs only years after being in an area of endemicity [3, 41]. Here, we achieved a 100% infection rate in beagles through controlled multiple vector transmissions. Although we acknowledge the limitation of this model, particularly concerning the restricted number of transmissions and the low number of investigated dogs, controlling the site of infected bites revealed aspects to CanL transmission that may otherwise have been overlooked.
Based on immunological, parasitological, and pathological data, the outcome of CanL varied in 8 dogs controlled for breed, age, sex, and source and subjected to a standardized infection pressure and equivalent standards of care, suggesting that the rate of disease progression in dogs is not only dependent on the number and frequency of infectious bites. This supports studies linking Leishmania susceptibility to genetic factors that predispose dogs to this infection [33, 42], although variation in the Slc11c1 locus did not account for the observed susceptibility to disease in dog 3080, dog 1605, or dog 1702. As shown by others [2, 23], a distinct leishmanin test reactivity and high levels of IFN-γ and IL-2 were good indicators of immunity in the infected dogs, while IL-10 levels correlated positively with splenic parasite burdens. Interestingly, at month 22, all 8 dogs contained a substantial and comparable parasite load in their lymph nodes, indicating its potential importance for maintaining immunity, as well as being an indicator of an active infection or disease [29, 38, 39].
We saw significant changes in known biochemical indicators of CanL in only 1 dog (dog 3080), suggesting that a sustained infection pressure such as that undergone by dogs in areas of endemicity may be needed to induce marked biochemical changes. Of note, these usually occur late under a natural infection setting [4]. Additionally, weight loss, a sign of advanced CanL [11], was not observed in any of the infected dogs. The high level of care received by the dogs in this study, including being fed when they became weak, may have played a factor in maintaining their weight.
A key observation inferred from this study relates to the potential relevance of infectious bite sites in the transmission of L. infantum back to vector populations. Although vital to understanding CanL propagation in disease foci, the transmissibility of infected dogs to sand flies remains poorly understood, complicated by the unknown location and frequency of infected bites in field settings. In this study, controlling the site and frequency of vector transmissions, combined with the range of clinical signs exhibited by infected dogs, presented an opportunity to assess their infectiousness to sand flies. Although skin parasitism has been associated with transmission of L. infantum to sand flies, it has been reported as a generalized condition resulting from dissemination of the parasite [7, 24, 26, 27, 29–32]. Here, we offer another possible explanation. Our data indicate that, early after infection, Leishmania parasites are accessible to sand flies through lesions that form at the site of most infectious bites. Persistence of pathogens at bite sites in the skin has been documented for other vector-borne diseases, including malaria, Lyme disease, and dengue [43–45]. In this study, small parasite-positive lesions, which can be overlooked without shaving the animal, formed at the site of bites in all infected dogs and persisted for months even in dogs that exhibited no other clinical signs of CanL. Additionally, bite site and distal lesions were both infective to sand flies, while normal skin, monitored bimonthly up to month 22, was not (except for 1 dog at month 14, when it was polysymptomatic). The prevalence, early appearance, and persistence of bite site lesions implicate them in propagation of Leishmania infections to sand flies. Although bite site lesions, sometimes referred to as “chancres,” have been reported in field dogs [15], they are mostly discussed as sites of parasite inoculation, and their persistence and role in transmissibility to sand flies has been underappreciated. Here, we demonstrate that primary bite site lesions persist, remaining parasite positive intermittently over the 2-year study period. We also demonstrate that parasites residing in these lesions are accessible to flies even a year after transmission. These observations may partially explain the contradictive reports regarding the importance of symptomatic versus asymptomatic dogs as a source of sand fly infection. Moreover, sand flies may preferentially feed on certain body parts, such as ears and nose, potentiating the likelihood of parasite recovery from previous bite sites. Further studies of the feeding behavior of vector sand flies in foci of disease are needed to validate this premise.
In conclusion, we propose that bite sites can retain parasites in the skin for months after a primary infection. These parasites are accessible to sand fly populations soon after transmission in most infected dogs, irrespective of disease status, providing a new perspective for the propagation of L. infantum in disease foci.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Tanya Burkholder and her team from the National Institute of Allergy and Infectious Diseases (NIAID) Comparative Medicine Branch, for assistance and continuous support during follow-up and monitoring of the dogs; Dr John Bacher and Dr Tom Thomas, for conducting surgical procedures; Dr Jose Luis Ramirez, for editing Supplementary Video 1; and Dr Javier Moreno and Dr Eugenia Carrillo Gallego, for generously providing their expert opinion whenever requested.
H. A., L. F., S. K. conceived and designed the experiments. H. A., F. O., C. M., P. C., R. G., C. T., C. A. D., M. O., L. G., and S. K. performed the experiments. H. A., F. O., G. O., L. G., L. F., J. G. V., and S. K. analyzed the data. G. O. contributed material. H. A., F. O., J. G. V., and S. K. wrote the manuscript.
Financial support. This work was supported by the Intramural Research Program of the Division of Intramural Research, NIAID, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol 2008; 24:324–30. [DOI] [PubMed] [Google Scholar]
- 2.Reis AB, Giunchetti RC, Carrillo E, Martins-Filho OA, Moreno J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol 2010; 26:341–9. [DOI] [PubMed] [Google Scholar]
- 3.Oliva G, Scalone A, Foglia Manzillo V et al. Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naive dogs exposed to three consecutive transmission seasons. J Clin Microbiol 2006; 44:1318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foglia Manzillo V, Di Muccio T, Cappiello S et al. Prospective study on the incidence and progression of clinical signs in naive dogs naturally infected by Leishmania infantum. PLoS Negl Trop Dis 2013; 7:e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis 2002; 186:1314–20. [DOI] [PubMed] [Google Scholar]
- 6.Chappuis F, Sundar S, Hailu A et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 2007; 5:873–82. [DOI] [PubMed] [Google Scholar]
- 7.Alvar J, Canavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Adv Parasitol 2004; 57:1–88. [DOI] [PubMed] [Google Scholar]
- 8.Riera C, Valladares JE, Gallego M et al. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet Parasitol 1999; 84:33–47. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes AP, Costa MM, Coelho EA et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 2008; 26:5888–95. [DOI] [PubMed] [Google Scholar]
- 10.Molano I, Alonso MG, Miron C et al. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet Immunol Immunopathol 2003; 92:1–13. [DOI] [PubMed] [Google Scholar]
- 11.Lemesre JL, Holzmuller P, Cavaleyra M, Goncalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine 2005; 23:2825–40. [DOI] [PubMed] [Google Scholar]
- 12.Moreno J, Nieto J, Masina S et al. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine 2007; 25:5290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maia C, Nunes M, Cristovao J, Campino L. Experimental canine leishmaniasis: clinical, parasitological and serological follow-up. Acta Trop 2010; 116:193–9. [DOI] [PubMed] [Google Scholar]
- 14.Campino L, Santos-Gomes G, Rica Capela MJ, Cortes S, Abranches P. Infectivity of promastigotes and amastigotes of Leishmania infantum in a canine model for leishmaniosis. Vet Parasitol 2000; 92:269–75. [DOI] [PubMed] [Google Scholar]
- 15.Killick-Kendrick R, Killick-Kendrick M, Pinelli E et al. A laboratory model of canine leishmaniasis: the inoculation of dogs with Leishmania infantum promastigotes from midguts of experimentally infected phlebotomine sandflies. Parasite 1994; 1:311–8. [DOI] [PubMed] [Google Scholar]
- 16.Costa DJ, Carvalho RM, Abbehusen M et al. Experimental infection of dogs with Leishmania and saliva as a model to study Canine Visceral Leishmaniasis. PLoS One 2013; 8:e60535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maia C, Seblova V, Sadlova J, Votypka J, Volf P. Experimental transmission of Leishmania infantum by two major vectors: a comparison between a viscerotropic and a dermotropic strain. PLoS Negl Trop Dis 2011; 5:e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Secundino NF, de Freitas VC, Monteiro CC, Pires AC, David BA, Pimenta PF. The transmission of Leishmania infantum chagasi by the bite of the Lutzomyia longipalpis to two different vertebrates. Parasit Vectors 2012; 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimblin N, Peters N, Debrabant A et al. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci U S A 2008; 105:10125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol 2001; 39:560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieto CG, Navarrete I, Habela MA, Serrano F, Redondo E. Pathological changes in kidneys of dogs with natural Leishmania infection. Vet Parasitol 1992; 45:33–47. [DOI] [PubMed] [Google Scholar]
- 22.Solano-Gallego L, Miro G, Koutinas A et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011; 4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinelli E, Killick-Kendrick R, Wagenaar J, Bernadina W, del Real G, Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect Immun 1994; 62:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am J Trop Med Hyg 2001; 64:119–24. [DOI] [PubMed] [Google Scholar]
- 25.Quinnell RJ, Courtenay O, Garcez L, Dye C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology 1997; 115 (Pt 2):143–56. [DOI] [PubMed] [Google Scholar]
- 26.da Costa-Val AP, Cavalcanti RR, de Figueiredo Gontijo N et al. Canine visceral leishmaniasis: relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Vet J 2007; 174:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vercosa BL, Lemos CM, Mendonca IL et al. Transmission potential, skin inflammatory response, and parasitism of symptomatic and asymptomatic dogs with visceral leishmaniasis. BMC Vet Res 2008; 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ. Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Negl Trop Dis 2014; 8:e2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurenti MD, Rossi CN, da Matta VL et al. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet Parasitol 2013; 196:296–300. [DOI] [PubMed] [Google Scholar]
- 30.Madeira MF, Figueiredo FB, Pinto AG et al. Parasitological diagnosis of canine visceral leishmaniasis: is intact skin a good target? Res Vet Sci 2009; 87:260–2. [DOI] [PubMed] [Google Scholar]
- 31.Molina R, Amela C, Nieto J et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg 1994; 88:491–3. [DOI] [PubMed] [Google Scholar]
- 32.Soares MR, de Mendonca IL, do Bonfim JM, Rodrigues JA, Werneck GL, Costa CH. Canine visceral leishmaniasis in Teresina, Brazil: Relationship between clinical features and infectivity for sand flies. Acta Trop 2011; 117:6–9. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Robert E, Altet L, Utzet-Sadurni M, Giger U, Sanchez A, Francino O. Slc11a1 (formerly Nramp1) and susceptibility to canine visceral leishmaniasis. Vet Res 2008; 39:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science 2000; 290:1351–4. [DOI] [PubMed] [Google Scholar]
- 35.Dapra F, Scalone A, Mignone W et al. Validation of a recombinant based antibody ELISA for diagnosis of human and canine leishmaniasis. J Immunoassay Immunochem 2008; 29:244–56. [DOI] [PubMed] [Google Scholar]
- 36.Semiao-Santos SJ, Abranches P, Silva-Pereira MC, Santos-Gomes GM, Fernandes JP, Vetter JC. Reliability of serological methods for detection of leishmaniasis in Portuguese domestic and wild reservoirs. Mem Inst Oswaldo Cruz 1996; 91:747–50. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y, Kamhawi S, Modi G et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med 1998; 188:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 1985; 7:545–55. [DOI] [PubMed] [Google Scholar]
- 39.Giunchetti RC, Correa-Oliveira R, Martins-Filho OA et al. A killed Leishmania vaccine with sand fly saliva extract and saponin adjuvant displays immunogenicity in dogs. Vaccine 2008; 26:623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokal JE. Editorial: Measurement of delayed skin-test responses. N Engl J Med 1975; 293:501–2. [DOI] [PubMed] [Google Scholar]
- 41.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol 2005; 35:1169–80. [DOI] [PubMed] [Google Scholar]
- 42.Quinnell RJ, Kennedy LJ, Barnes A et al. Susceptibility to visceral leishmaniasis in the domestic dog is associated with MHC class II polymorphism. Immunogenetics 2003; 55:23–8. [DOI] [PubMed] [Google Scholar]
- 43.Menard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol 2013; 11:701–12. [DOI] [PubMed] [Google Scholar]
- 44.Kern A, Schnell G, Bernard Q et al. Heterogeneity of Borrelia burgdorferi sensu stricto population and its involvement in Borrelia pathogenicity: study on murine model with specific emphasis on the skin interface. PLoS One 2015; 10:e0133195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frischknecht F. The skin as interface in the transmission of arthropod-borne pathogens. Cell Microbiol 2007; 9:1630–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.