Abstract
Background. The recommended schedule for receipt of 2-dose human rotavirus vaccine (HRV) coincides with receipt of the first and second doses of diphtheria, pertussis, and tetanus vaccine (ie, 6 and 10 weeks of age, respectively). Alternative schedules and additional doses of HRV have been proposed and may improve vaccine performance in low-income countries.
Methods. In this randomized trial in rural Ghana, HRV was administered at ages 6 and 10 weeks (group 1), 10 and 14 weeks (group 2), or 6, 10, and 14 weeks (group 3). We compared serum antirotavirus immunoglobulin A (IgA) seroconversion (≥20 U/mL) and geometric mean concentrations (GMCs) between group 1 and groups 2 and 3.
Results. Ninety-three percent of participants (424 of 456) completed the study per protocol. In groups 1, 2, and 3, the IgA seroconversion frequencies among participants with IgA levels of <20 U/mL at baseline were 28.9%, 37.4%, and 43.4%, respectively (group 1 vs group 3, P = .014; group 1 vs group 2, P = .163). Postvaccination IgA GMCs were 22.1 U/mL, 26.5 U/mL, and 32.6 U/mL in groups 1, 2, and 3, respectively (group 1 vs group 3, P = .038; group 1 vs group 2, P = .304).
Conclusions. A third dose of HRV resulted in increased seroconversion frequencies and GMCs, compared with 2 doses administered at 6 and 10 weeks of age. Since there is no correlate of protection, a postmarketing effectiveness study is required to determine whether the improvement in immune response translates into a public health benefit in low-income countries.
Clinical Trials Registration. NCT015751.
Keywords: rotavirus, rotavirus vaccines, randomized controlled trial, immunogenicity, infant, vaccines, developing countries, immunization schedules
(See the editorial commentary by Cunliffe and Kang on pages 1673–5, and major article by Zaman et al on pages 1686–93.)
Rotavirus is a leading cause of diarrhea-associated mortality among children <5 years of age in low-income countries (LICs) [1, 2], with countries in sub-Saharan Africa exhibiting the highest rates of rotavirus-associated mortality [2]. In Ghana, rotavirus is a common cause of diarrhea, circulating from approximately September/October to February/March [3–6]. Prior to widespread introduction of rotavirus vaccine, rotavirus was responsible for approximately 50% of diarrheal hospitalizations among children aged <5 years [7].
Two orally administered human rotavirus vaccines (HRVs), Rotarix (GSK Biologicals, Rixensart, Belgium) and RotaTeq (Merck, West Point, Pennsylvania), are recommended by the World Health Organization (WHO) for inclusion in the infant immunization series. While antirotavirus immunoglobulin A (IgA) seroconversion frequencies with these vaccines range between approximately 87% and 95% in high-income countries (HICs) [8, 9], rates in Africa and Asia have been consistently lower, between 36% and 78% [3, 10–12]. Similarly, IgA geometric mean concentrations (GMCs) in LICs are approximately 5–10 times lower than those in HICs [3], and, while vaccine efficacy against severe rotavirus diarrhea in HICs is high [8, 13], there is moderate efficacy among children in LICs [10, 14–16].
The reasons postulated for the observed reduced response to rotavirus vaccines in LICs include the presence of high maternal antirotavirus antibody titers, interference by the first dose of coadministered oral polio vaccine (OPV), altered gut microbiota, enteropathy, and malnutrition [17–20]. In LICs, HRV is administered in a 2-dose schedule at 6 and 10 weeks of age (WHO Expanded Programme on Immunization [EPI] visits 1 and 2). Pragmatic ways to overcome the effect of some of these factors might be to administer a third dose at 14 weeks of age (EPI visit 3) or, although not considered practical in maximizing EPI coverage, delay administration of the standard 2-dose schedule to a slightly older age, when maternal antibody levels have declined and when there is lower risk of interference from concomitant administration with the first OPV dose.
Previous studies assessed whether alternate schedules improve vaccine responses in infants from LICs. One study evaluated the immunogenicity and efficacy of HRV through 2 years of age in South Africa and Malawi, finding consistent trends indicating that a 3-dose HRV schedule (6, 10, and 14 weeks of age) may be optimal as compared to a 2-dose schedule administered at an older age (10 and 14 weeks of age) [21]. In an earlier South African immunogenicity study, infants receiving 2 doses of a lower-dose HRV at 6 and 10 weeks of age had lower immune responses as compared to infants receiving 2 doses at 10 and 14 weeks of age [19]. Thus, the increases in immunogenicity and efficacy of a 3-dose schedule identified in the South Africa and Malawi study may be even more pronounced when compared to a 2-dose schedule given at an earlier recommended schedule (6 and 10 weeks of age).
To investigate whether a 3-dose schedule of HRV administered at 6, 10, and 14 weeks of age is superior to a 2-dose schedule in which vaccine is given at the recommended earlier ages (6 and 10 weeks of age), we conducted a phase 4, single-center, individually randomized, open-label trial in rural Ghana. Secondary objectives included assessing whether 2 doses of HRV administered at a delayed schedule of 10 and 14 weeks of age were superior to 2 doses administered at 6 and 10 weeks of age, comparing the effect of high versus low levels of maternally derived antirotavirus immunoglobulin G (IgG) on the immune response to vaccination, and describing vaccine-type rotavirus shedding.
METHODS
Study Site and Subjects
This study was conducted in rural Navrongo (Upper East Region, Ghana). HRV (Rotarix) was introduced into the Ghana National EPI in May 2012 as a 2-dose schedule, given at 6 and 10 weeks of age. When infants were between 42 and 55 days old, parents were invited to learn more about the study and provide written informed consent. Infants were subsequently enrolled and vaccinated between September and December 2012. Infants eligible for study vaccination were between 6 and 7 weeks of age at the time of enrollment, generally healthy, free of contraindications to receipt of rotavirus vaccine, born at full term (≥36 weeks of gestation), not underweight (ie, <2000 g), the only child <16 weeks of age living in the compound, and had not previously received rotavirus vaccine. The trial was conducted according to the principles of the Declaration of Helsinki (2008), in compliance with good clinical practice guidelines, and approved by the following ethics review committees: the Ghana Health Service Ethics Review Board, the Navrongo Health Research Center Institutional Review Board (IRB), the Noguchi Memorial Institute for Medical Research (NMIMR) IRB, the Centers for Disease Control and Prevention IRB, and the Western IRB (Olympia, Washington).
Randomization and Procedures
All infants eligible for vaccination were randomly assigned to receive liquid HRV at 6 and 10 weeks of age (group 1), 10 and 14 weeks of age (group 2), or 6, 10, and 14 weeks of age (group 3). Randomization was conducted using a computer-generated algorithm, with infants assigned to study groups in blocks of 3 in a 1:1:1 ratio. A baseline blood specimen was collected prior to receipt of the first dose of HRV. A stool specimen was also collected before each HRV dose. All study groups received their rotavirus vaccinations concomitantly with routine EPI vaccines (OPV, pneumococcal conjugate vaccine, and pentavalent diphtheria, pertussis, tetanus, Haemophilus influenzae type b, and hepatitis B vaccine). Breastfeeding was not restricted.
Trained field workers visited participants at their homes to collect stool specimens 4 and 7 days (±1 day) after each HRV dose. All participants were asked to report to the study clinic 4 weeks (+2 weeks) following the last dose of HRV, to provide a postvaccination blood specimen (specimens were obtained from group 1 at 14 weeks and from groups 2 and 3 at 18 weeks); group 1 participants also had a blood specimen collected during their last visit, at 18 weeks of age. The last follow-up visit occurred in February 2013. Participants were followed throughout the study for serious adverse events (SAEs). All SAEs were reviewed and vetted by an independent safety monitor. Masked serum and stool specimens were shipped on dry ice and stored frozen (temperature, −20°C) at the Regional Rotavirus Reference Laboratory, NMIMR (University of Ghana, Legon) until testing (for stool specimens) or further shipment (for serum specimens).
Laboratory Assays
At the NMIMR laboratory, rotavirus antigen in stool was detected by an enzyme immunoassay (EIA; ProSpecT, Oxoid, United Kingdom). All stool samples testing positive for rotavirus by EIA were characterized by reverse transcription–polymerase chain reaction to identify the vaccine-like genotype G1P[8] [22, 23]. Sera were analyzed at Cincinnati Children's Hospital Medical Center Laboratory for Specialized Clinical Studies (Cincinnati, Ohio) by an enzyme-linked immunoassay, as previously described, to detect and quantify serum antirotavirus IgA or IgG antibody concentrations [24, 25]. Seroconversion was defined as the detection of an antirotavirus IgA concentration of ≥20 U/mL in infants with an IgA concentration of <20 U/mL at the time of receipt of their first rotavirus vaccine (6 weeks of age for groups 1 and 3 and 10 weeks of age for group 2).
Statistical Analyses
For serologic analyses, the per-protocol evaluation (PPE) population included participants satisfying the inclusion/exclusion criteria; baseline antirotavirus IgA <20 U/mL; receiving HRV concomitantly with OPV and according to group schedule, completing study visits according to protocol-defined window periods, and with valid serology results at the 14 (group 1) and 18 week (all groups) visits. For stool shedding analyses we included the PPE population of participants receiving each scheduled HRV dose, regardless of concomitant OPV administration, blood collection, and the window period for stool collection. The safety analysis data set included randomized participants who received at least one dose of HRV. Analyses were completed using SAS software (version 9; SAS Institute; Cary, North Carolina).
Proportions of participants seroconverting after vaccination were compared between groups, using the 2-sided Fisher exact test, and 95% confidence intervals (CIs) for the difference in rates were calculated using the Newcombe–Wilson method without continuity correction. To control for age-dependent seroconversion due to wild-type rotavirus infection during the follow-up period during the rotavirus season, the primary measure of seroconversion and GMCs in group 1 was based on the higher of the 2 postvaccination antirotavirus IgA concentrations measured in serum specimens obtained at 14 weeks or 18 weeks. GMCs were defined as the exponential of the mean logarithmic transformation of the antirotavirus IgA and IgG antibody responses. For quantitative analyses, serum antirotavirus IgA values of <20 U/mL were converted to 10 U/mL to produce results comparable to those of other studies, even though the limit of quantification of the assay was 7 U/mL. The GMCs and 95% CIs of the IgG antibody responses before vaccination and IgA antibody responses after vaccination were compared between groups, using the t test. The effect of maternally derived serum IgG on HRV IgA seroconversion was also evaluated, using (1) the Fisher exact test to compare IgA seroconversion frequencies within each group, stratified by IgG level before vaccination (ie, low [≤25th percentile] vs high [≥75th percentile]), with cut points determined by the distribution within each arm; and (2) a fixed-effects logistic regression model, with rotavirus IgA seroconversion after vaccination as the dependent variable and the level of rotavirus maternally derived IgG antibody before vaccination as an independent variable. In these analyses of effects of maternally derived antibody, all valid serum IgA and IgG values were used. SAEs were summarized by group and by relationship to HRV in terms of counts and percentages.
Enrollment of 152 participants per study group was estimated to provide 90% power to detect an increase in seroconversion from 35% in group 1 to 55% in group 3 [10, 19], using a 2-sided χ2 test (α = 0.05) for proportions and accounting for a 15% attrition rate.
RESULTS
Study Participants
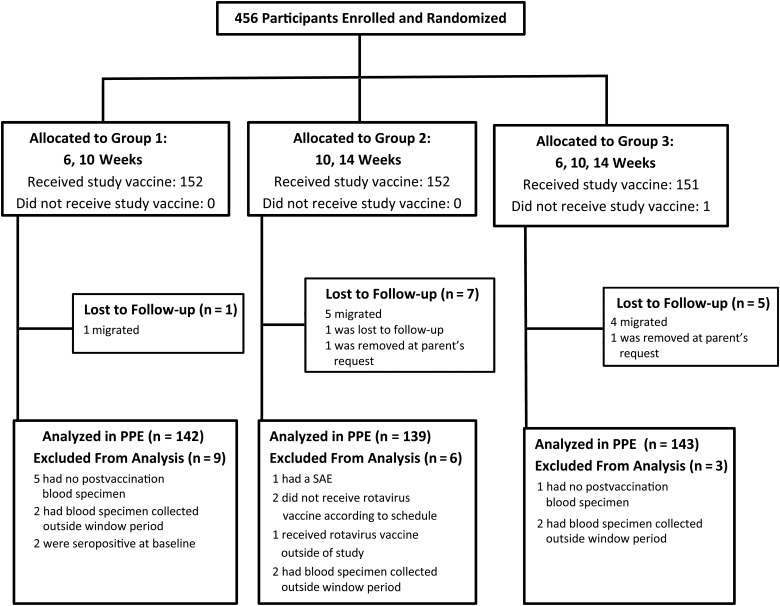
Of 456 participants screened and randomly assigned to the 3 study arms, 455 (99.8%) received HRV (Figure 1); 152, 152, and 151 participants were in groups 1, 2, and 3, respectively. The majority (93.2%) of randomized participants met the study criteria for inclusion in the PPE serologic analysis; only 2 participants were seropositive at baseline (Figure 1). Baseline characteristics were similar across groups and a very high proportion completed each follow-up visit within protocol-defined windows (Table 1). Per WHO recommendations, the majority of participants included in the PPE (>97% in each group) received a birth dose of OPV at ≤4 weeks of age. All participants received concomitant OPV.
Figure 1.
Study profile. Abbreviations: PPE, per-protocol evaluation; SAE, serious adverse event.
Table 1.
Characteristics of Study Participants
| Characteristic | Group 1 (n = 152) | Group 2 (n = 152) | Group 3 (n = 152) |
|---|---|---|---|
| Baseline | |||
| Age, wks | |||
| Mean ± SD | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.2 ± 0.2 |
| Range | 6.0–7.1 | 6.0–6.7 | 6.0–6.7 |
| Sex, % | |||
| Male, % | 69 (45.4) | 81 (53.3) | 73 (48.0) |
| Female, % | 83 (54.6) | 71 (46.7) | 79 (52.0) |
| Length, cm | |||
| Mean ± SD | 54.5 ± 2.0 | 54.5 ± 2.1 | 54.6 ± 2.2 |
| Range | 50.0–59.0 | 48.6–60.5 | 48.9–60.0 |
| Weight, kg | |||
| Mean ± SD | 4.5 ± 0.6 | 4.5 ± 0.6 | 4.5 ± 0.6 |
| Range | 3.0–6.1 | 3.1–5.8 | 2.9–5.6 |
| Group 1 (n = 142) | Group 2 (n = 139) | Group 3 (n = 143) | |
| Age at follow-up visit, wksa | |||
| Wk 10 visit | |||
| Mean ± SD | 10.2 ± 0.3 | 10.2 ± 0.2 | 10.2 ± 0.2 |
| Range | 10.0–11.1 | 10.0–10.7 | 10.0–10.7 |
| Wk 14 visit | |||
| Mean ± SD | 14.2 ± 0.3 | 14.3 ± 0.3 | 14.2 ± 0.3 |
| Range | 14.0–15.9 | 14.0–15.3 | 14.0–16.4 |
| Wk 18 visit | |||
| Mean ± SD | 18.3 ± 0.4 | 18.4 ± 0.4 | 18.3 ± 0.3 |
| Range | 18.0–19.9 | 18.0–20.1 | 18.0–20.4 |
Group 1 received rotavirus vaccine at ages 6 and 10 weeks, group 2 received vaccine at ages 10 and 14 weeks, and group 3 received vaccine at ages 6, 10, and 14 weeks.
Abbreviation: SD, standard deviation.
a Per-protocol evaluation.
Serum Antirotavirus IgA Responses
In the PPE population, 28.9% (95% CI, 22.1%–36.8%), 37.4% (95% CI, 29.8%–45.7%), and 43.4% (95% CI, 35.5%–51.6%) of participants in groups 1, 2, and 3, respectively, seroconverted, based on the primary measure (Table 2). Participants receiving 3 doses of HRV had a significantly higher seroconversion frequency (absolute rate difference, 14.5%; 95% CI, 3.3%–25.1%; P = .014) than those receiving 2 HRV doses at 6 and 10 weeks of age (group 1), but those receiving 2 HRV doses on a delayed schedule (group 2) did not have a significantly higher seroconversion frequency (absolute rate difference, 8.5%; 95% CI, −2.5% to 19.3%; P = .163) than those in group 1. When measuring seroconversion frequencies at 4 weeks following the final dose of HRV (14 weeks for group 1), there were larger increases in group 3 (43.4%) and group 2 (37.4%) as compared to group 1 (20.4%). While not as large, this also held true when seroconversion in group 1 was measured 8 weeks after dose 2.
Table 2.
Serum Antirotavirus Immunoglobulin A (IgA) Seroconversion After Vaccination
| Outcome | Group 1 (n = 142) |
Group 2 (n = 139) |
Group 3 (n = 143) |
Group 1 vs 3 |
Group 1 vs 2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects, No. | Subjects, % (95% CI) | Subjects, No. | Subjects, % (95% CI) | Subjects, No. | Subjects, % (95% CI) | Percentage Point Difference (95% CI) | P Value | Percentage Point Difference (95% CI) | P Value | |
| Primary measurea | 41 | 28.9 (22.1–36.8) | 52 | 37.4 (29.8–45.7) | 62 | 43.4 (35.5–51.6) | 14.5 (3.3–25.1) | .014b | 8.5 (−2.5 to 19.3) | .163b |
| Group 1 measured at wk 14 only | 29 | 20.4 (14.6–27.8) | … | … | … | … | 22.9 (12.2–33.0) | <.001c | 17.0 (6.4–27.1) | .002c |
| Group 1 measured at wk 18 only | 35 | 24.7 (18.3–32.3) | … | … | … | … | 18.7 (7.7–29.1) | .001d | 12.8 (1.2–23.2) | .028d |
Group 1 received rotavirus vaccine at ages 6 and 10 weeks, group 2 received vaccine at ages 10 and 14 weeks, and group 3 received vaccine at ages 6, 10, and 14 weeks.
Abbreviation: CI, confidence interval.
a Of group 1 participants who seroconverted, 56.1% (23) demonstrated seroconversion at weeks 14 and 18, 14.6% (6) at week 14 only, and 29.3% (12) at week 18 only.
b Highest IgA seroconversion value at either 14 or 18 weeks for group 1 and at 18 weeks in groups 2 and 3.
c For the comparison of seroconversion frequency at wk 14 in group 1 to the frequency at wk 18 for group 2 or group 3.
d For the comparison of seroconversion frequency at wk 18 in groups 1, 2, and 3.
In the PPE population, using the primary measure, GMCs were 22.1 U/mL (95% CI, 17.4–28.2 U/mL), 26.5 U/mL (95% CI, 20.7–34.0 U/mL), and 32.6 U/mL (95% CI, 24.7–43.2 U/mL) for participants in groups 1, 2, and 3, respectively (Table 3). Participants receiving 3 doses of HRV had significantly higher GMCs than those receiving 2 doses at 6 and 10 weeks of age (GMC ratio, 1.47; 95% CI, 1.02–2.13; P = .038), but participants receiving 2 doses on a delayed schedule did not have significantly higher GMCs than those receiving doses at 6 and 10 weeks of age (GMC ratio, 1.20; 95% CI, .85–1.69; P = .304). Based on comparisons between groups of GMCs at only the 14-week visit (GMC, 16.8 U/mL; 95% CI, 13.8–20.4 U/mL) or 18-week visit (GMC, 19.1 U/mL; 95% CI, 15.4–23.8 U/mL) for group 1, the differences between study groups increased, resulting in a significant difference between groups 1 and 2 when the 14-week measurement was used (GMC ratio, 1.58; 95% CI, 1.15–2.16; P = .005).
Table 3.
Serum Geometric Mean Concentrations (GMCs) of Antirotavirus Immunoglobulin A (IgA) After Vaccination
| Variable | Group 1 |
Group 2 |
Group 3 |
Groups 1 vs 3 |
Groups 1 vs 2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects, No. |
GMC (95% CI) |
Subjects, No. |
GMC (95% CI) |
Subjects, No. |
GMC (95% CI) |
GMC Ratio (95% CI) |
P Value | GMC Ratio (95% CI) |
P Value | |
| All subjects | ||||||||||
| Primary measurea | 142 | 22.1 (17.4–28.2) | 139 | 26.5 (20.7–34.0) | 143 | 32.6 (24.7–43.2) | 1.47 (1.02–2.13) | .038b | 1.20 (.85–1.69) | .304b |
| Group 1 measured at wk 14 only | 142 | 16.8 (13.8–20.4) | … | … | … | … | 1.94 (1.38–2.73) | <.001c | 1.58 (1.15–2.16) | .005c |
| Group 1 measured at wk 18 only | 142 | 19.1 (15.4–23.8) | … | … | … | … | 1.71 (1.20–2.43) | .003d | 1.39 (1.00–1.93) | .052d |
| Seroconverting subjects | ||||||||||
| Primary measurea | 41 | 156.7 (101.3–242.5) | 52 | 135.5 (95.0–193.3) | 62 | 153.1 (103.6–226.2) | 0.98 (.54–1.76) | .938b | 0.86 (.50–1.50) | .600b |
| Group 1 measured at wk 14 only | 29 | 126.4 (76.8–208.0) | … | … | … | … | 1.21 (.63–2.34) | .564c | 1.07 (.59–1.94) | .816c |
| Group 1 measured at wk 18 only | 35 | 139.0 (87.4–222.1) | … | … | … | … | 1.10 (.59–2.04) | .757d | 0.97 (.55–1.72) | .929d |
Group 1 received rotavirus vaccine at ages 6 and 10 weeks, group 2 received vaccine at ages 10 and 14 weeks, and group 3 received vaccine at ages 6, 10, and 14 weeks.
Abbreviation: CI, confidence interval.
a Of group 1 participants who seroconverted, 56.1% (23) demonstrated seroconversion at weeks 14 and 18, 14.6% (6) at week 14 only, and 29.3% (12) at week 18 only.
b Highest IgA seroconversion value at either 14 or 18 weeks for group 1 and at 18 weeks in groups 2 and 3.
c For the comparison of seroconversion frequency at wk 14 in group 1 to the frequency at wk 18 for group 2 or group 3.
d For the comparison of seroconversion frequency at wk 18 in groups 1, 2, and 3.
Among only participants who seroconverted based on the primary outcome measure, postvaccination GMCs were 156.7 U/mL (95% CI, 101.3–242.5 U/mL), 135.5 U/mL (95% CI, 95.0–193.3 U/mL), and 153.1 U/mL (95% CI, 103.6–226.2 U/mL) for groups 1, 2, and 3, respectively (Table 2). There were no significant differences between groups 1 and 3 (GMC ratio, 0.98; 95% CI, .54–1.76; P = .938) or groups 1 and 2 (GMC ratio, 0.86; 95% CI, .50–1.50; P = .600). These results were similar regardless of whether the final specimen used for comparison between study groups was based on only the week 14 or week 18 specimen for group 1.
Maternal Antibody Evaluations
The GMC of maternally derived rotavirus measured at 6 weeks of age was similar in both group 1 (279.9 U/mL; 95% CI, 237.2–330.4 U/mL) and group 3 (269.7 U/mL; 95% CI, 232.6–312.8 U/mL; P = .743; Table 4), while the IgG GMC measured at 10 weeks in group 2 was statistically significantly lower, at 193.4 U/mL, than that in group 1(P = .002) and group 3 (P = .003). Within each arm, when using the findings for serum specimens obtained 4 weeks after vaccination, the seroconversion frequency was higher among infants with low maternally derived IgG levels (≤25th percentile), compared with infants with high maternally derived IgG levels (≥75th percentile; group 1, 38.9% vs 5.6% [P = .001]; group 2, 60.0% vs 28.6% [P = .015]; and group 3, 52.8% vs 36.1% [P = .236]; Table 4). After controlling for maternally derived IgG level, infants vaccinated with 3 doses of HRV had a significantly increased likelihood of seroconversion as compared to those who received 2 doses at 6 and 10 weeks of age (odds ratio, 1.38; 95% CI, 1.07–1.76; P = .012), while infants who received 2 doses at 10 and 14 weeks of age did not have a significantly increased likelihood of seroconversion as compared to those vaccinated with 2 doses at 6 and 10 weeks of age (OR, 1.12; 95% CI, .86–1.45; P = .400).
Table 4.
Serum Antirotavirus Immunoglobulin A Seroconversion Frequency, by Maternally Derived Antirotavirus Immunoglobulin G (IgG) Concentration Quartile 4 Weeks After the Final Vaccine Dose, and Baseline Geometric Mean Concentration (GMC) of Maternally Derived IgG
| Variable | Group 1 (n = 142) | Group 2 (n = 139) | Group 3 (n = 143) |
|---|---|---|---|
| IgA seroconversion frequency, by maternally-derived IgG quartile | |||
| ≤25th | 14/36 (38.9a) | 21/35 (60.0b) | 19/36 (52.8c) |
| >25th to ≤50th | 6/35 (17.1) | 13/35 (37.1) | 14/36 (38.9) |
| >50th to <75th | 7/35 (20.0) | 8/34 (23.5) | 16/35 (45.7) |
| ≥75th | 2/36 (5.6) | 10/35 (28.6) | 13/36 (36.1) |
| Baseline GMC, U/mL (95% CI) | 279.9 (237.2–330.4) | 193.5 (163.9–228.1) | 269.7 (232.6–312.8) |
Data are no. of vaccine recipients who seroconverted/no. evaluated (%), unless otherwise indicated. Group 1 received rotavirus vaccine at ages 6 and 10 weeks, group 2 received vaccine at ages 10 and 14 weeks, and group 3 received vaccine at ages 6, 10, and 14 weeks.
Abbreviations: CI, confidence interval; IgA, immunoglobulin A.
a P = .001, compared with the frequency among those with values in the 75th quartile.
b P = .015, compared with the frequency among those with values in the 75th quartile.
c P = .236, compared with the frequency among those with values in the 75th quartile.
Postvaccination Shedding of Vaccine-Type Virus
Only 5% of participants (17 of 338) were found to be shedding vaccine-type rotavirus in stool, based on analysis of specimens collected 4 or 7 days after receipt of any vaccine dose (group 1, 6 of 121; group 2, 5 of 99; group 3, 6 of 118). All cases of shedding occurred after the first dose with the exception of one occurring after the second dose.
Safety
SAEs occurred in 8 participants; 1 (0.7%) in group 1 (a case of gastroenteritis), 4 (2.6%) in group 2 (1 case of abscess of the left jaw, 2 cases of sepsis, and 1 death from congenital hydrocephalus), and 3 (2.0%) in group 3 (2 cases of acute respiratory infection and 1 case of gastroenteritis). All SAEs were classified by the independent safety monitor as unrelated to HRV or study procedures.
DISCUSSION
Earlier studies have elicited mixed results regarding the immunological benefit of adding a third dose of HRV to the infant schedule so that the vaccine is provided at 6, 10, and 14 weeks of age [10, 12, 19]. In this study in rural Ghana, 3 doses of HRV resulted in significantly improved antirotavirus IgA seroconversion frequencies and GMCs as compared 2 doses given at the WHO-recommended ages of 6 and 10 weeks. A higher likelihood of seroconversion following 3 HRV doses was also found after controlling for maternally derived IgG concentration. In contrast, 2 doses of HRV given on a delayed schedule at 10 and 14 weeks of age increased the seroconversion frequency when compared to the 6- and 10-week arm, but this difference was not significant. As expected, the conservative approach of using the highest IgA titer in 6- and 10-week blood specimens collected 4 and 8 weeks following the last dose of HRV shifted the differences between study arms toward the null. Although there was significant improvement in the immune response (an additional 14.5% of infants seroconverted) when a third dose of HRV was added to the recommended 2-dose regimen in these Ghanaian children, the seroconversion frequency in this arm is still far lower than that among children receiving 2 doses in HICs [26].
While the results from this study in Ghana differ from a similar study conducted in Pakistan, where no significant differences in seroconversion frequencies or GMCs were observed between the standard schedule and a delayed 2-dose schedule or a 3-dose schedule [12], they are consistent with those from prior studies of HRV in Africa investigating the effects of a delayed 2-dose schedule (at ages 10 and 14 weeks) as compared to (1) 2 doses administered at younger ages (6 and 10 weeks) and (2) 3 doses (6, 10, and 14 weeks of age) [10, 19]. Although not powered to differentiate between the 6- and 10-week arm and the 10- and 14-week arm, results from a South African immunogenicity study demonstrated an even larger, though not statistically significant, increase in seroconversion frequencies and GMCs among participants receiving a lower-dose HRV at the older age as compared to the current study [19]. In an HRV efficacy and immunogenicity subset study conducted in South Africa and Malawi [10], 3 doses of HRV, administered at 6, 10, and 14 weeks of age, resulted in modestly increased antirotavirus IgA seroconversion frequencies (South Africa, 66.7%; Malawi, 57.1%) as compared to 2 doses given at 10 and 14 weeks of age (South Africa, 57.1%; Malawi, 47.2%). Although not powered to differentiate between the 2 HRV arms, the efficacy of the vaccine to prevent severe rotavirus gastroenteritis through 2 rotavirus seasons was also higher among 3-dose recipients (South Africa, 85.0%, Malawi, 42.3%), compared with 2-dose recipients (South Africa, 32.0%, Malawi, 34.0%) [21, 27], suggesting that a third dose of HRV, even when compared to 2 doses provided at an older age, provides a boost or provides another opportunity to better protect children through their second year of life.
In addition to concomitant administration of OPV with the first dose of rotavirus vaccine at 6 weeks of age, maternal antibody has also been cited as one of the probable causes of the low immune response to rotavirus vaccines in children in LICs, including in Africa [20]. The baseline IgG GMC was significantly lower in group 2, in which serum specimens were collected at 10 weeks of age, as compared to groups 1 and 3, in which serum specimens were collected at 6 weeks of age. The findings from this study, in which a third dose of HRV reduced the impact of maternally derived IgG antibodies on IgA seroconversion, are similar to those of studies in Pakistan and Vietnam, which found that the frequency of IgA seroconversion was reduced among participants with higher levels of maternally derived IgG before vaccination [12, 28]. In addition, IgA GMCs were not significantly different between the 3-dose and 2-dose arms among infants who seroconverted in the current study, indicating that infants receiving a third dose of HRV have an additional chance to respond to the vaccine at an older age, after maternal IgG antibody levels have waned and the first dose of OPV is administered.
Vaccine-like virus shedding in this study was similar to results from trials in other developing countries in which OPV was concomitantly administered. Like other trials, shedding primarily occurred following the first vaccination [29]. In Bangladesh and South Africa, where HRV doses were provided at 12 and 16 weeks of age and at 6, 10, and 14 weeks of age, respectively, approximately 5% and 13% of participants, respectively, shed rotavirus following the first dose [17, 19].
There were a few limitations to this study. First, we attempted to balance the person-time exposure to wild-type infection across study groups by using the highest titer of either the 14-week or 18-week visit for group 1, although the group 1 results from the 4-week postvaccination visit may be a more valid comparison than this more conservative measure. Second, a baseline blood sample was only collected immediately prior to the 10-week HRV dose in group 2, while it was collected prior to the 6-week HRV dose for groups 1 and 3, allowing participants in these groups an additional 4 weeks to be exposed to wild-type infection, which would have been falsely counted as an immune response to the vaccine. However, rotavirus season began later than usual in 2012, with the first rotavirus cases detected via routine surveillance in December and only 2 participants, both in group 1, seropositive prior to receipt of HRV. Finally, while serum IgA is not a mechanistic correlate of protection [30], it has been used in the development of all live attenuated rotavirus vaccines to demonstrate vaccine take and is the best surrogate marker available [31]. Thus, the improved immune responses observed in this and other African studies could indicate that there would be improved clinical protection, even if modest, in African populations.
Although there were only modest increases in immune responses in this study and mixed results in other trials, it is possible that administration of 2 doses on a delayed schedule or a third dose in LIC populations may result in a large clinical benefit. As administration of 2 doses of HRV on a delayed schedule (1) misses the opportunity to vaccinate children at the first EPI visit and prevent disease early in life and (2) was deemed operationally suboptimal by the WHO [32], inclusion of a third dose of HRV may be the most feasible option for improving clinical benefit. Since inclusion of a third dose of HRV in the EPI schedule would increase both cold chain capacity and vaccine costs, a postmarketing study designed to investigate the effectiveness and operational considerations of a third HRV dose could determine whether this schedule results in public health benefit and provide compelling evidence for policy change in LICs.
Notes
Acknowledgments. We thank all families who participated in this trial; the research team in Ghana, Ms Margaret Atta-Poku, Mr Edward Sobe, Mr Clement Narh, and the entire rotavirus study group at the Noguchi Memorial Institute for Medical Research and the Navrongo Health Research Centre, for their contributions to the study; and Dr Eileen Klein, from the University of Washington and Seattle Children's Hospital, for her role as the independent medical monitor for this study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Gates Foundation, PATH, or the Centers for Disease Control and Prevention.
Financial support. This work was supported by PATH through funding from the Bill & Melinda Gates Foundation (grant OPP1017334).
Potential conflicts of interest. M. C. M. has a laboratory service agreement with GSK and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Rotavirus vaccines WHO position paper. Wkly Epidemiol Rec 2007; 82:285–96.17691162 [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136–41. [DOI] [PubMed] [Google Scholar]
- 3.Armah GE, Breiman RF, Tapia MD et al. . Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012; 30(suppl 1):A86–93. [DOI] [PubMed] [Google Scholar]
- 4.Armah GE, Kapikian AZ, Vesikari T et al. . Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis 2013; 208:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binka FN, Anto FK, Oduro AR et al. . Incidence and risk factors of paediatric rotavirus diarrhoea in northern Ghana. Trop Med Int Health 2003; 8:840–6. [DOI] [PubMed] [Google Scholar]
- 6.Armah GE, Mingle JA, Dodoo AK et al. . Seasonality of rotavirus infection in Ghana. Ann Trop Paediatr 1994; 14:223–9. [DOI] [PubMed] [Google Scholar]
- 7.Mwenda JM, Ntoto KM, Abebe A et al. . Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J Infect Dis 2010; 202(suppl):S5–11. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Matson DO, Dennehy P et al. . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 9.Clark HF, Bernstein DI, Dennehy PH et al. . Safety, efficacy, and immunogenicity of a live, quadrivalent human-bovine reassortant rotavirus vaccine in healthy infants. J Pediatr 2004; 144:184–90. [DOI] [PubMed] [Google Scholar]
- 10.Madhi SA, Cunliffe NA, Steele D et al. . Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 11.Shin S, Anh DD, Zaman K et al. . Immunogenicity of the pentavalent rotavirus vaccine among infants in two developing countries in Asia, Bangladesh and Vietnam. Vaccine 2012; 30(suppl 1):A106–13. [DOI] [PubMed] [Google Scholar]
- 12.Ali SA, Kazi AM, Cortese MM et al. . Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J Infect Dis 2014; 210:1772–9. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR et al. . Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 14.Armah GE, Sow SO, Breiman RF et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 15.Zaman K, Dang DA, Victor JC et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 16.Tapia MD, Armah G, Breiman RF et al. . Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub-Saharan Africa. Vaccine 2012; 30(suppl 1):A79–85. [DOI] [PubMed] [Google Scholar]
- 17.Zaman K, Sack DA, Yunus M et al. . Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine 2009; 27:1333–9. [DOI] [PubMed] [Google Scholar]
- 18.Wood D. WHO informal consultation on quality, safety and efficacy specifications for live attenuated rotavirus vaccines Mexico City, Mexico, 8-9 February 2005. Vaccine 2005; 23:5478–87. [DOI] [PubMed] [Google Scholar]
- 19.Steele AD, De VB, Tumbo J et al. . Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010; 28:6542–8. [DOI] [PubMed] [Google Scholar]
- 20.Parashar UD, Glass RI. Rotavirus vaccines--early success, remaining questions. N Engl J Med 2009; 360:1063–5. [DOI] [PubMed] [Google Scholar]
- 21.Madhi SA, Kirsten M, Louw C et al. . Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30(suppl 1):A44–51. [DOI] [PubMed] [Google Scholar]
- 22.Gentsch JR, Glass RI, Woods P et al. . Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 1992; 30:1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouvea V, Glass RI, Woods P et al. . Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 1990; 28:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward RL, Bernstein DI, Shukla R et al. . Protection of adults rechallenged with a human rotavirus. J Infect Dis 1990; 161:440–5. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein DI, Smith VE, Sherwood JR et al. . Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 1998; 16:381–7. [DOI] [PubMed] [Google Scholar]
- 26.Vesikari T, Karvonen A, Korhonen T et al. . Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004; 22:2836–42. [DOI] [PubMed] [Google Scholar]
- 27.Cunliffe NA, Witte D, Ngwira BM et al. . Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30(suppl 1):A36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anh DD. National Development and Licensure of a Human Monovalent Rotavirus Vaccine (ROTAVIN-M1) in Vietnam. 2012.
- 29.Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 2008; 8:642–9. [DOI] [PubMed] [Google Scholar]
- 30.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol 2012; 2:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheuvart B, Neuzil KM, Steele AD et al. . Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum Vaccin Immunother 2014; 10:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Meeting of the immunization Strategic Advisory Group of Experts, April 2009 - Conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:220–36. [PubMed] [Google Scholar]