Abstract
Background. The burden of rotavirus morbidity and mortality is high in children aged <5 years in developing countries, and evaluations indicate waning protection from rotavirus immunization in the second year. An additional dose of rotavirus vaccine may enhance the immune response and lengthen the period of protection against disease, but coadministration of this dose should not interfere with immune responses to concurrently given vaccines.
Methods. A total of 480 9-month-old participants from Matlab, Bangladesh, were enrolled in a study with a primary objective to establish noninferiority of concomitant administration of measles-rubella vaccine (MR) and a third dose of human rotavirus vaccine (HRV; MR + HRV), compared with MR given alone. Secondary objectives included noninferiority of rubella antibody seroconversion and evaluating rotavirus IgA/IgG seroresponses in MR + HRV recipients.
Results. Two months after vaccination, 75.3% and 74.3% of MR + HRV and MR recipients, respectively, had seroprotective levels of measles virus antibodies; 100.0% and 99.6%, respectively, showed anti–rubella virus immunoglobulin G (IgG) seroprotection. In the MR + HRV group, antirotavirus immunoglobulin A and IgG seropositivity frequencies before vaccination (52.7% and 66.3%, respectively) increased to 69.6% and 88.3% after vaccination.
Conclusions. Vaccine-induced measles and rubella antibody responses are not negatively affected by concomitant administration of HRV. The HRV dose increases antirotavirus serum antibody titers and the proportion of infants with detectable antirotavirus antibody.
Clinical Trials Registration. NCT01700621.
Keywords: rotavirus vaccine, measles vaccine, rubella vaccine, vaccine co-administration, non-interference
(See the editorial commentary by Cunliffe and Kang on pages 1673–5, and major article by Armah et al on pages 1678–85.)
Rotavirus-associated morbidity and mortality are high in infants in developing countries and continue to be important through the second year of life [1, 2]. In rural Bangladesh, for example, 45% of the cases of severe rotavirus disease in children <5 years of age occur after the first year of life [3]. Developing world clinical trials and postlicensure evaluations of currently available human rotavirus vaccines (HRVs) indicate that protection is sub-optimal and may wane in the second year of life [4–6]. An additional dose of HRV given at 9 months of age along with other Expanded Program on Immunization (EPI) vaccines such as measles-rubella vaccine (MR) might enhance immunity to rotavirus and extend the duration of protection. To consider an additional dose of HRV for inclusion in the current schedule requires the demonstration that co-administration does not interfere with the immune response to the existing MR [7, 8]. We therefore tested the primary hypothesis that the immunogenicity of measles vaccine coadministered with monovalent HRV (Rotarix, GSK Biologicals, Dresden, Germany) at 9 months of age was not inferior to that of measles vaccine (given as MR) when administered alone. Secondary aims included (1) describing the safety profile of concomitant administration of MR and HRV, (2) assessing the noninferiority of the immune response to rubella immunization (as MR) when given concomitantly with HRV, and (3) evaluating the immune response to an additional dose of HRV given at 9 months of age.
METHODS
Study Design and Participants
This single-center, open-label, randomized, parallel-group trial was conducted from January through September 2013 among the infant population of the Matlab Health and Demographic Surveillance System in rural Bangladesh. Healthy infants 9 months (+1 month) of age who had previously received 2 doses of HRV—along with EPI vaccines, including oral polio vaccine (OPV)—at approximately 6 and 10 weeks of age through a 3-year pilot rotavirus vaccination program were eligible to participate in the study [9]. Infants were excluded if they had a history of hypersensitivity to any study vaccine component; had a history of intussusception, intestinal malformations, or abdominal surgery; had a history of measles and/or rubella or receipt of measles and/or rubella vaccine; used any immunosuppressive drugs or immunoglobulin and/or blood products prior to or during the study; were currently enrolled in any other interventional trial; or planned to leave the study area before study completion. Temporary exclusions included acute diarrhea (defined as ≥3 loose stools within a 24-hour period) or vomiting (defined as projectile vomiting or any vomiting at the discretion of the clinician) at the time of enrollment or within the last 24 hours, and/or acute febrile illness (defined as a temperature of ≥38°C) at the time of enrollment.
The trial was approved by the ethics review boards of the International Centre for Diarrhoeal Disease Research, Bangladesh (Dhaka); the Western Institutional Review Board (Puyallup, Washington); and the Centers for Disease Control and Prevention (Atlanta, Georgia). The study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with good clinical practice guidelines. Written informed consent was obtained from parents or guardians prior to enrollment. The trial was registered with ClinicalTrials.gov (NCT01700621).
Procedures
Participants were recruited and sequentially assigned in a 1:1 ratio, via block randomization with a block size of 6, to receive either subcutaneous injection of MR (Measles and Rubella Virus Vaccine Live USP, Serum Institute of India, Pune, India) plus lyophilized oral HRV (hereafter, “MR + HRV”) or MR only. Group assignment was masked for all laboratory personnel responsible for immunogenicity and stool testing.
All infants were observed for 30 minutes after vaccination, and parents were instructed to seek care at the study clinic for any illnesses in participants during the study period. Local and systemic adverse events were assessed on days 4 (±1), 7 (±1), 11 (±2), 14 (±2), and 28 (±3) after vaccination, through home visits by trained field workers. Solicited adverse events included diarrhea, fever, vomiting, loss of appetite, irritability, and signs of intussusception (defined as colicky abdominal pain or paroxysms of crying and/or screaming, abdominal distention or mass, intermittent irritability or lethargy with behavioral changes, stool with blood or “red currant jelly,” and/or vomiting ≥2 episodes/day). All serious adverse events (SAEs) through 2 months after vaccination were triaged by study physicians and reported to an independent safety monitor, who characterized the possible relationship to study product, in addition to the characterization made by the study physicians and investigator.
Serum specimens were collected from each infant on day 0, prior to study vaccinations, and 2 months after vaccination (at 11–12 months of age). To provide an additional possible measure of rotavirus vaccine take in the MR + HRV group, stool specimens were collected on days 0, 4 (±1), and 7 (±1) after vaccination, to assess vaccine shedding [10, 11].
Outcomes
Sera were analyzed for the presence of measles virus serum neutralizing antibodies (SNAs), using a standardized plaque-reduction neutralization (PRN) assay, in which PRN titers, defined as the serum dilutions that reduced the number of plaques by 50%, were calculated using the Kärber method [12]. A 1:100 dilution of World Health Organization (WHO) Second International Standard Anti-Measles Serum (IS, coded 66/202, supplied by the National Institute for Biological Standards and Control, South Mimms, United Kingdom) was tested in parallel with each serum specimen to calculate the reciprocal of the 50% end point titer determined by the PRN test, and based on the validation of the WHO standard, titers were expressed as mIU/mL. Measles virus SNA seropositivity was defined as a PRN concentration of ≥8 mIU/mL and seroprotection as a PRN concentration of >120 mIU/mL [12, 13].
Serum specimens were also analyzed in duplicate for both measles virus and rubella virus immunoglobulin G (IgG) antibodies, using commercially available indirect enzyme-linked IgG immunoassays (EIAs; Wampole Laboratories, Princeton, New Jersey). An index standard ratio (ISR) of ≥1.10 on both runs of the respective assay was considered evidence of seropositivity for measles virus or seroprotection against rubella. In the case of rubella, an ISR of at least 1.10 represents 10 IU/mL of rubella virus antibody, consistent with a protective level. All measles virus and rubella virus assays were performed at the National Measles and Rubella Reference Laboratories, Measles, Mumps, Rubella, and Herpesviruses Branch, Division of Viral Diseases, Centers for Disease Control and Prevention (Atlanta, Georgia).
Antirotavirus immunoglobulin A (IgA) and IgG were measured by enzyme-linked immunosorbent assay at the Laboratory of Specialized Clinical Studies at the Cincinnati Children′s Hospital Medical Center (Cincinnati, Ohio), as described previously [14–16]. A standard serum pool was used to determine arbitrary units of rotavirus IgA or IgG in each sample. A subject was considered seropositive if the IgA or IgG rotavirus antibody concentration was ≥20 U/mL [14, 16]. Rotavirus antigen in stool was detected by EIA by the laboratory at the International Centre for Diarrhoeal Disease Research, Bangladesh, using a commercially available EIA (ProSpecT Rotavirus Microplate Assay, Oxoid Ltd, Basingstoke, United Kingdom). Rotavirus G and P types were confirmed by reverse-transcription polymerase chain reaction [17]; G1P[8] rotavirus detected in day 4 or 7 specimens (but not day 0 specimens) were assumed to be vaccine type and confirmed on the basis of partial VP7 gene sequencing.
Statistical Analyses
The primary analysis was conducted on the per-protocol cohort, which included infants meeting all inclusion and no exclusion criteria, having less than seroprotective levels of measles virus SNAs before vaccination, and receiving study vaccines and undergoing blood specimen collection according to schedule. Rubella virus–associated secondary analyses were conducted on the same per-protocol cohort, except that infants were required to have less than seroprotective levels of anti–rubella virus IgG before vaccination instead of measles virus SNAs. For the measles vaccine and rubella vaccine immunogenicity analyses, proportions of participants reaching seroprotective levels of measles virus SNAs or anti–rubella virus IgG 2 months after vaccination, respectively, were compared between groups, using the Newcombe-Wilson method without continuity correction. A noninferiority margin of −10% was chosen as the maximal absolute reduction in proportion seroprotection allowed in the concomitant MR + HRV group as compared to the MR alone group. Geometric mean concentrations (GMCs) were calculated for measles virus SNAs and rubella virus IgG.
Rotavirus vaccine immunogenicity analyses were conducted on the per-protocol cohort without the requirement for less than seroprotective levels of measles virus SNAs before vaccination. Antirotavirus IgA and IgG geometric mean titers (GMTs) and the proportion of infants who were seropositive were compared before vaccination and 2 months after vaccination in each group, using the McNemar test for correlated proportions; for GMT calculations, concentrations of <20 U/mL were converted to 10 U/mL. Percentages of individuals with G1P[8] rotavirus detected in stool specimens on day 4 or 7 after vaccination were also calculated with 95% confidence intervals (CIs) using the Wilson score method without continuity correction.
Under the assumptions that 12% of infants would have seroprotective levels of measles virus SNAs at baseline, that 90% of infants without seroprotective levels of measles virus SNA would reach seroprotective levels following receipt of MR alone, and that 10% of participants would withdraw from the study, 480 infants were enrolled to provide at least 90% statistical power to rule out a difference in measles virus seroconversion of −10% between the groups (calculated as the proportion in the MR group minus that in the MR + HRV group), using a 1-sided significance level of 0.025.
RESULTS
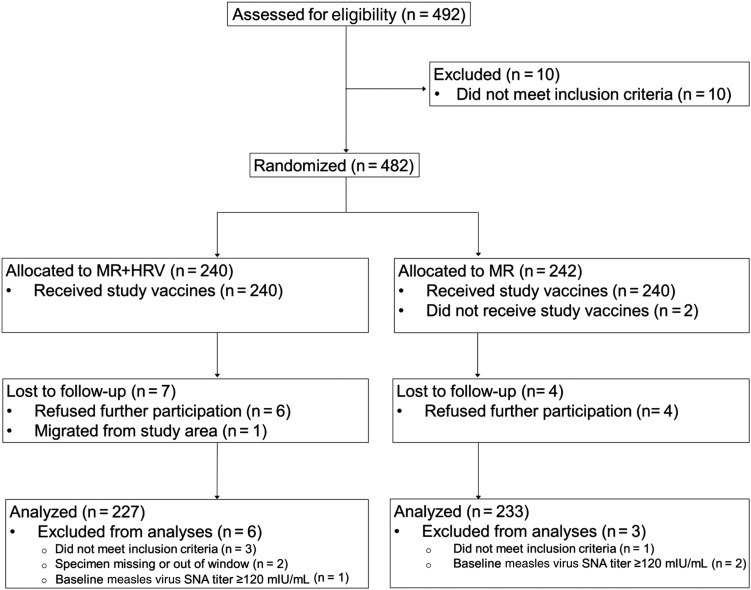
The trial profile is presented in Figure 1. Of 482 participants enrolled and randomized, 227 (94.6%) from the MR + HRV group and 233 (96.3%) from the MR group were included in per-protocol analysis of measles vaccine immunogenicity. Table 1 describes baseline characteristics of randomized participants, which were similar across groups.
Figure 1.
Trial profile. Abbreviations: HRV, human rotavirus vaccine; MR, measles-rubella vaccine; SNA, serum neutralizing antibody.
Table 1.
Baseline Characteristics of Randomized Participants Receiving Study Vaccine
| Characteristic | MR + HRV (n = 240) | MR (n = 240) |
|---|---|---|
| Male sex | 130 (54.2) | 117 (48.8) |
| Age, mo | 9.4 ± 0.25 | 9.4 ± 0.25 |
| Ever breastfed | 240 (100.0) | 240 (100.0) |
| Length, cm | 69.6 ± 2.49 | 69.8 ± 2.79 |
| Weight, kg | 8.0 ± 1.10 | 8.0 ± 0.99 |
| Mother's age, y | 28.1 ± 5.66 | 28.0 ± 6.06 |
Data are no. (%) of children or mean value ± SD.
Abbreviations: HRV, human rotavirus vaccine; MR, measles-rubella vaccine.
Measles Virus and Rubella Virus
Baseline measles virus SNA seroprotection in the MR + HRV and MR groups were low (0.42% [1 of 240 infants] and 1.25% [3 of 240], respectively), as was preexisting baseline anti–rubella virus IgG seroprotection (0% [0 of 240 infants] and 0.42% [1 of 240], respectively). After vaccination, 75.3% of infants (95% CI, 69.3%–80.5%) in the MR + HRV group and 74.3% (95% CI, 68.3%–79.4%) in the MR group reached seroprotective levels of measles virus SNAs (Table 2). Measles virus SNA GMCs and anti–measles virus IgG seropositivity after vaccination were similar in the 2 groups (Table 2). Seroprotective levels of anti–rubella virus IgG were reached by nearly all participants in both groups after vaccination, and anti–rubella virus IgG GMCs were comparable (Table 2).
Table 2.
Postvaccination Proportions of Infants Seroprotected Against Measles and Rubella, With Accompanying Between-Group Differences and Geometric Mean Concentrations (GMCs)
| Immune Measure | MR + HRV |
MR Alone |
Difference in Seroconversion |
||||
|---|---|---|---|---|---|---|---|
| Subjects, Proportion | % (95% CI) | GMC (95% CI) | Subjects, Proportion | % (95% CI) | GMC (95% CI) | % (95% CI) | |
| Measles virus SNAsa | 171/227 | 75.3 (69.3–80.5) | 196.3 (175.3–219.7) | 173/233 | 74.3 (68.3–79.4) | 194.2 (174.0–216.7) | 1.1 (−6.9 to 9.0) |
| Anti–measles virus IgGb | 219/227 | 96.5 (93.2–98.2) | Not defined | 227/232 | 97.8 (95.1–99.1) | Not defined | −1.4 (−4.9 to 1.9) |
| Anti–rubella virus IgGc | 228/228 | 100.0 (98.3–100.0) | 191.9 (174.8–210.7) | 233/234 | 99.6 (97.6–99.9) | 177.8 (161.8–195.4) | 0.4 (−1.3 to 2.4) |
Data are for all children meeting inclusion/exclusion criteria.
Abbreviations: CI, confidence interval; HRV, human rotavirus vaccine; IgG, immunoglobulin G; MR, measles-rubella vaccine.
a Defined as a measles virus serum neutralization antibody (SNA) concentration of >120 mlU/mL.
b Defined by operating characteristic of the enzyme-linked immunoassay (EIA). Seroprotective levels are not defined for the EIA.
c Defined as a rubella virus IgG concentration of ≥10 IU/mL.
Rotavirus
Prevaccination antirotavirus IgA and IgG seropositivity (defined as a titer of ≥20 U/mL) frequencies at 9 months of age were 52.7% (95% CI, 46.4%–59.0%) and 66.3% (95% CI, 60.1%–71.9%), respectively, in the MR + HRV group. These increased to 69.6% (95% CI, 63.3%–75.2%; P <.001) and 88.3% (95% CI, 83.5%–91.8%; P <.001) after vaccination (Table 3). The antirotavirus IgA and IgG GMTs also increased significantly from prevaccination levels (Table 3). In the MR only group (ie, the group that received no HRV), antirotavirus IgA and IgG seropositivity frequencies and GMTs after vaccination remained virtually unchanged from those before vaccination (Table 3).
Table 3.
Rotavirus Seropositivity and Geometric Mean Titers (GMT), Before and 2 Months After Vaccination, and Immunoglobulin A (IgA) and Immunoglobulin G (IgG) Seroconversion
| Immune Measure | MR + HRV |
MR Alone |
||||
|---|---|---|---|---|---|---|
| Subjects, Proportion | % (95% CI) | GMTa (95% CI) | Subjects, Proportion | % (95% CI) | GMTa (95% CI) | |
| Seropositivityb,c | ||||||
| Antirotavirus IgA | ||||||
| Before vaccination | 126/239 | 52.7 (46.4–59.0) | 43.6 (34.8–54.5) | 113/240 | 47.1 (40.9–53.4) | 39.2 (31.3–49.1) |
| After vaccination | 160/230 | 69.6 (63.3–75.2) | 60.6 (49.4–74.2) | 110/236 | 46.6 (40.4–53.0) | 38.1 (30.5–47.6) |
| Antirotavirus IgG | ||||||
| Before vaccination | 159/240 | 66.3 (60.1–71.9) | 79.2 ( 61.4–102.1) | 140/240 | 58.3 (52.0–64.4) | 63.2 (49.1–81.4) |
| After vaccination | 204/231 | 88.3 (83.5–91.8) | 168.6 (137.3–207.2) | 135/236 | 57.2 (50.8–63.4) | 60.1 (46.7–77.5) |
| Subjects, Proportion | % (95% CI) | Subjects, Proportion | % (95% CI) | |||
| Seroconversiond | ||||||
| IgA | 48/110 | 43.6 (34.7–53.0) | 8/126 | 6.4 (3.3–12.0) | ||
| IgG | 55/80 | 68.8 (57.9–77.9) | 7/99 | 7.1 (3.5–13.9) | ||
Abbreviations: CI, confidence interval; HRV, human rotavirus vaccine; MR, measles-rubella vaccine.
a Rotavirus IgA and IgG values of <20 U/mL are converted to 10 U/mL for calculation purposes.
b Defined as titers of ≥20 U/mL. Data are for all randomized children, including 3 children in the MR + HRV group and 1 child in the MR group who did not meet the primary objective inclusion criteria of receiving the first dose of their primary rotavirus vaccine series between 6 and 10 weeks of age (42–70 days; vaccines were received on days 72, 79, and 102 for children in the MR + HRV group and on day 72 for the child in the MR group).
c Differences in denominators before and after vaccination are due to insufficient serum or to withdrawals from study.
d Defined as titers of ≥20 U/mL in previously seronegative subjects. Data are for all randomized children with serum specimens available before and after vaccination.
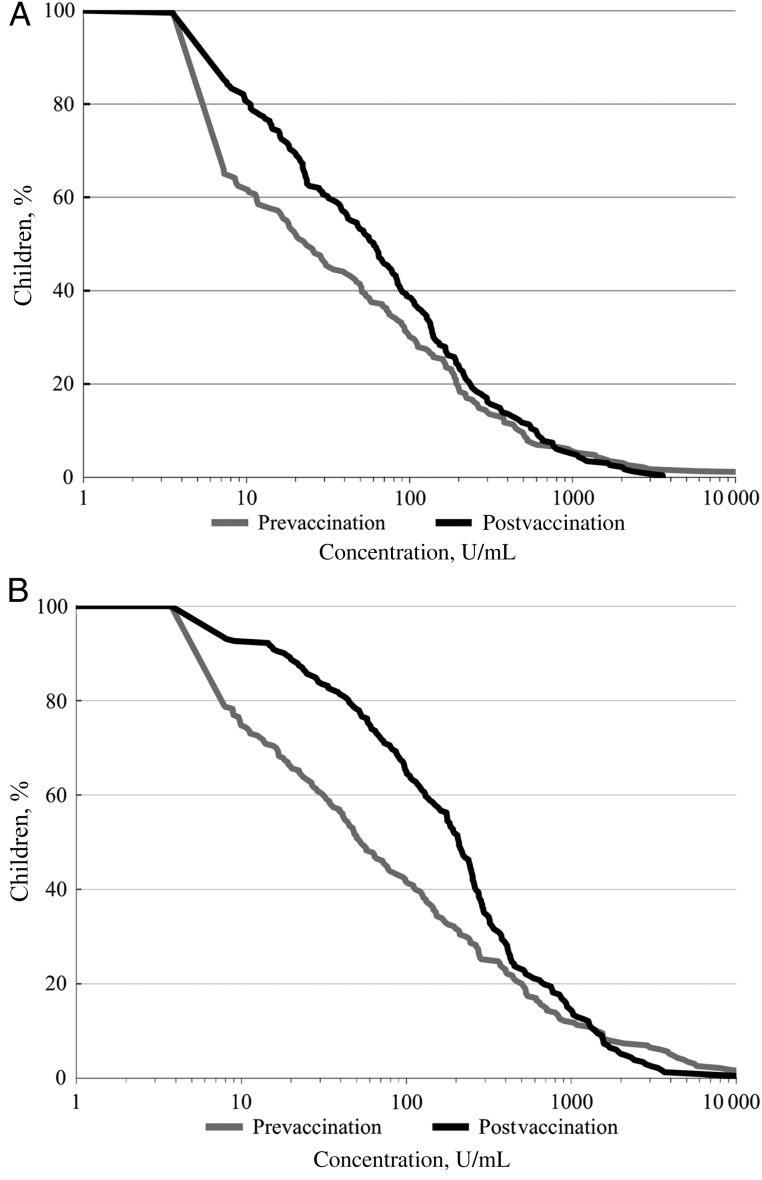
Reverse cumulative distribution plots of antirotavirus IgA and IgG titers in the MR + HRV group demonstrated that a large portion of the improvement in rotavirus immune status occurred among participants who were seronegative before vaccination (IgA/IgG titer, <20 U/mL) or those with seropositive concentrations at the lower end of the distributions (Figure 2A and 2B). Among infants in the MR + HRV group who were seronegative for antirotavirus IgA or IgG before vaccination, 43.6% (95% CI, 34.7%–53.0%; P <.001) became seropositive for antirotavirus IgA, and 68.8% (95% CI, 57.9%–77.9%; P <.001) became seropositive for antirotavirus IgG, respectively, after the 9 month HRV vaccination (Table 3). A total of 62 infants were seronegative for antirotavirus IgA at baseline and did not seroconvert after vaccination. Among these, 18 (29.0%) were seropositive for antirotavirus IgG before vaccination, and 44 (71.0%) were seronegative for IgG before vaccination. Among the latter subset, 24 of 44 (54.5%) became antirotavirus IgG seropositive after vaccination.
Figure 2.
Reverse cumulative distribution plot of antirotavirus immunoglobulin A (IgA) concentrations (U/mL) (A) and anti-rotavirus IgG concentrations (U/mL) (B) before vaccination and 2 months after vaccination among infants who received measles-rubella vaccine plus human rotavirus vaccine. Data are for all randomized children with prevaccination and postvaccination serum samples and rotavirus test results available.
In stool specimens, HRV shedding was not identified in any of the 140 specimens collected at day 0 from infants in the MR + HRV group, but was confirmed in 4 of 238 specimens (1.7%; 95% CI, 0.7%–4.2%) and 6 of 240 specimens (2.5%; 95% CI, 1.2%–5.4%) collected in the MR + HRV group on days 4 and 7, respectively.
Safety
There were no immediate reactions following vaccination and no differences in the frequency of solicited or unsolicited nonserious adverse events between study groups (Table 4). Sixteen SAEs were reported, of which 10 were in the MR + HRV group, and 6 were in the MR group; none of the SAEs were considered related to vaccine (results not shown). No suspected or confirmed cases of intussusception were identified, and no deaths occurred.
Table 4.
Solicited Adverse Events of Any Grade (Safety Population), by Day After Vaccination
| Adverse Event, Time | MR + HRV, Subjects, No. (%) (n = 240) |
HRV, Subjects, No. (%) (n = 240) |
|---|---|---|
| Any reaction | ||
| Day 0–3 | 31 (12.9) | 38 (15.8) |
| Day 4–14 | 86 (17.9) | 84 (17.5) |
| Loss of appetite | ||
| Day 0–3 | 1 (0.4) | 0 (0.0) |
| Day 4–14 | 5 (1.1) | 2 (0.4) |
| Diarrhea | ||
| Day 0–3 | 7 (2.9) | 12 (5.0) |
| Day 4–14 | 23 (4.8) | 28 (5.8) |
| Fever | ||
| Day 0–3 | 21 (8.8) | 27 (11.3) |
| Day 4–14 | 62 (12.9) | 53 (11.0) |
| Intussusception | ||
| Day 0–3 | 0 (0.0) | 0 (0.0) |
| Day 4–14 | 0 (0.0) | 0 (0.0) |
| Irritability | ||
| Day 0–3 | 0 (0.0) | 0 (0.0) |
| Day 4–14 | 1 (0.2) | 3 (0.6) |
| Vomiting | ||
| Day 0–3 | 6 (2.5) | 7 (2.9) |
| Day 4–14 | 20 (4.2) | 24 (5.0) |
Abbreviations: HRV, human rotavirus vaccine; MR, measles-rubella vaccine.
DISCUSSION
This study demonstrates that concomitant administration of MR and HRV was safe and that vaccine-induced measles and rubella immunity was not negatively affected by concomitant use of an additional dose of HRV at 9 months of age in this population. The study′s prespecified noninferiority criteria, a reduction in immune response of not more than 10% for both measles and rubella vaccines, were met. Additionally, a large majority of infants demonstrated improvements in measures for antirotavirus serum antibodies, with increases particularly focused among the infants with low levels before vaccination. Finally, this study demonstrated an excellent reactogenicity and safety profile for MR and HRV when given concomitantly to infants. The majority of local and systemic reactions were mild and transient, and none of the SAEs were considered by independent safety monitors to be associated with vaccine administration.
While EIA is often the primary test used to evaluate measles vaccine noninferiority, in this study we chose to also measure measles virus SNAs, as this is the gold standard and provides a measure of seroprotection. While the proportion of infants achieving seroprotective levels of measles virus SNAs after vaccination was similar between groups, it was below that assumed during the design of the study. However, EIA revealed that a very high proportion (>95%) of infants in both groups demonstrated seroconversion with measles vaccination, and regardless of the method used, noninferiority of stand-alone and concomitant vaccination was achieved. In this study, the measles vaccine seroresponse was measured 2 months after vaccination [18, 19], and it is unknown whether measurement of SNAs at an even later time point would have resulted in increased measured proportions of infants seroprotected. Other studies in low-resource populations have found that the immune response to measles vaccination matures over the course of many months [19–23]. On the contrary, rotavirus IgA titers generally peak earlier, at 14–28 days after vaccination, so it is possible that antirotavirus responses may have waned somewhat by the time our samples were collected [24].
Our study was designed as a proof-of-concept investigation of whether additional HRV doses might provide some benefit to infants, which was indicated. Another indication of a positive vaccine response, the detection of HRV shedding in stool, was also measured on day 4 and day 7 after vaccination, but very little shedding was observed. This is consistent with previous findings of very low shedding in infants from this same population after a second dose of HRV, presumably due to preexisting antibodies suppressing growth of attenuated vaccine virus [25]. While the lack of shedding does not seem congruent with the low proportion of children with detectable preexisting antibody before vaccination and the generally positive immune responses we measured serologically, one of our study inclusion criteria was receipt of 2 primary rotavirus vaccine doses, and the epidemiology of rotavirus in Bangladesh indicates that a large proportion of infants would have been exposed naturally.
This study found that antirotavirus antibody levels several months after receipt of the 2-dose HRV at 6 and 10 weeks of age (and before administration of an additional dose of HRV) were low or essentially undetectable in a substantial proportion of infants. It was these infants who demonstrated much of the improvement in antirotavirus serum IgA and IgG levels. Unfortunately, without an established serologic immune correlate of protection for rotavirus, it is not clear the degree to which these improvements in antibody levels might translate to clinical protection from severe diarrhea.
Coadministration of live attenuated rotavirus vaccines have been shown to not interfere with immune responses to other routine childhood vaccines, including diphtheria-pertussis-tetanus vaccine, Haemophilus influenzae type b vaccine, hepatitis B vaccine, and both inactivated and oral polio vaccines [26–29], but this is the first evaluation of coadministration with injected measles vaccine or measles-rubella vaccine. Studies in Brazil have found that yellow fever vaccine can interfere with rubella vaccine [30], but we found no such effect of HRV on the response to the rubella component of MR. Previous studies in infants have indicated a modest interference of oral polio vaccine on rotavirus vaccine immunogenicity, but this is offset with multiple doses [31, 32].
Developing-world trials of currently available rotavirus vaccines, including HRV, have demonstrated efficacy at moderate levels that are below those measured in efficacy trials in the United States, Europe, and Latin America [4–6]. Additionally, postmarketing observational studies of rotavirus vaccination in poverty-challenged populations have further confirmed that effectiveness is modest in infants from these settings and may wane in subsequent years [33–36]. Many factors have been postulated to account for the waning immunity and clinical protection seen in developing settings [37], but this is the first study evaluating the potential for additional doses of rotavirus vaccine provided outside early infancy to improve immunity levels among infants or young children.
The strength of our study was that it was a randomized trial conducted in a population representative of the target low-resource populations that might benefit most from future use of additional doses of rotavirus vaccine to improve protection, particularly into the second year of life.
Our study had some limitations. First, the study outcome was immunogenicity, and there is no accepted serological immune correlate of protection against rotavirus, although serum IgA is regarded as the best surrogate marker of protection available [38, 39]. We did not have a 14-week serum sample (after the 6- and 10-week HRV doses were given) to measure the baseline immune response for our study; this would have allowed us to better interpret the serum results at 9 months of age and the results after the additional HRV dose. Second, although this small study could not rule out an increased risk of intussusception following HRV immunization on the order of magnitude that might be expected from other reports of HRV-associated intussusception [40], we monitored for this, given that the additional dose of rotavirus vaccine was provided to older age infants, when intussusception is epidemiologically most common [41]. Postlicensure evaluations have not identified an increased risk of intussusception from the third dose of a licensed bovine pentavalent vaccine (RotaTeq, Merck, Kenilworth, New Jersey), which was usually given at 6 months of age in these evaluations [42, 43].
Novel rotavirus vaccine candidates in development that might show higher efficacy in developing populations are still many years away. Thus, a programmatic change to provide additional doses might be a feasible approach to optimizing protection of the currently available live attenuated rotavirus vaccines. Before such an additional dose can be considered programmatically, further studies are needed to evaluate the improvement in protection such a dose may provide, as well as to monitor the safety of such an approach and characterize the programmatic feasibility and cost-protection/cost-benefit.
Notes
Acknowledgments. We thank Sun Bae Sowers and Lijuan Hao, for technical expertise in the performance of the measles and rubella assays and assistance in analyzing the data for quality assurance purposes; and the study participants and caregivers, for their valuable time and information. The International Centre for Diarrhoeal Disease Research, Bangladesh, thanks PATH, for commitment to its research efforts; and the governments of Australia, Bangladesh, Canada, Sweden, and the United Kingdom, for providing core/unrestricted support.
K. M. N., A. D. S., J. A. F., U. P., K. Z., and M. M. C. conceived and designed the study. K. Z., T. A., M. Y., M. R., S. M. N. M., and J. A. F. undertook, supervised, or monitored the study. J. A. F., K. Z., J. C. V., M. Y., T. I. A. B., W. J. B., J. P. I., B. L., U. P., and K. M. N. analyzed and interpreted data. M. M. supervised the analysis of the rotavirus antibody assays and reviewed all data prior to reporting. All authors contributed to the drafting of the report and approved the final submitted manuscript.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the US Department of Health and Human Services.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant OPP1017344 to PATH).
Potential conflicts of interest. M. M. has laboratory service agreements with Merck, Sanofi Pasteur, and GlaxoSmithKline. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet ID 2012; 12:136–41. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K, Yunus M, Faruque ASG et al. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine 2009; 27(suppl 5):F31–4. [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Cunliffe NA, Steele AD et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 5.Zaman K, Dang DA, Victor JC et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 6.Armah GE, Sow SO, Breiman RF et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 7.Gatchalian S, Yao Y, Zhou B et al. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine 2008; 26:2234–41. [DOI] [PubMed] [Google Scholar]
- 8.Victor JC, Gatchalian S, Yao Y et al. Corrigendum to “Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants” [Vaccine 26 (2008) 2234–2241] Vaccine 2014; 32:306–8. [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, Yunus M, Sack D et al. Effectiveness of the oral human rotavirus vaccine (Rix4414) in Bangladeshi infants: a cluster-randomized trial [abstract 39]. Presented at: Tenth International Rotavirus Symposium, Bangkok, Thailand, 2012. [Google Scholar]
- 10.Dennehy PH, Brady RC, Halperin SA et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J 2005; 24:481–8. [DOI] [PubMed] [Google Scholar]
- 11.O'Ryan M. Rotarix (RIX4414): An oral human rotavirus vaccine. Expert Rev Vaccines 2007; 6:11–9. [DOI] [PubMed] [Google Scholar]
- 12.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 2007; 26:59–66. [DOI] [PubMed] [Google Scholar]
- 13.Chen RT, Markowitz LE, Albrecht P et al. Measles antibody: reevaluation of protective titers. J Infect Dis 1990; 162:1036–42. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein DI, Smith VE, Sherwood JR et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89–12. Vaccine 1998; 16:381–7. [DOI] [PubMed] [Google Scholar]
- 15.Knowlton DR, Spector DM, Ward RL. Development of an improved method for measuring neutralizing antibody to rotavirus. J Virol Methods 1991; 33:127–34. [DOI] [PubMed] [Google Scholar]
- 16.Ward RL, Bernstein DI, Shukla R et al. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis 1989; 159:79–88. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Sultana R, Ahmed G et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg Infect Dis 2007; 13:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helfand RF, Kebede S, Gary HE Jr, Beyene H, Bellini WJ. Timing of development of measles-specific immunoglobulin M and G after primary measles vaccination. Clin Diagn Lab Immunol 1999; 6:178–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson MM, Spady DW, Albrecht P et al. Measles vaccination of infants in a well-vaccinated population. Pediatr Infect Dis J 1995;14:17–22. [DOI] [PubMed] [Google Scholar]
- 20.Wijesinghe PR, Abeysinghe MRN, Yoksan S et al. Safety and immunogenicity of live-attenuated Japanese encephalitis SA 14-14-2 vaccine co-administered with measles vaccine in 9-month-old infants in Sri Lanka. Vaccine 2014; 32:4751–7. [DOI] [PubMed] [Google Scholar]
- 21.Whittle HC, Campbell H, Rahman S, Armstrong JR. Antibody persistence in Gambian children after high-dose Edmonston-Zagreb measles vaccine. Lancet 1990; 336:1046–8. [DOI] [PubMed] [Google Scholar]
- 22.Martins C, Garly ML, Bale C et al. Measles antibody levels after vaccination with Edmonston-Zagreb and Schwarz measles vaccine at 9 months or at 9 and 18 months of age: a serological study within a randomised trial of different measles vaccines. Vaccine 2013; 31:5766–71. [DOI] [PubMed] [Google Scholar]
- 23.Fowlkes A, Witte D, Beeler J et al. Persistence of vaccine-induced measles antibody beyond age 12 months: a comparison of response to one and two doses of Edmonston-Zagreb measles vaccine among HIV-infected and uninfected children in Malawi. J Infect Dis 2011; 204:S149–57. [DOI] [PubMed] [Google Scholar]
- 24.Davidson GP, Hogg RJ, Kirubakaran CJ. Serum and intestinal immune response to rotaviral enteritis in children. Infect Immun 1983; 40:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaman K, Sack DA, Yunus M et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine 2009; 27:1333–9. [DOI] [PubMed] [Google Scholar]
- 26.Ciarlet M, Sani-Grosso R, Yuan G et al. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J 2008; 27:874–80. [DOI] [PubMed] [Google Scholar]
- 27.Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008; 122:e1062–6. [DOI] [PubMed] [Google Scholar]
- 28.Tregnaghi MW, Abate HJ, Valencia A et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 2011; 30:e103–8. [DOI] [PubMed] [Google Scholar]
- 29.Salinas B, Perez SI, Linhares AC et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24:807–16. [DOI] [PubMed] [Google Scholar]
- 30.Nascimento Silva JR, Camacho LAB, Siqueira MM et al. Mutual interference on the immune response to yellow fever vaccine and a combined vaccine against measles, mumps and rubella. Vaccine 2011; 29:6327–34. [DOI] [PubMed] [Google Scholar]
- 31.Steele AD, De VB, Tumbo J et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010; 28:6542–8. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Rotavirus vaccines—WHO position paper- January 2013. WEER 2013; 88:49–64. [Google Scholar]
- 33.Patel M, Patzi M, Pastor D et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Palma O, Cruz L, Ramos H et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel M, Pedreira C, De Oliveira LH et al. Association between pentavalent rotavirus vaccine and sever rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243–51. [DOI] [PubMed] [Google Scholar]
- 36.Groome M, Page N, Cortese M et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014; 14:1096–104. [DOI] [PubMed] [Google Scholar]
- 37.Neuzil KM, Zaman K, Victor JC. A proposed framework for evaluating and comparing efficacy estimates in clinical trials of new rotavirus vaccines. Vaccine 2014; 32(suppl 1):A179–84. [DOI] [PubMed] [Google Scholar]
- 38.Desselberger U. Rotaviruses. Virus Res 2014; 190:75–96. [DOI] [PubMed] [Google Scholar]
- 39.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol 2012; 2:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel MM, López-Collada VR, Bulhões MM et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 364:2283–92. [DOI] [PubMed] [Google Scholar]
- 41.Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines 2009; 8:1555–64. [DOI] [PubMed] [Google Scholar]
- 42.Buttery JP, Danchin MH, Lee KJ et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29:3061–6. [DOI] [PubMed] [Google Scholar]
- 43.Loughlin J, Mast C, Doherty M, Wang F, Wong J, Seeger J. Postmarketing evaluation of the short-term safety of the pentavalent rotavirus vaccine. Pediatr Infect Dis J 2012; 31:292–6. [DOI] [PubMed] [Google Scholar]