Abstract
Background. Mycoplasma genitalium is an emerging sexually transmitted pathogen implicated in inflammatory syndromes of the female reproductive tract. The objective of this study was to investigate human immunodeficiency virus (HIV)–infected women for an association between M. genitalium and cervicitis, a putative mechanism for enhanced HIV transmission efficiency to an uninfected partner.
Methods. Using a longitudinal cohort of antiretroviral therapy–adherent New Orleans women, we retrospectively screened for M. genitalium and quantitatively characterized several markers of cervical inflammation, including secreted cytokines and cytological and histological signs of leukocyte infiltration.
Results. We observed a high prevalence of M. genitalium (7.4%) among HIV-infected New Orleans women. Chronic M. genitalium infection was associated with increased secretion of proinflammatory cytokines, including interleukin 1β, interleukin 6, and interleukin 8, and marked inflammatory cervical infiltrates in the cervix with enrichment of HIV target cells. Cure of M. genitalium infection resulted in ablation of all signs of inflammation.
Conclusions. These findings implicate M. genitalium as an etiologic agent of cervicitis in HIV-infected women, providing a potential mechanism for enhanced HIV transmission to an uninfected partner. Screening and treatment of M. genitalium among HIV-infected individuals may be warranted to further understand this coinfection scenario, improve cervical health, and reduce the spread of HIV.
Keywords: Mycoplasma genitalium, cervicitis, inflammation, sexually transmitted infection, HIV, AIDS, cervical inflammation
Mycoplasma genitalium is a prevalent and emerging etiologic agent of inflammatory sexually transmitted diseases of the female urogenital tract, including cervicitis, pelvic inflammatory disease, and tubal factor infertility [1–3] . The recent 2015 meta-analysis by Manhart et al showed a significant association between M. genitalium and cervicitis, regardless of the clinical definition used, which increased after controlling for coinfections (pooled odds ratio [OR], 1.99; 95% confidence interval [CI], 1.39–2.84) [1]. Although cervicitis is a relatively common clinical finding (prevalence, up to 41%) in women at high risk for sexually transmitted infections (STIs) [4], it is detectable only during pelvic examination, by the presence of mucopurulent cervical discharge and/or easily induced bleeding after sample collection. Despite the prevalence of this syndrome, our understanding of cervicitis is lacking in terms of its basic pathophysiological characteristics as a disease syndrome and whether it is a risk factor for HIV transmission. The objective of the current study was to evaluate HIV-infected women to define whether M. genitalium infection elicits cervical inflammation, a putative mechanism for enhanced HIV transmission to a sex partner.
Both clinical and basic experimental evidence support the aforementioned epidemiological implications of M. genitalium as an inflammatory pathogen of the cervix. First, both acute [5–7] and chronic [8] M. genitalium infection elicits the robust secretion of several potent proinflammatory cytokines. As the first line of defense against invading pathogens, the epithelium serves as a physical barrier and is charged with signaling the early innate immune responses following pathogen insult. For M. genitalium infections, this epithelial cell response has been shown to include several potent chemokines [5–8], including interleukin 8 (IL-8), and leads to marked signs of cervical leukocytosis in cervical specimens [9]. Collectively, these observations largely corroborate and provide mechanistic insight into the epidemiological associations of M. genitalium infection with microscopic signs of cervicitis measured on Gram stains of endocervical swab specimens [3].
The cervix is also a common and well-documented site of HIV transmission, where M. genitalium infection has been shown to increase the frequency of HIV shedding [10]. In a study of heterosexual US couples, Perez et al showed that M. genitalium seropositivity was more common among HIV-seroconcordant couples, compared with HIV-serodiscordant couples (OR, 3.44; 95% CI, 1.68–7.04), suggesting that M. genitalium is a risk factor for HIV transmission [11]. Next, a 2008 study showed that HIV-positive Kenyan women coinfected with M. genitalium, specifically those with a high organism burden, were more likely to test positive for HIV-1 DNA in cervical swab specimens [10]. Similarly, and in the only longitudinal analysis to date, M. genitalium infection was positively associated with HIV-1 RNA detection in cervical swab specimens (OR, 2.67; 95% CI, .99–7.20) [12]. A cross-sectional study of antiretroviral therapy (ART)–adherent New Orleans women [13] failed to find a similar association between M. genitalium infection and HIV shedding (OR, 0.39; 95% CI, .31–2.79), suggesting that ART may mitigate this effect. Nonetheless, M. genitalium coinfection is extremely common among HIV-infected adults (prevalence, 11%–33%) [14], and substantial rationale exists for the mechanistic investigation of M. genitalium as a risk factor for HIV transmission.
MATERIALS AND METHODS
Subject Enrollment and Specimen Collection
A retrospective cohort study approved by the Louisiana State University (LSU) Health Sciences Center Institutional Review Board and the Research Review Committee of the Interim LSU Hospital in New Orleans. The subjects included in this study originally consented to participate and enrolled in a cervical health study directed by Dr Michael Hagensee (Section of Infectious Diseases, Department of Medicine, LSU Health Sciences Center). Briefly, 109 HIV-infected, ART-adherent women were enrolled at the HIV Outpatient Program clinic in New Orleans, Louisiana, between 2009 and 2014 and asked to return at 3-month intervals, when they underwent physical and pelvic examinations.
At each visit, 4 types of specimens were collected, in the following order. First, a cervical broom or spatula/cytobrush was collected into ThinPrep PreservCyt, for conventional Papanicolaou smear. Second, a single vaginal and endocervical swab specimen was collected for DNA purification. Third, a cervicovaginal lavage (CVL) specimen was collected by repeated irrigation of the external cervical os. CVL specimens were centrifuged at 2500 x g (at 4°C for 8 minutes). Supernatants were filtered through a 0.5-µM membrane and then frozen in aliquots at −80°C. Fourth, a peripheral blood specimen was obtained to quantify circulating CD4+ T cells (using the FC500 flow cytometer; Beckman Coulter, Pasadena, California) and the plasma HIV load (using the Cobas AmpliPrep/Cobas TaqMan HIV-1 Test, v2.0; Roche Diagnostics, Indianapolis, Indiana). Urine specimens were obtained for Chlamydia trachomatis and Neisseria gonorrhoeae testing once annually as described below. Subjects were referred for colposcopy and cervical biopsy/curettage as indicated.
Nucleic Acid Amplification Tests (NAATs) for STI Detection
To detect and enumerate M. genitalium and T. vaginalis organisms, DNA was purified from endocervical swab specimens collected at each visit (using the DNeasy Blood and Tissue Kit; Qiagen, Valencia, California) and used in real-time quantitative polymerase chain reaction (PCR) assays as described previously [9]. PCR was performed using the Cobas Z480 analyzer with User Defined Workflow software (Roche Diagnostics; Indianapolis, Indiana). Human papillomavirus (HPV) was detected by conventional PCR, using the PGMY09/11 L1 consensus primers, and then genotyped using the reverse line blot assay (Roche Diagnostics). High-risk (HR) HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were included in the screening test; HPV16 and 18 were categorized separately from the other HR types. HIV proviral DNA was quantified from DNA in endocervical swab specimens as described previously [15]. C. trachomatis and N. gonorrhoeae testing was performed once annually (with the Hologic APTIMA Combo 2 test; Hologic, Bedford, Massachusetts), using urine specimens. Herpes simplex virus type 2 testing was performed on DNA extracted from selected ThinPrep PreservCyt specimens, using a PCR assay targeting the gene encoding glycoprotein B [16]. The presence of Epstein-Barr Virus (EBV) DNA in endocervical swab specimens was determined by PCR amplification of the EBNA1 gene, as described previously [17].
Cervical Leukocyte and Secreted Cytokine Quantification
Leukocytes were quantified from ThinPrep PreservCyt specimens as described previously [9]. First, we performed a composite analysis of all 16 subjects positive for M. genitalium, regardless of the duration of infection, and then compared their leukocyte to epithelial cell ratios to those from a randomly selected subset of 22 subjects in the cohort who never tested positive in the study. Longitudinal analyses were performed similarly for 3 subjects chronically infected with M. genitalium, which was defined by M. genitalium–positive NAAT results for at least 4 consecutive endocervical swab specimens, corresponding to an infection duration of at least 1 year.
Secreted cytokines were quantified in CVL specimens from a subset of specimens (n = 18) from M. genitalium–positive subjects and a subset (n = 22) from M. genitalium–negative subjects, using cytometric bead arrays as detailed in the Supplementary Data. Statistical comparisons were conducted using the Student t-test (GraphPad Prism, version 6.0d; La Jolla, California).
Cervical Histological and Immunohistochemical Analyses
Paraffin-embedded endocervical curettage specimens were used for histological analyses, using standard hematoxylin and eosin staining, and for immunohistochemical (IHC) analysis. For IHC analysis, antigen retrieval was performed for all probed antigens, using a decloaking chamber (Biocare Medical; Concord, California) with the exception of neutrophil-elastase IHC analysis, in which slides were deparaffinized with xylene and ethanol. Tissue sections were incubated overnight at 4°C with antibodies specific for the following cellular antigens: CD3, CD8, and CD68 (Biocare Medical); CD4 (Abcam, Cambridge, United Kingdom); and neutrophil-elastase (Dako North America, Carpinteria, California). Species-specific negative control antibodies (Biocare Medical) were processed in parallel for each antigen. The open-source image analysis software ImmunoRatio (version 1.0c) was used for quantification of a DAB-positive signal from tissue sections, enabling a quantitative comparison of immunostaining results. Statistical analyses were conducted using the Student t test (GraphPad Prism, version 6.0d).
RESULTS
Cohort Demographics and STIs
The cohort (n = 109) consisted predominately of black women (88.1%), and the median age was 42 years (range, 22–65 years) at enrollment; neither age nor race differed significantly relative to M. genitalium infection status (Table 1). All women were either receiving ART at enrollment or had therapy initiated at the enrollment visit. No significant differences were observed among the tested parameters between M. genitalium–positive and –negative subjects at enrollment, except EBV infection was more common among M. genitalium–positive subjects (P = .03; Table 1).
Table 1.
Demographic and Laboratory Characteristics at Enrollment Among Women With and Those Without Mycoplasma genitalium Infection Attending the Human Immunodeficiency Virus (HIV) Outpatient Clinic in New Orleans, Louisiana, from 2008 to 2014
| Characteristic | M. genitalium Positive (n = 7) | M. genitalium Negative (n = 102) | P Valuea |
|---|---|---|---|
| Age, y | |||
| <25 | 0.0 (0/7) | 2.0 (2/102) | .13 |
| 26–35 | 57.1 (4/7) | 18.6 (19/102) | |
| 36–50 | 42.9 (3/7) | 58.8 (60/102) | |
| >50 | 0.0 (0/7) | 20.6 (21/102) | |
| Race | |||
| Black | 85.7 (6/7) | 88.1 (89/101) | .60 |
| White | 14.2 (1/7) | 10.9 (11/101) | |
| Hispanic | 0.0 (0/7) | 1.0 (1/101) | |
| Peripheral CD4+ T-cell count | |||
| Cells/mm3b | 295 (29–505) | 482 (2–1509) | .12c |
| Below cohort mean | 80.0 (4/5) | 48.4 (45/93) | .36 |
| Plasma HIV-1 load | |||
| Copies/mLd | 1049 (0–87 469) | 186 (0–1 125 953) | .34c |
| Above cohort median | 66.7 (4/6) | 49.5 (45/91) | .68 |
| Cervical cytologic finding | |||
| Normal | 16.7 (1/6) | 55.3 (47/85) | .10 |
| ASCUS | 33.3 (2/6) | 20.0 (17/85) | .60 |
| LSIL | 50.0 (3/6) | 23.5 (20/85) | .17 |
| HSIL | 0 (0/6) | 1.2 (1/85) | 1.00 |
| ASCUS or worse | 83.3 (5/6) | 44.7 (38/85) | .10 |
| HR-HPV infection | |||
| Positive | 100.0 (6/6) | 83.2 (79/95) | .59 |
| HPV16 | 33.3 (2/6) | 9.5 (9/95) | .13 |
| HPV18 | 16.7 (1/6) | 12.6 (12/95) | .57 |
| Other HR HPV | 50.0 (3/6) | 47.4 (45/95) | 1.00 |
| T. vaginalis | 0.0 (0/7) | 14.1 (12/85) | .59 |
| Epstein-Barr virus | 100.0 (6/6) | 51.5 (51/99) | .03 |
Data are percentage of subjects (no. with characteristic/no. evaluated), unless otherwise indicated.
Abbreviations: ASCUS, atypical cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; T. vaginalis, Trichomonas vaginalis.
a By the Fisher exact test, unless otherwise indicated.
b Data expressed as mean CD4+ T cells per mm3 of peripheral blood (range).
c By the Wilcoxon rank sum test.
d Data are median HIV-1 RNA copies per mL of plasma (range). For positive HIV-1 test results below the lower limit of quantification (LLQ), the LLQ defined in the package insert was used for statistical comparisons.
M. genitalium–positive subjects had a reduced mean CD4+ T-cell count and increased median viral load as compared to those testing negative, but the difference for each measure was not statistically significant. Approximately half of the subjects had an abnormal Papanicolaou smear (defined as detection of atypical squamous cells of undetermined significance or worse), likely reflective of the HR-HPV prevalence of 84% and age of the cohort. The prevalence of cervical T. vaginalis infection at enrollment was 14.1%. No prevalent or incident C. trachomatis or N. gonorrhoeae infections were identified.
The incidence rate for M. genitalium infections was 80.2 cases per 1000 persons per year. Over the duration of the study, 3 subjects were identified as chronically infected with M. genitalium, defined as testing positive during at least 4 consecutive visits (>1 year). HPV prevalence is also detailed in Table 1. The mean M. genitalium load (±SD) in chronically infected subjects (3.5 × 104 ± 1.9 × 104 DNA copies per swab) was significantly higher than in subjects infected for <1 year (1.3 × 104 ± 6.8 × 103; P = .01, by the Wilcoxon rank sum test). One subject chronically infected with M. genitalium (subject 3) had a history of genital herpes and was not excluded from the study, because she was receiving acyclovir (400 mg twice daily) for the duration of the study, had no visible lesions on physical examination, and had negative results of all HSV1/2 NAATs for 13 liquid cytology specimens collected over 3.5 years (data not shown). Of subjects with available DNA for HIV provirus quantification, cervical HIV DNA was detected during 2 of 16 visits (12.5%) among M. genitalium–positive subjects, compared with 12 of 288 visits (4.2%) among subjects never testing positive for M. genitalium (P = .12, by the z test).
M. genitalium Infection Was Associated With Inflammatory Cervicovaginal Secretions
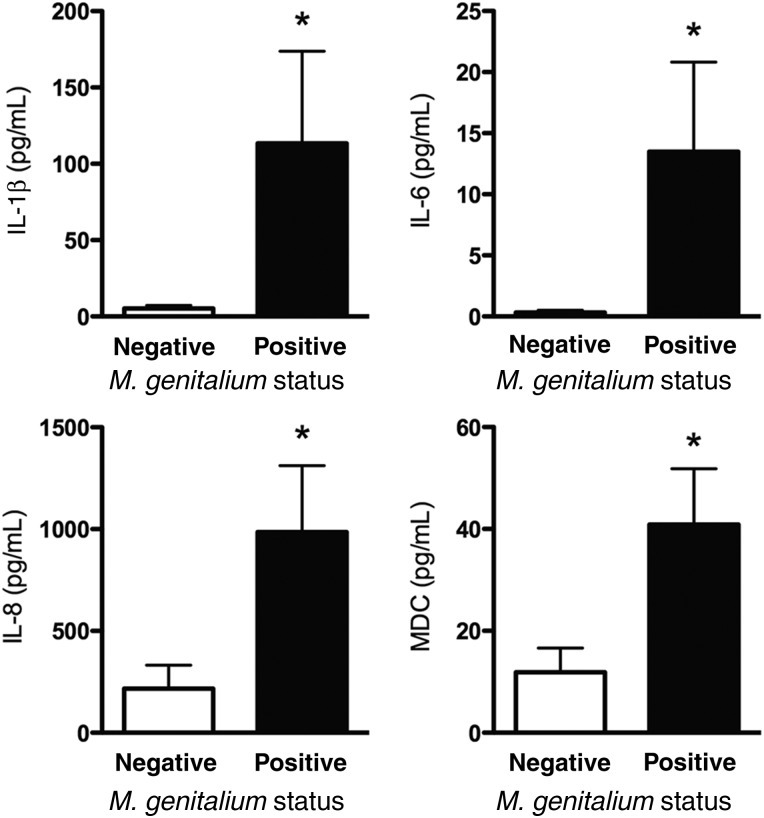
Among a panel of 49 different cytokines and chemokines, the levels of several proinflammatory cytokines were significantly higher in cervical secretions from 3 women with chronic M. genitalium infection (18 visits), compared with 4 subjects who never tested positive (22 visits). Specifically, interleukin 1β (IL-1β), IL-6, IL-8, monocyte chemotactic protein (MCP-2), and macrophage-derived chemokine (MDC) levels were significantly higher in M. genitalium–positive women (selected analytes are shown in Figure 1 ; P < .05, by the Student t test). Levels of several of the tested analytes were either below the limit of detection or not significantly different between any comparison groups; the complete data set for this analysis is presented in Supplementary Table 1. Cytokine values normalized to total protein level quantified from CVL specimens directly were not significantly different than raw cytokine values (comparative data not shown). Therefore, raw cytokine values are presented and were used for statistical analyses. In contrast to women with chronic infection, secreted cytokine levels in women who tested positive for M. genitalium at only a single or 2 consecutive visits were not significantly different than levels for M. genitalium–negative subjects (data not shown; P > .05).
Figure 1.
Secreted cervical cytokine response during chronic Mycoplasma genitalium infection. A comprehensive panel of cytokines was quantitatively analyzed in cervicovaginal lavage specimens from women chronically infected with M. genitalium, using multiplex cytometric bead arrays. The complete results are shown in the Supplementary Data. *P < .05, by the Student t test. Abbreviations: IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8; interleukin 8; MDC, macrophage-derived chemokine.
Cure of Chronic M. genitalium Infection Ablated Cervical Inflammation
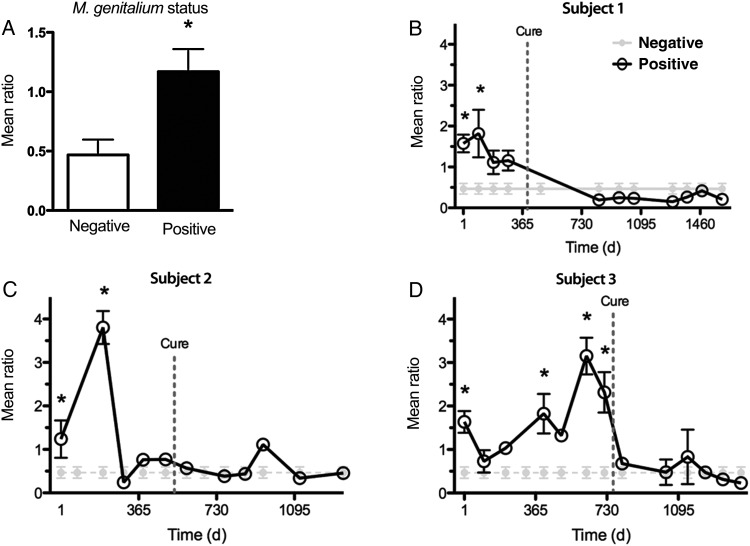
We next sought to quantify cytological and histological signs of cervical inflammation. In a composite analysis, women who were chronically infected with M. genitalium had significantly higher leukocyte to epithelial cell (EC) ratios at those visits as compared to women who never tested positive (Figure 2). Analysis of individual chronically infected subjects showed that leukocyte counts were, on most M. genitalium–positive visits, significantly elevated relative to those in women who never tested positive (P < .0001, by analysis of variance; Figure 2). Cervical inflammation in the liquid cytology specimen from subject 2 appeared to be ablated several months before M. genitalium cure (Figure 2C), which may be explained by aggressive treatment of a low-grade cervical lesion during the 3 visits immediately prior to M. genitalium cure. Direct epithelial cell counts during these 3 visits were approximately 16-fold higher than those at the visits before and after this aggressive management, which likely skewed the ratio of leukocytes to epithelial cells. All 3 chronically infected women were cured of M. genitalium infection during the study: subject 1 was cured after receipt of ciprofloxacin treatment for management of an unrelated postsurgical pelvic hematoma; no antibiotic dose was noted for subjects 2 and 3, and therefore it is possible that these subjects received antibiotic therapy from another clinical setting or spontaneously cleared the infection. Nonetheless, cure of M. genitalium reduced cytological signs of inflammation to baseline levels measured at least 3 months after the last positive NAAT result (Figure 2).
Figure 2.
Cervical leukocyte quantification during chronic Mycoplasma genitalium infection and after cure. The ratio of leukocytes to epithelial cells was calculated using ThinPrep PreservCyt specimens collected from women with chronic M. genitalium infection and a randomly selected subset of 22 negative specimens. A, Composite analysis of specimens from all M. genitalium–positive subjects and those who never tested positive. B–D, Individual longitudinal analyses of 3 study subjects who were chronically infected with M. genitalium and individuals who never tested positive. Data are expressed as the mean ratio of leukocytes to epithelial cells per high-powered microscope field. *P < .05, by the Student t test.
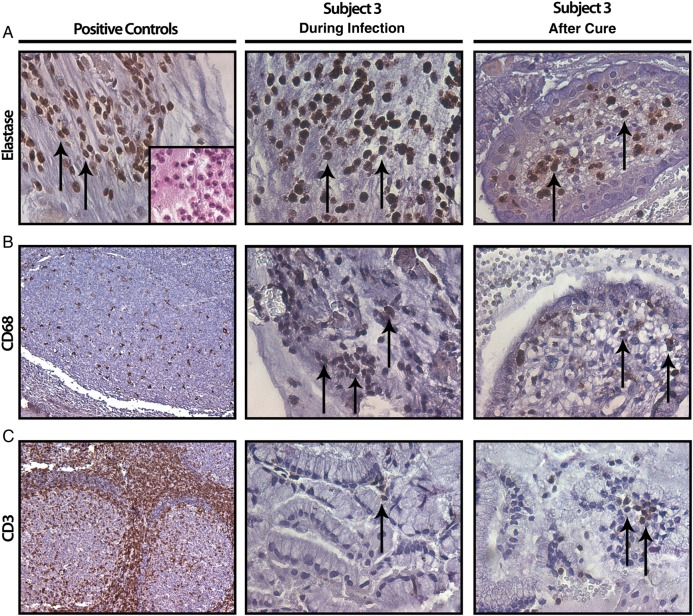
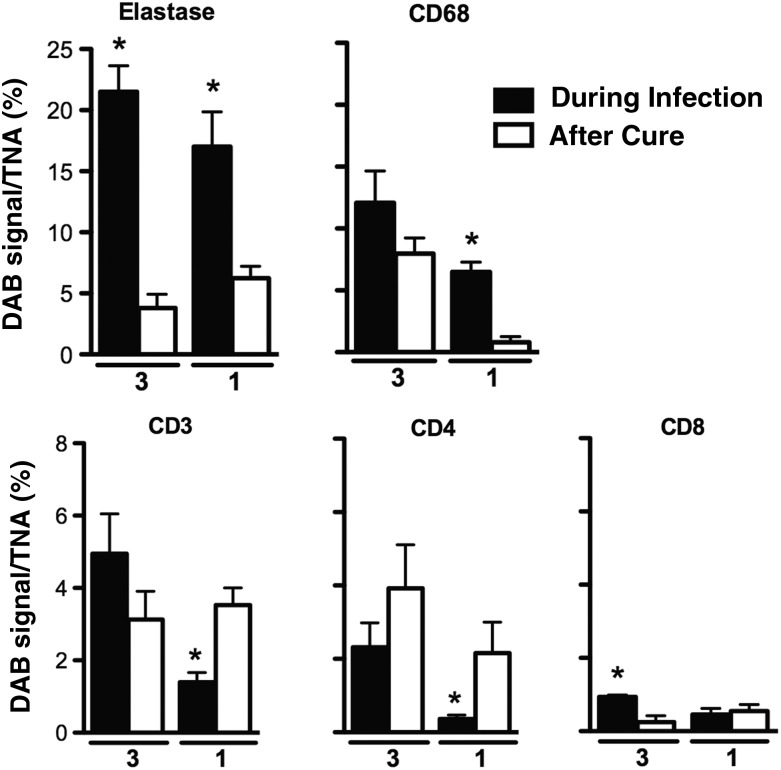
Despite the retrospective nature of the study, we identified curettage specimens collected before and after cure of M. genitalium infection from 2 subjects chronically infected with M. genitalium (subjects 1 and 3). For both subjects, marked increases in infiltrating leukocyte counts were observed in specimens collected at the visit in which M. genitalium infection was present, compared with the specimens collected after cure, which lacked signs of leukocytosis (data not shown). As representative results, IHC micrographs from subject 3 are shown in Figure 3, with quantitative results for both subjects presented in Figure 4. By targeting elastase via IHC analysis, we observed neutrophil infiltrates in the M. genitalium–positive curettage specimens that were significantly increased relative to levels in the M. genitalium–negative, postcure specimen from both of the chronically infected women (P < .05, by the Student t test; Figure 4). Significant increases in CD68+ monocyte/macrophage infiltrates were observed in endocervical curettage specimens obtained during chronic M. genitalium infection (P < .05; Figure 4); a statistically significant difference was observed for subject 1. In contrast, T-lymphocyte (CD3+, CD4+, and CD8+) levels were very low; significant differences are noted in Figure 4 (P > .05).
Figure 3.
Characterization of cervical leukocytic infiltrates by immunohistochemical analysis. Representative paraffin-embedded cervical curettage specimens from subject 3 were probed using markers of neutrophils (A; elastase), monocytes/macrophages (B; CD68), and T cells (C; CD3) while chronically infected with Mycoplasma genitalium (middle column) and after cure (right column). The positive controls for each antibody (left column) are demonstrated by positive staining in human tonsil sections for CD68 (B) and CD3 (C); specificity of the neutrophil elastase antibody is verified by positive staining of distinct polymorphonuclear cell infiltrates in endocervical curettage specimens (A, inset). Arrows denote cells staining positive for the indicated cellular marker. Tonsil fields in panels B and C are 100× original magnification; all other fields 400× original magnification.
Figure 4.
Quantitative comparison of leukocytic infiltrates during chronic Mycoplasma genitalium infection and after cure. Immunohistochemical findings for neutrophils (elastase), monocytes/macrophages (CD68), and T cells (CD3+, CD4+, and CD8+) in endocervical curettage specimens were quantitatively analyzed for 2 chronically infected subjects (subjects 1 and 3). The 3,3′-diaminobenzidine (DAB) signal was compared between curettage specimens obtained while chronically infected with M. genitalium and following cure. Results are expressed as the DAB signal per total nuclear area (TNA) in 5 high-powered microscope fields. P < .05, by the Student t test, compared with after cure.
DISCUSSION
With a prevalence of 7.4% (7 of 95 women), M. genitalium infections were expectedly more common in this cohort of HIV-infected, ART-adherent women than observed in our 2014 study of low-risk Louisiana women (1.5%) [9] but had similar prevalence to that in other cross-sectional studies of high-risk populations [14]. The prevalence was slightly lower than that (9.9%) observed previously in 324 patients from the same outpatient clinic from 2002 to 2005 [13], although vaginal swab specimens and a different testing platform were used in the earlier study and may account for this difference. Importantly, chronic infections (duration, >12 months) were identified in 3 subjects (3.2%) in the current study and collectively substantiate that M. genitalium remains a common coinfection in HIV-infected women, particularly considering the extraordinary level of care these patients receive (4 examinations per year) and routine use of macrolide antibiotics. In univariate analyses of the cohort demographic characteristics, HIV-related outcomes, and cervical coinfections, only the detection of EBV DNA in the cervix was significantly associated with M. genitalium infection. We hypothesize this enhanced EBV detection frequency is due to EBV-infected B-lymphocyte trafficking to the cervix in the context of chronic inflammation.
Our findings of inflammation corroborate previous clinical [1, 2, 4, 9, 18] and experimental [6–8] evidence implicating M. genitalium as a cause of cervicitis. Observations in the current study extend these findings to HIV-infected women and, based on the profile of the cytokine response, support the hypothesis that epithelial cells play an important role in initiating the response to infection, including coordinating leukocyte recruitment. Importantly, several studies have implicated IL-6, IL-8, and/or IL-1β as markers or predictors of increased HIV shedding from female reproductive tract tissues [19–21], with levels of all increased during chronic M. genitalium coinfection in our cohort, and they may also enhance HIV transmission to an uninfected partner [22]. The observed inflammatory response in our HIV-infected cohort was only increased in women chronically coinfected with M. genitalium, suggesting that longer-term infection may be necessary to elicit or detect the response in CVL specimens.
Mucosal inflammation, including the secretion of proinflammatory cytokines and leukocytic infiltrates, collectively has been shown to exacerbate HIV disease progression and/or transmission of HIV to a sex partner [19, 21, 23–25]. For instance, proinflammatory cytokines are secreted following activation of the NF-κB protein complex in multiple cell types, including infiltrating leukocytes, and can act in a paracrine manner to directly activate NF-κB in neighboring cells. NF-κB has been shown previously to bind the long terminal repeat region of latent HIV particles, resulting in enhanced virus replication [26, 27]. In the current study, we observed elevated levels of several cytokines implicated in enhanced HIV replication and shedding, providing some mechanistic support for M. genitalium′s role in HIV shedding [10]. In addition, a study by Totten et al has shown that the M. genitalium organism burden may correlate with HIV shedding intensity [10]. The mean organism burden in our New Orleans women with chronic M. genitalium coinfection was approximately 3 times higher than that in cohort subjects who were infected for <1 year, suggesting that a high organism burden may be related to chronicity of infection and intensity of the host response. We intermittently detected HIV proviral DNA in cervical swab specimens, likely because these women were adherent to ART and had low circulating HIV loads, so we are unable to make conclusions about HIV shedding intensity or frequency from this study.
Similar to our previous study of low-risk Louisiana women [9], we found significant increases in cervical leukocyte count in women infected with M. genitalium. Interestingly, the baseline ratio of leukocytes to ECs was lower in the HIV-infected cohort as compared to the low-risk group, but the approximately 3-fold increase in leukocyte count was similar in both groups. Longitudinal analyses of HIV-infected individuals with chronic M. genitalium coinfection showed cytological and histological evidence of leukocyte infiltration that was ablated after M. genitalium cure. Although it was not possible to control for all potentially inherent confounders in this retrospective analysis, these results are compelling indicators that treatment of M. genitalium eliminates the associated cervicitis—an important outcome, considering that cervicitis is associated with HIV shedding [20, 21, 28].
The role of cervicitis-associated enhancement of HIV transmission is likely heavily dependent on which cell types are involved in the immune response to M. genitalium infection. Using IHC analysis, we found neutrophils to be the primary constituent of the cellular infiltrate, as suggested initially by the increased secretion of IL-8 in CVL specimens. Activated neutrophils are known to release oxidants and hydrolytic enzymes; although this is an important antibacterial mechanism, chronic exposure can also lead directly to tissue damage [29, 30] and may enhance transmission of HIV. In addition, neutrophils release proinflammatory cytokines, which perpetuate local inflammatory responses leading to enrichment of both HIV-infected and naive target cells to potentially enhance local HIV replication [31] and/or transmission to an uninfected partner. Our observation of CD68(+) monocyte/macrophage enrichment during chronic M. genitalium infection indicates that M. genitalium infection results in HIV target cell recruitment to the cervical mucosa. In contrast, T cells from endocervical curettage specimens, overall, were very low in abundance, irrespective of M. genitalium infection status, which was somewhat surprising considering that T cells are known to be a common constituent of the endocervix [31, 32].
Together, the findings in this study corroborate M. genitalium as an etiologic agent of cervicitis and extend these observations to HIV-infected women. The biggest limitation to this study is the relatively small number of subjects with chronic M. genitalium infection (n = 3), and therefore substantiation of these conclusions in a larger cohort is warranted. However, the overall prevalence of M. genitalium (7.4%) in this cohort of HIV-infected women is of particular concern because macrolide resistance is rapidly emerging [33] and because undiagnosed/untreated infections may potentiate the spread of HIV in populations at high risk of acquiring STIs. Considering the expanding body of evidence linking M. genitalium with inflammation and HIV acquisition or transmission, screening and treatment of HIV-infected individuals may be a priority in advance of large-scale programmatic screening in the United States.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are grateful to Rafael F. Velasquez, MD, Lyndsey Buckner, PhD, and Alison J. Quayle, PhD, for their scientific input and critical reading of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of General Medical Sciences of the NIH (grant 1 U54 GM104940 to the Louisiana Clinical and Translational Science Center).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 2.McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 2011; 7:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis 2009; 36:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowin CL, Ma L, Martin DH, Pyles RB. Mycoplasma genitalium-encoded MG309 activates NF-kappaB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect Immun 2009; 77:1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol 2009; 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGowin CL, Radtke AL, Abraham K, Martin DH, Herbst-Kralovetz M. Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3–dimensional human endocervical epithelial cell model. J Infect Dis 2013; 207:1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGowin CL, Annan RS, Quayle AJ et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun 2012; 80:3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehon PM, McGowin CL. Mycoplasma genitalium infection is associated with microscopic signs of cervical inflammation in liquid cytology specimens. J Clin Microbiol 2014; 52:2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manhart LE, Mostad SB, Baeten JM, Astete SG, Mandaliya K, Totten PA. High Mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis 2008; 197:733–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez G, Skurnick JH, Denny TN et al. Herpes simplex type II and Mycoplasma genitalium as risk factors for heterosexual HIV transmission: report from the heterosexual HIV transmission study. Int J Infect Dis 1998; 3:5–11. [DOI] [PubMed] [Google Scholar]
- 12.Napierala Mavedzenge S, Muller EE, Lewis DA, Chipato T, Morrison CS, Weiss HA. Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis 2015; 211:1388–98. [DOI] [PubMed] [Google Scholar]
- 13.Gatski M, Martin DH, Theall K et al. Mycoplasma genitalium infection among HIV-positive women: prevalence, risk factors and association with vaginal shedding. Int J STD AIDS 2011; 22:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–20. [DOI] [PubMed] [Google Scholar]
- 15.Henning TR, Lacour N, Amedee AM. Efficient methodologies for sensitive HIV-1 RNA quantitation from plasma and vaginal secretions. J Clin Virol 2009; 46:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey L, Huang ML, Selke S, Wald A. Differentiation of herpes simplex virus types 1 and 2 in clinical samples by a real-time taqman PCR assay. J Med Virol 2005; 76:350–5. [DOI] [PubMed] [Google Scholar]
- 17.Ryan JL, Fan H, Swinnen LJ et al. Epstein-Barr virus (EBV) DNA in plasma is not encapsidated in patients with EBV-related malignancies. Diagn Mol Pathol 2004; 13:61–8. [DOI] [PubMed] [Google Scholar]
- 18.Manhart LE. Mycoplasma genitalium: an emergent sexually transmitted disease? Infect Dis Clin North Am 2013; 27:779–92. [DOI] [PubMed] [Google Scholar]
- 19.Cummins JE, Christensen L, Lennox JL et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses 2006; 22:788–95. [DOI] [PubMed] [Google Scholar]
- 20.Gumbi PP, Nkwanyana NN, Bere A et al. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J Virol 2008; 82:8529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herold BC, Keller MJ, Shi Q et al. Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr 2013; 63:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty H, Sen PK, Helms RW et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 2001; 15:621–7. [DOI] [PubMed] [Google Scholar]
- 23.Arnold KB, Burgener A, Birse K et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2015; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 24.Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab Sci 2014; 51:98–111. [DOI] [PubMed] [Google Scholar]
- 25.Masson L, Passmore JA, Liebenberg LJ et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahabieh MS, Ooms M, Brumme C et al. Direct non-productive HIV-1 infection in a T-cell line is driven by cellular activation state and NFkappaB. Retrovirology 2014; 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin GE, Leung K, Folks TM, Kunkel S, Nabel GJ. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 1989; 339:70–3. [DOI] [PubMed] [Google Scholar]
- 28.Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis 2010; 202:1543–52. [DOI] [PubMed] [Google Scholar]
- 29.Campillo-Gimenez L, Casulli S, Dudoit Y et al. Neutrophils in antiretroviral therapy-controlled HIV demonstrate hyperactivation associated with a specific IL-17/IL-22 environment. J Allergy Clin Immunol 2014; 134:1142–52 e5. [DOI] [PubMed] [Google Scholar]
- 30.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 1994; 56:672–86. [DOI] [PubMed] [Google Scholar]
- 31.Nkwanyana NN, Gumbi PP, Roberts L et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology 2009; 128:e746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poppe WA, Drijkoningen M, Ide PS, Lauweryns JM, Van Assche FA. Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical study. Eur J Obstet Gynecol Reprod Biol 1998; 81:277–82. [DOI] [PubMed] [Google Scholar]
- 33.Manhart LE, Gillespie CW, Lowens MS et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis 2013; 56:934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.