Abstract
Background. Oral vaccination with live-attenuated Salmonella Typhi strain Ty21a is modestly efficacious, but the mechanisms of protection are currently unknown. While humoral and cellular immune responses are well described in peripheral blood, the cellular response at the intestinal mucosa has never been directly assessed.
Methods. We vaccinated healthy adults with Ty21a and assessed humoral and cellular immunity in vaccinated volunteers and controls after 18 days. Immunoglobulin levels were assessed in peripheral blood by an enzyme-linked immunosorbent assay. Cellular responses were assessed in peripheral blood and at the duodenal and colonic mucosa by flow cytometry.
Results. We demonstrate the generation of Ty21a-responsive and heterologous influenza virus–responsive CD4+ and CD8+ T cells at the duodenal mucosa. All duodenal responses were consistently correlated, and no responses were observed at the colonic mucosa. Peripheral anti-lipopolysaccharide immunoglobulin G and immunoglobulin A responses were significantly correlated with duodenal responses. The assessment of integrin β7 expression intensity among peripheral and duodenal T-cell subsets revealed varied capacities for mucosal homing and residence.
Conclusions. The breadth of duodenal cellular responses was not reflected peripherally. The direct evaluation of mucosal immune defense may yield functional correlates of protection and could provide insight into mechanisms that may be manipulated to enhance vaccine immunogenicity.
Keywords: Salmonella, typhoid, Ty21a, cellular immunity, T cells, cytokines, humoral immunity, immunoglobulins, heterologous immunity
Salmonella enterica serovar Typhi is a human-host-restricted intracellular pathogen and the causative agent of typhoid fever. Following ingestion, the bacteria cause systemic illness following invasion via the mucosal surface of the small intestine [1]. In the 1970s, through chemical mutagenesis of pathogenic S. Typhi strain Ty2, a live-attenuated oral typhoid vaccine, Ty21a, was developed [2]. Vaccination with 3 doses of Ty21a is moderately protective, and although estimates of efficacy vary [3–5], a recently published review calculated a cumulative efficacy of 48% 3 years following vaccination [6].
Ty21a is able to induce humoral and cellular immune responses, both of which have been implicated in protection against disease. While opsonophagocytic antibody function [7], cellular cytotoxicity, proliferation, and cytokine production functionality have been assessed in peripheral blood following vaccination with Ty21a [8–13], cellular immunity at the human intestinal mucosa has never been directly assessed. Numerous studies have demonstrated that cellular immune responses generated through vaccination with Ty21a are primed for mucosal homing [13–15], highlighting the importance of mucosal immunity in defense against disease. Furthermore, it has been demonstrated that the assessment of vaccine immunogenicity by peripheral sampling alone provides an incomplete reflection of vaccine immunogenicity [16].
It has previously been observed in murine models that previously primed T cells of heterologous specificities are recruited to the lung during influenza virus infection [17]. Although this phenomenon has not been observed in humans, we hypothesized that vaccination with Ty21a could enhance T-cell responses to heterologous antigens at the mucosal surface by a similar mechanism.
Through the direct assessment of immunity at the intestinal mucosa, it may be possible to identify mechanisms involved in the induction of protective immunity, which may be manipulated to improve oral vaccine immunogenicity. Here, we have assessed cellular immunity in vaccinated volunteers and controls at the duodenal and colonic mucosa and in peripheral blood after 18 days. We have compared and correlated peripheral and mucosal cellular responses with accepted peripheral humoral measures of vaccine efficacy, providing a unique insight into the relationship between human mucosal and peripheral immune defense.
MATERIALS AND METHODS
Ethical Approval, Recruitment, and Study Protocol
All volunteers provided written informed consent. This study was approved by the United Kingdom National Research Ethics Service (10/H1005/20). Twenty-three healthy adult volunteers were enrolled into the study. Eleven volunteers (6 males and 5 females [2 of whom were previously vaccinated against influenza]; median age, 24 years) were randomly selected for oral vaccination with live-attenuated S. Typhi (Ty21a; Vivotif). A single oral capsule was taken on days 0, 2, and 4, approximately 1 hour before a meal, with a cold or lukewarm drink. Twelve volunteers (3 males and 9 females [3 of whom were previously vaccinated against influenza]; median age, 23 years) were randomly assigned to an unvaccinated control group.
Mucosal Mononuclear Cell (MMC) Isolation
Mucosal samples were acquired at day 18. O2 was administered nasally, and saturation was monitored throughout endoscopic biopsy. Sedation was offered to all volunteers; those who requested sedation were given up to 5 mg of midazolam intravenously. By use of large-capacity forceps (Boston Scientific), 12–15 single-bite mucosal biopsy specimens were acquired during flexible video-endoscopy from the duodenal mucosa at parts D2–D3 (n = 20) and from the sigmoid colon at an insertion distance of 20–25 cm (n = 17). MMCs were isolated from biopsy specimens, using a modified version of a previously described method [31]. Full details are presented in the Supplementary Materials and Methods.
Peripheral Blood Mononuclear Cell (PBMC) Isolation
Peripheral blood samples were collected in sodium heparin Vacutainers (BD Biosciences) at day 0 (n = 23) and, after overnight fasting, at day 18 (n = 21). PBMCs were isolated using Lymphoprep (Axis-Shield), according to the manufacturers instructions. Full details are presented in the Supplementary Materials and Methods.
Antigenic Stimulation and Incubation
PBMCs (1 × 106 cells/well) and MMCs (between 0.5 × 106 and 1 × 106 cells/well) were seeded in complete medium in 96-well flat-bottomed plates. Cells in each well were stimulated with either 1 × 106 colony-forming units (CFU) heat-killed Salmonella Typhi Ty21a (Vivotif; bacteria were suspended in Dulbecco's phosphate-buffered saline [PBS], quantified using the Miles and Misra technique, and killed by incubation at 95°C for 30 minutes) or 45 ng influenza virus hemagglutinin and neuraminidase antigens (Influvac, containing, in equal quantities, a A/Brisbane/59/2007 H1N1-like strain, a A/Brisbane/10/2007 H3N2-like strain, and a B/Brisbane/60/2008-like strain). One positive control well was stimulated with 100 ng staphylococcal enterotoxin B (SEB; Sigma-Aldrich). One negative control well was left untreated to adjust for non–antigen-specific background cytokine production. Cells were then incubated at 37°C in 5% CO2. After 2 hours, 1 µL brefeldin A (BD GolgiPlug; BD Biosciences) was added to each well, and the plate was incubated for a further 16 hours at 37°C in 5% CO2.
Flow Cytometric Analyses
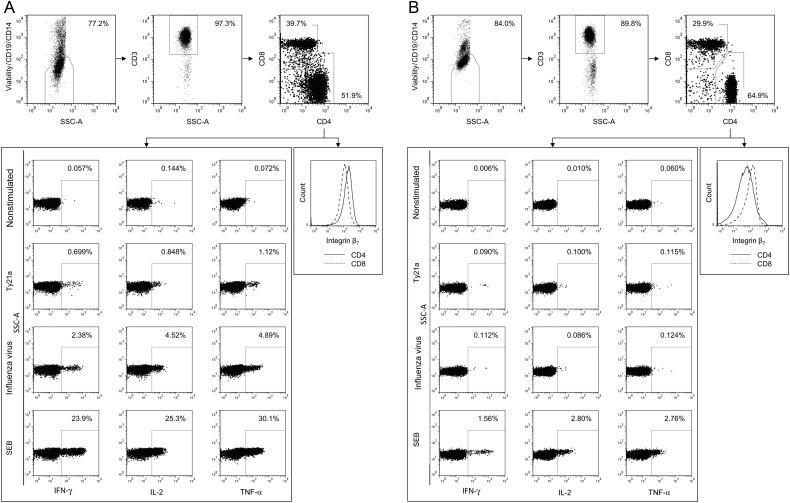
Following incubation, PBMCs and MMCs were washed, stained for viability and surface phenotype, and, following fixation and permeabilization, stained for intracellular cytokine production. Details of the antibodies that were used are presented in the Supplementary Materials and Methods. Cells were washed, resuspended, and stored in the absence of light at 4°C until data were acquired using a LSR II flow cytometer (BD Biosciences). Compensation beads (BD Biosciences) were used to create compensation matrices, and sequential cell isolation was used to identify populations of interest (Figure 2). Full details are presented in the Supplementary Materials and Methods.
Figure 2.
Representative flow cytometric gating strategy. Dot plots are shown for cells isolated from the duodenal mucosa (A) and peripheral blood (B). Dead cells, B cells, and monocytes were removed by staining for viability (Vivid), CD19, and CD14 and gating on the negative population. T cells were identified according to the expression of CD3. T cells were classified according to the expression of CD4 and CD8 and the expression of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and/or interleukin 2 (IL-2) assessed in nonstimulated and in live-attenuated Salmonella Typhi strain Ty21a (Ty21a)-, influenza virus–, and staphylococcal enterotoxin B (SEB)–stimulated samples.
Enzyme-Linked Immunosorbent Assay (ELISA)
Each well in flat-bottomed 96-well microtitre plates (Nunc) was coated with 100 µL of carbonate-bicarbonate buffer containing either 50 ng S. Typhi lipopolysaccharide (LPS; Sigma-Aldrich) or 25 ng influenza virus hemagglutinin and neuraminidase antigens (Influvac, containing, in equal quantities, a A/Brisbane/59/2007 H1N1-like strain, a A/Brisbane/10/2007 H3N2-like strain, and a B/Brisbane/60/2008-like strain) and incubated at 4°C overnight. Plates were washed 3 times with PBS-Tween. Plates were blocked with 1.0% bovine serum albumin and incubated for 2 hours at room temperature. A standard was created using serum obtained from a convalescent patient with a diagnosis of typhoid. Volunteer samples were diluted 4 times across an optimized range for optimum comparison against the standard. Plates were washed, and samples were added in duplicate and incubated at 4°C overnight. For detection of immunoglobulin G (IgG), plates were washed and incubated with 1:4000 anti-human IgG–alkaline phosphatase (Sigma-Aldrich) for 2 hours. For detection of immunoglobulin A (IgA), plates were washed and incubated with 1:4000 anti-human-IgA (AbD Serotec) for 2 hours; plates were washed again and then incubated with 1:2000 streptavidin to alkaline phosphatase (AbD Serotec) for 1 hour. For detection of both IgG and IgA, plates were washed and incubated with 100 µL p-nitrophenyl phosphate (Sigma-Aldrich). Optical density was measured at 405 nm, using a FLUOstar Omega microplate reader (BMG Labtech).
Statistical Analyses
Comparisons were made using paired and unpaired t tests, as indicated. Associations were measured using the Pearson correlation coefficient. Statistical analyses were performed using Prism v5.03 (GraphPad). P values are 2-tailed and considered significant at P < .05.
RESULTS
Serum Immunoglobulin Specificity
Ty21a-mediated protection is dependent upon the expression of LPS [2], and, in field trials, humoral responses to LPS were shown to correlate with vaccine efficacy [18]. We compared levels of serum anti-LPS IgG and IgA prior to and following vaccination. We also measured levels of serum IgG and IgA specific to influenza virus, a common naturally encountered pathogen, to assess the impact of vaccination on humoral immunity to a heterologous pathogen. Influenza virus was selected since the majority, if not all, volunteers would have been exposed to this pathogen in the community.
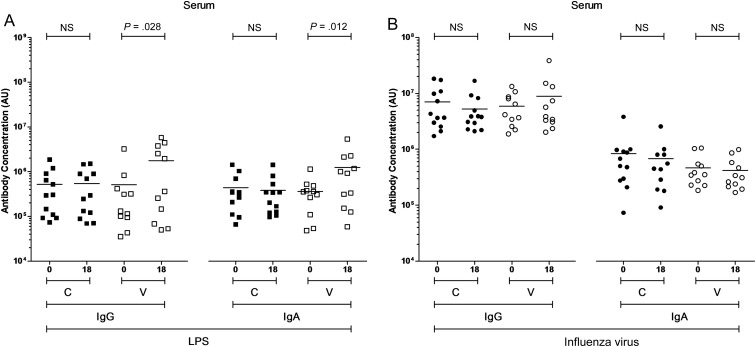
While levels of anti-LPS serum IgG and IgA among unvaccinated subjects were not different between day 18 and day 0, levels among the vaccinated were higher at day 18 than at day 0 (P = .03 and P = .01; Figure 1A). Levels of anti-influenza serum IgG or IgA among the unvaccinated and the vaccinated subjects were not different between day 18 and day 0 (Figure 1B).
Figure 1.
Levels of serum immunoglobulin G (IgG) and immunoglobulin A (IgA) to specific antigens. The levels of IgG and IgA specific to Salmonella Typhi lipopolysaccharide (LPS; A) and influenza virus (B) in serum, expressed in arbitrary units (AU). For control (C; closed squares and circles) and vaccinated (V; open squares and circles) volunteers, paired comparisons were made between day 0 and day 18 values. Horizontal bars represent mean values (paired t tests were performed using logarithmically transformed data). Abbreviation: NS, not significant.
Peripheral Blood and Gut Mucosal Cellular Responses
We compared the frequency of Ty21a-responsive T cells in vaccinated volunteers and controls, at the duodenal and colonic mucosa and in peripheral blood. We also measured the frequency of influenza virus–responsive T cells. A combinatorial gating strategy was used to identify the proportion of CD4+ and CD8+ T cells positive for any combination of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and/or interleukin 2 (IL-2; Figure 2). Cytokine production in nonstimulated samples (negative control) was minimal, did not differ between vaccinated and unvaccinated subjects, and was subsequently subtracted from other conditions. Cytokine production in staphylococcal enterotoxin B–stimulated samples (positive control) was high and did not differ between vaccinated and unvaccinated subjects.
At day 0, in peripheral blood, the frequency of Ty21a-responsive and heterologous influenza virus–responsive CD4+ and CD8+ T cells in the vaccinated group was not different from the frequency in the unvaccinated control group (Supplementary Figure 1). These data suggest that groups were well matched for prior exposure to Ty21a and influenza virus antigens and that any differences observed thereafter may be attributed to an effect of vaccination with Ty21a. Paired comparisons between day 0 and day 18 were not made in peripheral blood, as overnight fasting, required prior to endoscopy, is known to influence cytokine production in response to restimulation with bacterial and viral antigens [19].
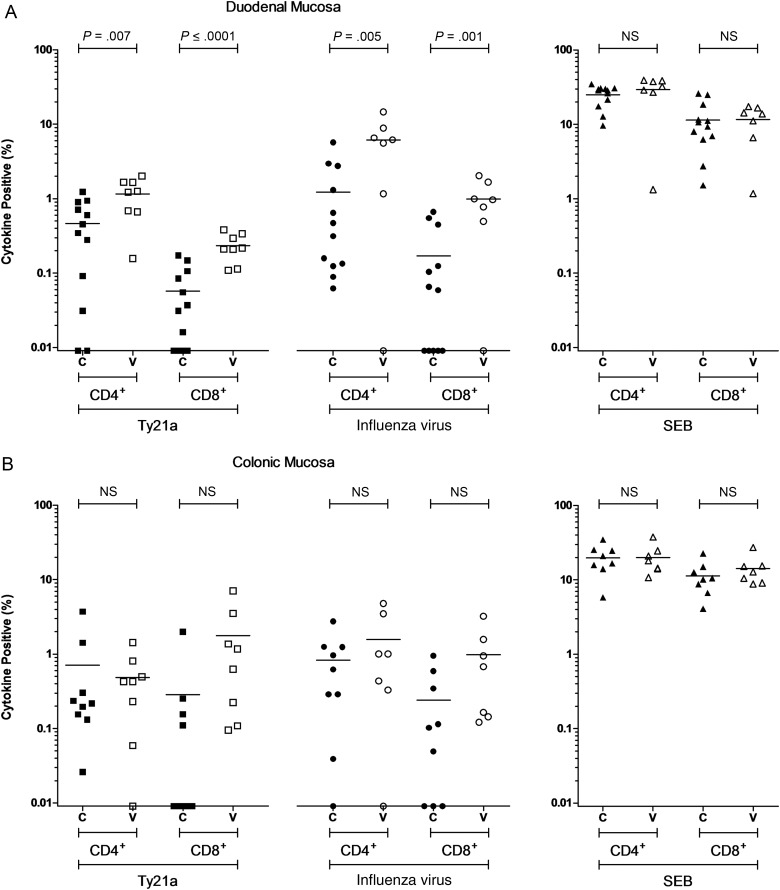
At day 18, at the duodenal mucosa, the frequency of Ty21a-responsive CD4+ T cells was 3-fold higher and the frequency of Ty21a-responsive CD8+ T cells 5-fold higher in vaccinated volunteers, compared with unvaccinated volunteers (P = .007 and P = <.0001, respectively; Figure 3A). The frequency of heterologous influenza virus–responsive CD4+ T cells was 5-fold higher and the frequency of heterologous influenza virus–responsive CD8+ T cells 6-fold higher in vaccinated volunteers, compared with unvaccinated volunteers (P = .005 and P = .01, respectively; Figure 3A). At the colonic mucosa, there was no significant difference between the frequencies of Ty21a-responsive or heterologous influenza virus–responsive T cells in vaccinated volunteers, compared with unvaccinated volunteers (Figure 3B).
Figure 3.
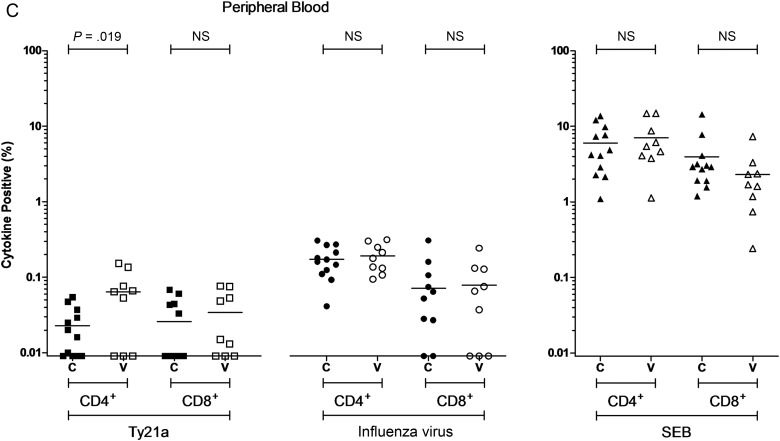
Antigen-specific cytokine-producing populations at day 18. The frequency of CD4+ and CD8+ live-attenuated Salmonella Typhi strain Ty21a (Ty21a)-responsive and heterologous influenza virus–responsive populations expressing any combination of interferon γ, tumor necrosis factor α, and/or interleukin 2 above background. Staphylococcal enterotoxin B (SEB)–stimulated control data is also included. For control (C; closed squares, circles, and triangles) and vaccinated (V; open squares, circles, and triangles) volunteers, measurements were made at the duodenal mucosa (A), the colonic mucosa (B), and in peripheral blood (C). Horizontal bars represent mean values (comparisons were made using unpaired t tests). Abbreviation: NS, not significant.
Figure 3.
In peripheral blood, the frequency of Ty21a-responsive CD4+ T cells was 4-fold higher in vaccinated volunteers, compared with unvaccinated volunteers (P = .019; Figure 3C). Vaccination did not influence the frequency of peripheral Ty21a-responsive CD8+ T cells, nor the frequency of heterologous influenza virus–responsive CD4+ or CD8+ T cells at day 18.
Characteristics and Functionality of Cellular Responses
Polyfunctional T cells, defined as cells that express multiple cytokines simultaneously, have been shown to correlate with vaccine-mediated protection against other intracellular infections [20, 21]. We compared the cytokine expression profile of vaccinated volunteers with that of unvaccinated volunteers to assess the functionality of responses to Ty21a and influenza virus antigens.
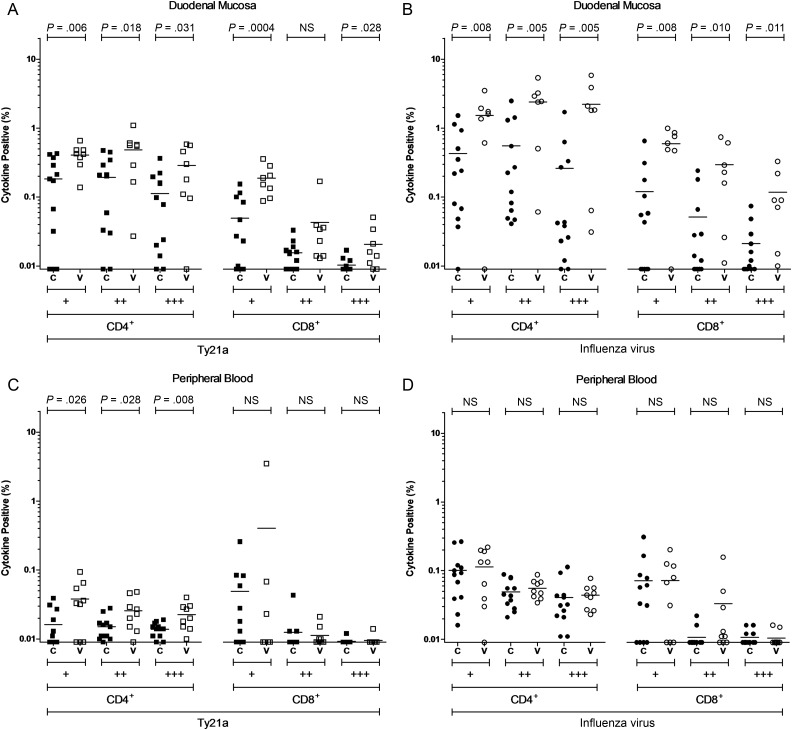
At the duodenal mucosa, the CD4+ T-cell response to Ty21a was functionally heterogeneous, with the frequency of cells expressing 1, 2, or 3 cytokines being higher in vaccinated volunteers, compared with unvaccinated volunteers (P = .006, P = .018, and P = .031, respectively; Figure 4A). The duodenal CD8+ T-cell response to Ty21a antigens was, in contrast, largely attributable to cells expressing just 1 cytokine (P = .0004; Figure 4A). Duodenal CD4+ and CD8+ T-cell responses to influenza virus antigens were also functionally heterogeneous, with the frequency of cells expressing 1, 2, or 3 cytokines being higher in vaccinated volunteers, compared with unvaccinated volunteers (CD4+ T cells, P = .008, P = .005, and P = .005, respectively; CD8+ T cells, P = .0008, P = .010, and P = .011; Figure 4B).
Figure 4.
Combinations of antigen-specific cytokine production at day 18. The frequency of CD4+ and CD8+ live-attenuated Salmonella Typhi strain Ty21a (Ty21a)-responsive and heterologous influenza virus–responsive populations expressing 1 (+), 2 (++), or 3 (+++) cytokines (interferon γ, tumor necrosis factor α, and/or interleukin 2) above background. For control (C; closed squares, circles, and triangles) and vaccinated (V; open squares, circles, and triangles) volunteers, measurements were made at the duodenal mucosa (A and B) and in peripheral blood (C and D). Horizontal bars represent mean values (comparisons were made using unpaired t tests). Abbreviation: NS, not significant.
In peripheral blood, the CD4+ T-cell response to Ty21a antigens was functionally heterogeneous, with the frequency of cells expressing 1, 2, or 3 cytokines being higher in vaccinated volunteers, compared with unvaccinated volunteers (P = .026, P = .028, and P = .008, respectively; Figure 4C). Analysis of expression profiles by individual cytokine demonstrated that responses to Ty21a and influenza virus antigens were attributable to cells producing combinations of IFN-γ, TNF-α, and IL-2 but that no one cytokine predominated (Supplementary Figure 2).
Correlations Between Cellular Populations at the Duodenal Mucosa
We explored the relationship between the generation of Ty21a-responsive and heterologous influenza virus–responsive T cells at the duodenal mucosa. The frequencies of Ty21a-responsive CD4+ and CD8+ T cells were correlated (r2 = 0.537 and P = .015; Table 1), as were the frequencies of heterologous influenza virus–responsive CD4+ and CD8+ T cells (r2 = 0.930 and P = <.0001; Table 1). The frequencies of Ty21a-responsive CD4+ and CD8+ T cells also correlated robustly with the frequencies of corresponding influenza virus–responsive T cells (CD4+ T cells, r2 = 0.74 and P = .0003; CD8+ T cells, r2 = 0.677 and P = .001; Table 1).
Table 1.
Pearson Correlation Analysis of Cellular and Humoral Immune Responses
| Variable | Duodenal Mucosa |
Peripheral Blood |
Serum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ty21a |
Influenza Virus |
Ty21a |
Influenza Virus |
S. Typhi LPS |
Influenza Virus |
|||||||
| CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | IgG | IgA | IgG | IgA | |
| Duodenal mucosa | ||||||||||||
| Ty21a | ||||||||||||
| CD4+ T cells | 1 | 0.537a | 0.741b | 0.724b | 0.530a | 0.319 | −0.102 | −0.223 | 0.542a | 0.710a | 0.160 | 0.008 |
| CD8+ T cells | … | 1 | 0.587b | 0.677b | 0.395 | 0.126 | −0.117 | −0.232 | 0.315 | 0.396 | 0.177 | −0.348 |
| Influenza virus | ||||||||||||
| CD4+ T cells | … | … | 1 | 0.929b | 0.327 | 0.299 | −0.025 | −0.122 | 0.392 | 0.512a | −0.245 | −0.228 |
| CD8+ T cells | … | … | … | 1 | 0.394 | 0.515 | 0.099 | 0.080 | 0.392 | 0.585b | −0.149 | −0.300 |
Unless otherwise indicated, values were not statistically significant.
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; LPS, lipopolysaccharide; S. Typhi, Salmonella enterica serovar Typhi; Ty21a, live-attenuated Salmonella Typhi strain Ty21a.
a P < .05.
b P < .001.
Correlations Between Peripheral and Mucosal Immunity
We explored the relationship between the generation of peripheral and duodenal immune responses. The frequency of peripheral Ty21a-responsive CD4+ T cells was modestly correlated with the frequency of duodenal Ty21a-responsive CD4+ T cells (r2 = 0.530 and P = .024; Table 1) but not with the frequency of duodenal Ty21a-responsive CD8+ T cells or with the frequency of heterologous influenza virus–responsive CD4+ and CD8+ T cells. Levels of serum anti-LPS IgG and IgA correlated with the frequency of duodenal Ty21a-responsive CD4+ T cells (IgG, r2 = 0.542 and P = .014; IgA, r2 = 0.710 and P = .0004; Table 1). Levels of serum IgA specific to LPS also correlated with the frequency of duodenal heterologous influenza virus–responsive CD4+ and CD8+ T cells (CD4+ T cells, r2 = 0.512 and P = .025; CD8+ T cells, r2 = 0.585 and P = .008; Table 1). Levels of anti-influenza virus IgG and IgA did not, however, correlate with either the frequency of Ty21a-responsive or heterologous influenza virus–responsive T cells at the duodenal mucosa in either subset.
Cellular Potential for Mucosal Homing and Residence
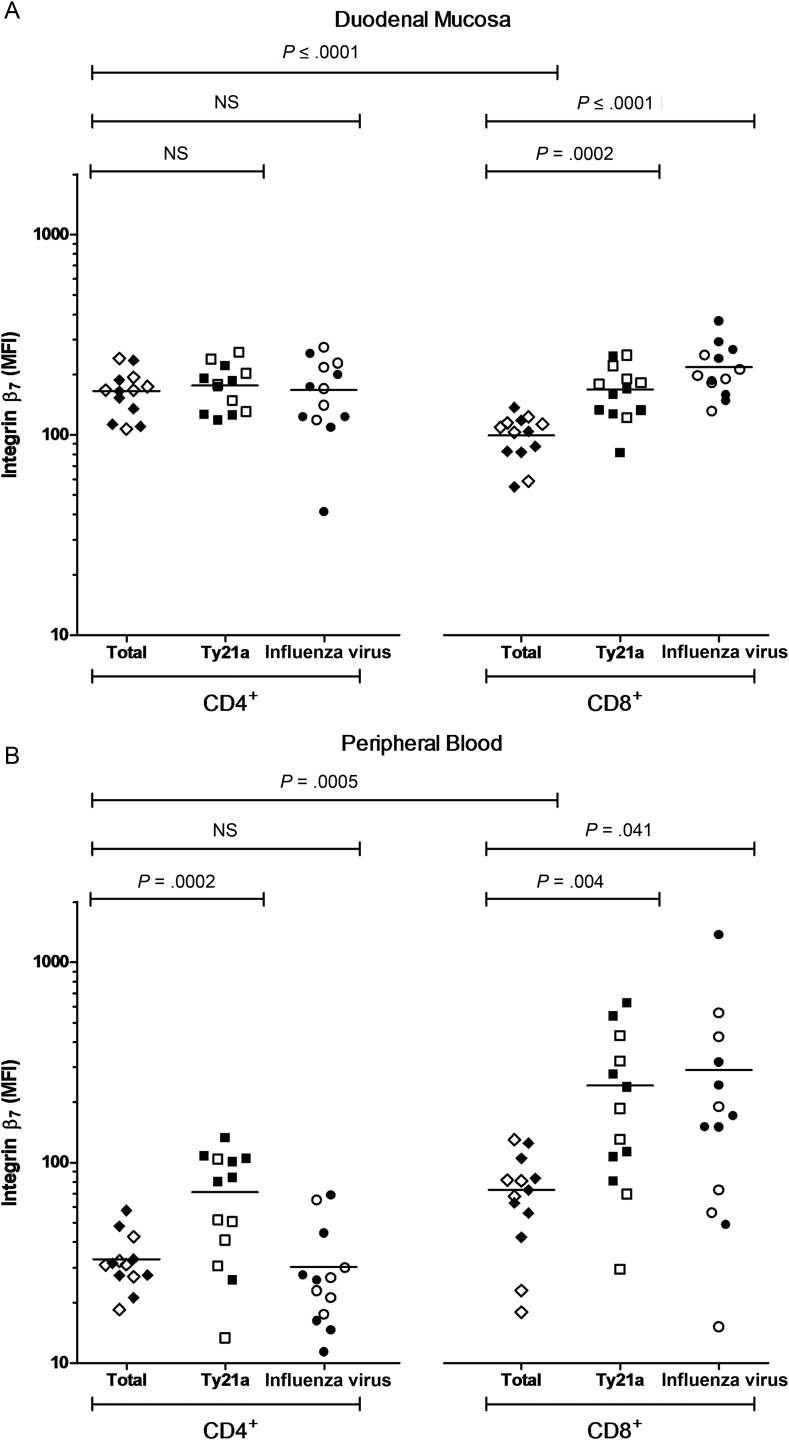
Integrin β7 plays a prominent role in mucosal cellular immune defense: α4β7 facilitates peripheral trafficking to the intestinal mucosa, and αεβ7 helps maintain resident intraepithelial lymphocyte populations [22, 23]. Since duodenal CD4+ and CD8+ T-cell responses to Ty21a and influenza virus antigens were closely correlated, we assessed the cellular capacity for mucosal homing and residence, which is a potential mechanism by which Ty21a vaccination may have influenced trafficking to the duodenal mucosa. We assessed integrin β7 expression intensity, a component of both α4β7 in peripheral blood and αεβ7 at the duodenal mucosa. A combinatorial gating strategy was used to determine the level of integrin β7 expression among CD4+ and CD8+ T-cell populations (Figure 2).
At the duodenal mucosa, integrin β7 expression was more intense among the total CD4+ T-cell population than among the total CD8+ T-cell population (P = <.0001; Figure 5A). Integrin β7 expression intensity among Ty21a-responsive and influenza virus–responsive CD4+ T-cell subpopulations was not different from that among the total mucosal CD4+ T-cell population. In contrast, integrin β7 expression intensity among Ty21a-responsive and influenza virus–responsive CD8+ T-cell subpopulations was greater than that among the total mucosal CD8+ T-cell population (P = .0002 and P = <.0001, respectively; Figure 5A).
Figure 5.
Integrin β7 expression intensity among T-cell populations at day 18. Geometric mean fluorescence intensity (MFI) expression of integrin β7 among nonstimulated total CD4+ and total CD8+ T-cell populations (control volunteers, closed diamonds; vaccinated volunteers, open diamonds) and in live-attenuated Salmonella Typhi strain Ty21a (Ty21a)-stimulated (control volunteers, closed squares; vaccinated volunteers, open squares) and heterologous influenza virus–stimulated (control volunteers, closed circles; vaccinated volunteers, open circles) cytokine-producing subpopulations. Measurements were made at the duodenal mucosa (A) and in peripheral blood (B). Horizontal bars represent mean values (comparisons were made using unpaired t tests). Abbreviation: NS, not significant.
In contrast with observations made at the duodenal mucosa, integrin β7 expression was more intense among the total CD8+ T-cell population than among the total CD4+ T-cell population in peripheral blood (P = .0005; Figure 5B). While integrin β7 expression intensity among the Ty21a-responsive CD4+ T-cell subpopulation was greater than that among the total CD4+ T-cell population (P = .002; Figure 5B), expression intensity among the influenza virus–responsive CD4+ T-cell subpopulation was not different from that among the total CD4+ T-cell population. Integrin β7 expression intensity among the Ty21a-responsive and heterologous influenza virus–responsive CD8+ T-cell subpopulations was greater than that among the total CD8+ T-cell population (P = .004 and P = .041, respectively; Figure 5B).
DISCUSSION
We have described, for the first time, the cellular response to oral vaccination at the human intestinal mucosa. We demonstrate that Ty21a vaccination generates both polyfunctional Ty21a-responsive and heterologous influenza virus–responsive T cells at the duodenal mucosa. The frequency of Ty21a-responsive and influenza virus–responsive T cells at the duodenal mucosa were robustly and consistently correlated. In contrast, cellular and humoral measurements made in peripheral blood were poorly correlated with one another and did not provide insight into the induction of heterologous responses at the duodenal mucosa.
While robust Ty21a and heterologous influenza virus–responsive CD4+ and CD8+ T cells were observed at the duodenal mucosa, no cellular response was observed at the colonic mucosa to either antigen in either T-cell subset. This suggests that duodenal responses were compartmentalized to the embryological midgut, which includes the terminal ileum, the likely site of active invasion by Ty21a [1]. Interestingly, the frequency of Ty21a-responsive CD4+ and CD8+ T cells correlated robustly with one another and with the frequency of heterologous influenza virus–responsive CD4+ and CD8+ T cells at the duodenal mucosa. This suggests that the mechanisms responsible for the generation of these mucosal responses were localized and capable of influencing T cells of different phenotypes and specificities.
Polyfunctionality among Ty21a-responsive T cells at the duodenal mucosa and in peripheral blood may point toward an important mechanism through which Ty21a confers protection against disease [20, 21]. Further, the generation of anti-LPS immunoglobulin, an accepted indication of vaccine efficacy, was closely associated with cellular responses at the duodenal mucosa, suggesting that mucosal cellular activity may be associated with the generation of protective immunity.
In murine models, influenza virus infection results in the recruitment of previously primed heterologous T cells to the lung [17]. We propose that a similar mechanism was responsible for the generation of heterologous responses at the duodenal mucosa. Although only 2 individuals had previously been vaccinated against influenza, all were likely to have been exposed to this mucosal pathogen in the community—indeed, at baseline, anti-influenza virus IgG and IgA, as well as influenza virus–responsive CD4+ T cells, were detected in all volunteers, not just those who had been previously vaccinated against influenza.
Trafficking to the mucosa is largely dependent upon the expression of α4β7 and its ligand, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), as well as C-C chemokine receptor 9 and its ligand, chemotaxis chemokine ligand 25 [24]. We hypothesize that vaccination with Ty21a upregulated the expression of mucosal homing ligands resulting in the nonspecific migration of previously primed heterologous influenza virus–responsive T cells, carrying mucosal homing receptors, to the duodenal mucosa. This effect may or may not be restricted to influenza virus–responsive T-cell subsets, and further study is warranted.
We observed that integrin β7 expression intensity was generally higher among both Ty21a-responsive and influenza virus–responsive cells, compared with total cell populations, in peripheral blood and at the intestinal mucosa. Multiphasic peripheral CD8+ T-cell responses have previously been described and attributed to the trafficking of immune cells from peripheral blood to the mucosa [8, 9]. In keeping with this, integrin β7 expression among peripheral CD8+ T cells, particularly antigen-specific subpopulations, was more intense than that among peripheral CD4+ T cells. Integrin β7 expression among CD4+ T-cell subsets was highly polarized, being relatively low among peripheral subsets and high among duodenal subsets. Differential integrin β7 expression among antigen-specific CD4+ T-cell populations may ensure that some peripheral T cells persist in blood and avoid sequestration. We and others have demonstrated the presence of intracellular intravascular and bone-marrow bacteria in early, late, and recurrent invasive Salmonella disease [25, 26]. Thus, while T cells trafficking to the mucosa likely play a key role in the early response to invasion, CD4+ T-cell populations that persist in peripheral blood may help to prevent intravascular dissemination and persistence at secondary systemic sites of infection.
Prior to vaccination, all volunteers had detectable baseline levels of serum immunoglobulin specific to S. Typhi LPS. This may be caused by antibody binding to the core or a result of environmental exposure to nontyphoidal strains bearing the same LPS O-antigens as S. Typhi (O-9 and O-12); S. enterica serovar Enteritidis expresses both O-9 and O-12 antigens, and S. enterica serovar Typhimurium expresses the O-12 antigen [27]. Not all vaccinated volunteers generated peripheral humoral anti-LPS responses, which is likely a reflection of the limited efficacy of this vaccine. It was interesting that mucosal cellular responses correlated strongly with serum anti-LPS immunoglobulin responses, as humoral responses to LPS have been associated with vaccine efficacy [18]. This suggests that, in individuals in whom Ty21a vaccination is not efficacious, the failure encompasses both cellular and humoral mechanisms of defense. It has previously been demonstrated that the generation of immunoglobulin following oral vaccination is dependent upon α4β7 [28]. Thus, the close association between cellular and humoral immune responses suggests that the strength of each volunteer's cellular and humoral response to vaccination was influenced by a mucosal mechanism, possibly the expression intensity of α4β7 and/or MAdCAM-1 at the time of vaccination.
It is possible that, through Toll-like receptor engagement, vaccination may have nonspecifically activated mucosal antigen-presenting cells and T cells, resulting in enhanced cytokine production following experimental restimulation [29, 30]. However, as background cytokine production in the negative control was minimal, and as cytokine production in the positive control was not different in the vaccinated volunteers as compared to the unvaccinated volunteers, we believe this is unlikely to be the primary factor responsible for the observed heterologous cellular response. Owing to the invasive nature of endoscopic biopsy, we were limited to sampling from intestinal sites at a single time point. Data presented here and elsewhere [8] indicate that the assessment of mucosal immunity at alternate time points could provide further insight into the generation of protective immune responses. We assessed the expression of just 3 cytokines and, as a result, the T-cell populations identified here are unlikely to represent the responsive populations in their entirety.
In future studies, we plan to study the effect of oral vaccination with Ty21a on the cellular response to a range of heterologous antigens. We will comprehensively assess cellular phenotype and functionality through the inclusion of additional surface markers and a broader repertoire of cytokines/chemokines. We also plan to further investigate the timing and longevity of peripheral and mucosal immune responses.
Taken together, our data demonstrate that oral vaccination with Ty21a generates Ty21a-responsive T cells as well as heterologous influenza virus–responsive T cells at the duodenal mucosa. We propose that heterologous influenza virus–responsive T cells previously primed through natural exposure were recruited to the duodenal mucosa through compartmentalized upregulation of homing ligands. The direct evaluation of mucosal cellular immune defense provides the opportunity to identify functional correlates of protection and may offer new insights into mechanisms that may be manipulated to improve oral vaccine immunogenicity, either through the development of oral adjuvants or the development of multivalent Salmonella-based vectors.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all volunteers who participated in this study; the staff of the of the Gastroenterology Unit at the Royal Liverpool University Hospital, for their assistance; and Dr Sreedhar Subramanian (Royal Liverpool University Hospital), for helpful contributions.
Financial support. This work was supported by the Sir Jules Thorn Charitable Trust, the Rosetrees Trust, and the Wellcome Trust.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med 2002; 347:1770–82. [DOI] [PubMed] [Google Scholar]
- 2.Germanier R, Fuer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis 1975; 131:553–8. [DOI] [PubMed] [Google Scholar]
- 3.Wahdan MH, Serie C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis 1982; 145:292–5. [DOI] [PubMed] [Google Scholar]
- 4.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet 1987; 1:1049–52. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin Infect Dis 2007; 45(suppl 1):S24–8. [DOI] [PubMed] [Google Scholar]
- 6.Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2014; 1:CD001261. [DOI] [PubMed] [Google Scholar]
- 7.Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol 2014; 21:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArthur MA, Sztein MB. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One 2012; 7:e38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salerno-Goncalves R, Wahid R, Sztein MB. Ex Vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol 2010; 17:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 2004; 173:5852–62. [DOI] [PubMed] [Google Scholar]
- 11.Salerno-Goncalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun 2005; 73:3521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 2002; 169:2196–203. [DOI] [PubMed] [Google Scholar]
- 13.Lundin BS, Johansson C, Svennerholm AM. Oral immunization with a Salmonella enterica serovar typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect Immun 2002; 70:5622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantele A, Zivny J, Hakkinen M, Elson CO, Mestecky J. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J Immunol 1999; 162:5173–7. [PubMed] [Google Scholar]
- 15.Kantele A, Kantele JM, Savilahti E et al. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol 1997; 158:574–9. [PubMed] [Google Scholar]
- 16.Thome JJ, Yudanin N, Ohmura Y et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 2014; 159:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology 2005; 340:296–306. [DOI] [PubMed] [Google Scholar]
- 18.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis 1989; 11(suppl 3):S552–67. [DOI] [PubMed] [Google Scholar]
- 19.van den Brink GR, van den Boogaardt DE, van Deventer SJ, Peppelenbosch MP. Feed a cold, starve a fever? Clin Diagn Lab Immunol 2002; 9:182–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darrah PA, Patel DT, De Luca PM et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843–50. [DOI] [PubMed] [Google Scholar]
- 21.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81:8468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlin C, Berg EL, Briskin MJ et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74:185–95. [DOI] [PubMed] [Google Scholar]
- 23.Cepek KL, Shaw SK, Parker CM et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 1994; 372:190–3. [DOI] [PubMed] [Google Scholar]
- 24.Mavigner M, Cazabat M, Dubois M et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest 2012; 122:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon MA, Kankwatira AM, Mwafulirwa G et al. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 2010; 50:953–62. [DOI] [PubMed] [Google Scholar]
- 26.Wain J, Diep TS, Ho VA et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantele A, Pakkanen SH, Siitonen A, Karttunen R, Kantele JM. Live oral typhoid vaccine Salmonella Typhi Ty21a - a surrogate vaccine against non-typhoid salmonella? Vaccine 2012; 30:7238–45. [DOI] [PubMed] [Google Scholar]
- 28.Wyant T, Leach T, Sankoh S et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut 2015; 64:77–83. [DOI] [PubMed] [Google Scholar]
- 29.Lore K, Betts MR, Brenchley JM et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol 2003; 171:4320–8. [DOI] [PubMed] [Google Scholar]
- 30.Caron G, Duluc D, Fremaux I et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol 2005; 175:1551–7. [DOI] [PubMed] [Google Scholar]
- 31.Kaltsidis H, Cheeseman H, Kopycinski J et al. Measuring human T cell responses in blood and gut samples using qualified methods suitable for evaluation of HIV vaccine candidates in clinical trials. J Immunol Methods 2011; 370:43–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.