Abstract
Background. Models of controlled human malaria infection (CHMI) initiated by mosquito bite have been widely used to assess efficacy of preerythrocytic vaccine candidates in small proof-of-concept phase 2a clinical trials. Efficacy testing of blood-stage malaria parasite vaccines, however, has generally relied on larger-scale phase 2b field trials in malaria-endemic populations. We report the use of a blood-stage P. falciparum CHMI model to assess blood-stage vaccine candidates, using their impact on the parasite multiplication rate (PMR) as the primary efficacy end point.
Methods. Fifteen healthy United Kingdom adult volunteers were vaccinated with FMP2.1, a protein vaccine that is based on the 3D7 clone sequence of apical membrane antigen 1 (AMA1) and formulated in Adjuvant System 01 (AS01). Twelve vaccinees and 15 infectivity controls subsequently underwent blood-stage CHMI. Parasitemia was monitored by quantitative real-time polymerase chain reaction (PCR) analysis, and PMR was modeled from these data.
Results. FMP2.1/AS01 elicited anti-AMA1 T-cell and serum antibody responses. Analysis of purified immunoglobulin G showed functional growth inhibitory activity against P. falciparum in vitro. There were no vaccine- or CHMI-related safety concerns. All volunteers developed blood-stage parasitemia, with no impact of the vaccine on PMR.
Conclusions. FMP2.1/AS01 demonstrated no efficacy after blood-stage CHMI. However, the model induced highly reproducible infection in all volunteers and will accelerate proof-of-concept testing of future blood-stage vaccine candidates.
Clinical Trials Registration. NCT02044198.
Keywords: malaria, AMA1, vaccine, blood stage, CHMI
The development of an effective vaccine against Plasmodium falciparum malaria remains a key strategic goal to aid the control, local elimination, and eventual eradication of this disease [1]. Next-generation vaccine strategies are currently seeking to improve on the moderate levels of efficacy reported from phase 3 clinical trials of the RTS,S/AS01 malaria vaccine [2]. The complexity of the parasite's life cycle within the vertebrate host and mosquito vector has made the development of vaccines against malaria challenging but also offers opportunities for numerous points of immune intervention [3]. One long-standing strategy has been to develop vaccines against the pathogenic blood stage of P. falciparum by inducing antibodies against the merozoite form of the parasite that invades erythrocytes [4]. Such vaccines could complement preerythrocytic immunity afforded by RTS,S/Adjuvant System 01 (AS01), ameliorate disease severity, and/or reduce or prevent transmission by accelerating the control and clearance of blood-stage parasitemia [5].
Numerous factors have hindered development of vaccines against the merozoite, including substantial levels of polymorphism in candidate antigens, redundant erythrocyte invasion pathways, and the apparent need for very high antibody concentrations to prevent rapid erythrocyte invasion [3, 4]. Furthermore, the best approach to assess the efficacy of blood-stage parasite vaccines in humans has been widely debated [5]. Historically, controlled human malaria infection (CHMI) models initiated by mosquito bite have been widely used to assess efficacy of preerythrocytic vaccine candidates in small, proof-of-concept, phase 2a clinical trials [6, 7]. In contrast, the efficacy testing of blood-stage vaccines has typically relied on larger-scale phase 2b field trials in endemic populations, although a few trials of the mosquito-bite CHMI model have been performed [8, 9]. Reasons for this include the assumption that the efficacy of blood-stage vaccines could not be assessed in the short interval between parasite emergence from the liver (around 6–7 days after mosquito bite) and diagnosis of blood-stage infection by thick-film microscopy (typically 4–6 days later).
CHMI models are being increasingly used for the testing of antimalarial drugs [10], as well as vaccines [6]: infection can now be initiated by mosquito bite, injection of cryopreserved sporozoites, or injection of blood-stage parasites [6, 11–14]. A growing number of P. falciparum strains are being tested, to complement the historical focus on the laboratory reference clone 3D7 (or its parental strain, NF54) [15, 16], and genetically modified parasites have entered the clinical arena [17]. CHMI studies are also being undertaken in malaria-endemic countries [18, 19], and new models are being developed for Plasmodium vivax [20]. Here, we sought to build on previous experience [12–14, 21, 22] and further develop the blood-stage P. falciparum CHMI model to enable more-accurate and rapid efficacy assessment of blood-stage vaccine candidates prior to field trial assessment. The rationale for this study was that an effective blood-stage vaccine should demonstrate a measurable effect on the parasite multiplication rate (PMR) in malaria-naive individuals, especially against homologous challenge. Notably, adults with naturally acquired immunity in a malaria-exposed population showed substantially lower PMRs than nonimmune United Kingdom adults [23]. PMR can be modeled for each individual on the basis of quantitative real-time polymerase chain reaction (qPCR) data on blood-stage parasitemia prior to patency and detection by thick-film microscopy [24]. This CHMI model should allow for a longer period of qPCR monitoring, homologous challenge, and, compared with the mosquito-bite CHMI model, consistency in the initial number of blood-stage parasites in all volunteers. The uniformity of the known starting inoculum and the increased number of data points available for modeling should also lead to improved confidence of the calculated PMRs and, thus, to greater power to observe partial vaccine efficacy [5, 13, 21].
Apical membrane antigen 1 (AMA1) has been a long-standing vaccine antigen candidate, supported by a wealth of data from immunoepidemiological and in vitro studies, as well as rodent and nonhuman primate models [25]. A recombinant protein vaccine, known as FMP2.1, based on the 3D7 clone sequence of AMA1 [26] and formulated in AS01 or AS02 from GlaxoSmithKline (GSK) has previously been developed and tested in a series of phase 1a/b safety and immunogenicity trials [8, 27–29]. Both adjuvants contain the immune enhancers monophosphoryl lipid A and QS-21 Stimulon (Quillaja saponaria Molina, fraction 21; licensed by GSK from Agenus). A subsequent phase 2b field trial involving 400 Malian children that used the FMP2.1/AS02 formulation reported strain-specific efficacy against parasites with a 3D7 AMA1-like sequence in a secondary efficacy end point analysis [30]. Here, we report an assessment of the PMR in healthy United Kingdom adults following FMP2.1/AS01 receipt and CHMI with homologous 3D7 clone blood-stage parasites in a phase 1/2a trial.
METHODS
FMP2.1/AS01 Vaccine
The protein vaccine FMP2.1 has been previously reported [26] and encodes amino acids 83–531 of P. falciparum AMA1 (3D7 clone sequence). The batch of FMP2.1 protein was 130 months old and passed repeat evaluation by all release assays prior to use in this trial (Supplementary Table 1). As in previous trials [8, 30], 50 µg of FMP2.1 was administered intramuscularly with AS01.
Study Design and Approvals
The VAC054 study was an open-label, nonrandomized phase 1/2a trial of the blood-stage malaria vaccine candidate FMP2.1/AS01, with efficacy assessed by blood-stage CHMI in vaccinated volunteers and compared to that for infectivity controls (Supplementary Figure 1). The vaccine was administered at days 0, 28, and 56 (nominal study days are reported throughout). Volunteers underwent CHMI 2 weeks after the final vaccination (on day 70 or the day of challenge [dC + 0]). The study received ethical approval from the United Kingdom National Health Service (NHS) Research Ethics Service (Oxfordshire Research Ethics Committee A, reference 13/SC/0596) and the Western Institutional Review Board in the United States (reference 20131985). The study was approved by the United Kingdom Medicines and Healthcare Products Regulatory Agency (reference 21584/0326/001-0001). The trial was registered with Clincaltrials.gov (NCT02044198) and was conducted according to the principles of the current revision of the Declaration of Helsinki 2008 and in full conformity with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines for good clinical practice.
Participants
Healthy, malaria-naive males and nonpregnant females aged 18–45 years were invited to participate in the study. All volunteers gave written informed consent prior to participation. A full list of inclusion and exclusion criteria is reported in Supplementary Methods. The original donor of the blood-stage inoculum was seropositive for cytomegalovirus (CMV) and Epstein-Barr virus (EBV), so in previous CHMI trials performed in the United Kingdom [14, 21], volunteers were excluded on the basis of CMV or EBV seronegativity [12, 13]. However, the inoculum bank has tested negative for both viruses by PCR, so, for this study, subjects were deemed eligible regardless of serostatus. Data on adverse events (AEs) were collected throughout a volunteer's participation in the trial, either on diary cards or at follow-up visits (Supplementary Table 2).
Blood-Stage CHMI
A single vial of blood-stage inoculum was thawed, washed, and diluted under aseptic conditions (Supplementary Methods). Sequencing of the parasite gene encoding AMA1 was performed, and 100% identity with the 3D7 clone sequence in the FMP2.1 vaccine was confirmed. The intended inoculum was 1000 parasitized erythrocytes per volunteer. A limiting dilution assay performed on the inoculum at the time the last volunteer was infected demonstrated 69% viability (ie, an effective inoculum of 690 parasites per volunteer). Following CHMI, blood samples were collected once on dC + 1 and twice daily from dC + 2 for qPCR analysis. Diagnosis of malaria was made on the basis of positive findings on a thick blood film, confirmed by qPCR findings of ≥500 parasites/mL (Supplementary Methods).
Parasite qPCR and PMR Modeling
qPCR was conducted as previously described [9], with some minor modifications (Supplementary Methods). Raw data are reported in Supplementary Table 3. qPCR data for 4 previous mosquito-bite CHMI trials (MAL034A, MAL034B, VAC039, and VAC045) have been previously reported [9, 31, 32]. PMR was calculated using a linear model fitted to log10-transformed qPCR data [24] according to methods prespecified in the protocol (Supplementary Methods). Fitted lines were constrained to pass through the known starting parasitemia level, calculated from the results of the viability assay of the inoculum and a weight-based estimate of each volunteer's blood volume [9].
Ex Vivo Interferon γ (IFN-γ) Enzyme-Linked Immunspot (ELISpot) Assay
T-cell responses to AMA1 were assessed over time by ex vivo IFN-γ ELISpot analysis following restimulation of peripheral blood mononuclear cells (PBMCs) for 18–20 hours with overlapping peptides spanning the entire AMA1 3D7 sequence present in the vaccine. Assays were performed as previously described [9], with some minor modifications (Supplementary Methods). Results are expressed as IFN-γ spot-forming units (SFU) per million PBMCs.
Enzyme-Linked Immunosorbent Assay (ELISA) of Total Immunoglobulin G (IgG), Avidity, and Isotypes
ELISAs of total AMA1 3D7 IgG in serum were performed in Oxford as previously described, with reporting in micrograms/milliliter, using the same calibration-free concentration analysis conversion factor [33]. Serum IgG ELISAs at the National Institutes of Health (NIH) and the Walter Reed Army Institute of Research (WRAIR) were also performed as previously described, with reporting in micrograms/milliliter, using conversion factors generated with affinity-purified AMA1-specific human IgG [8, 34]. Avidity (sodium thiocyanate–displacement) ELISA and isotype ELISAs were performed in Oxford as previously described [33, 35].
In Vitro Assay of Growth Inhibitory Activity (GIA)
The ability of antibodies to inhibit growth of P. falciparum 3D7 clone parasites in vitro was assessed at the NIH GIA Reference Center by a standardized GIA assay, using purified IgG as previously described [34]. Briefly, each test IgG was incubated with synchronized P. falciparum parasites for a single growth cycle, and relative parasitemia levels were quantified by biochemical determination of parasite lactate dehydrogenase. All samples were tested at 10 mg/mL in a final test well, followed by a dilution series for positive samples to determine the concentration that gave 50% GIA (half maximal effective concentration [EC50]). GIA assays were also conducted at the WRAIR, using 20% serum dilution as reported previously [8]. Serum IgG concentrations were measured using the ADI Human IgG ELISA Kit (catalog no. 1750) as per the manufacturer's protocol but with the inclusion of an extra sample dilution (1:100 000).
Statistical Analysis
Full analyses are described in the Supplementary Methods. The study was powered to detect a 33% decrease in mean PMR with ≥80% power.
RESULTS
Participant Flow
Forty-five volunteers were screened in total across 3 trial sites (Supplementary Figure 1). Fifteen volunteers were recruited to each group, with more males recruited than females in both (group 1, 66.7% male; group 2, 73.3% male). The age range of volunteers in group 1 was 23–43 years (mean, 33 years) and 19–34 years in group 2 (mean, 22 years). Three volunteers in group 1 withdrew from the trial prior to completing the vaccination phase, and 1 volunteer in group 1 also withdrew after CHMI (at dC + 8.5), all for personal reasons.
Vaccine and CHMI Safety
There were no serious AEs or unexpected reactions during the course of the trial, and no volunteers withdrew due to vaccine- or CHMI-related AEs. The safety profile of FMP2.1/AS01 was similar to that reported previously in healthy US adult volunteers [8], with the second and third vaccinations reported as more reactogenic than the first. The solicited AEs are shown in Supplementary Table 2. The majority of solicited AEs occurred within the first 2 days after vaccination and resolved within 72 hours. There were no severe unsolicited AEs or laboratory-detected AEs reported following vaccination. Symptoms relating to malaria parasite infection following CHMI were similar across both groups and peaked after initiation of antimalarial therapy. EBV and CMV serological findings were checked before and after CHMI, and, as expected, there were no cases of seroconversion (Supplementary Table 4), adding to the growing safety database that supports the use of this inoculum in volunteers regardless of their EBV/CMV serostatus [12].
Blood-Stage CHMI and Vaccine Efficacy
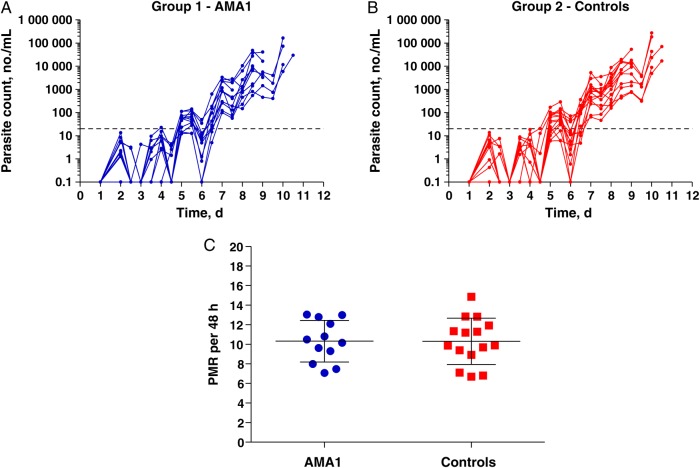
All volunteers developed patent blood-stage parasitemia following CHMI and received a diagnosis on the basis of findings of thick blood film microscopy by dC + 10.5, except for the one volunteer who withdrew on dC + 8.5 (Figure 1A and 1B and Supplementary Figure 2A). This volunteer was asymptomatic, and the thick blood film was negative for parasites, so the volunteer had not reached the criteria for commencing treatment; however, the volunteer had positive qPCR results at the time of withdrawal and was included in the primary analysis. There was neither a delay to diagnosis in vaccinees as compared to controls, nor any difference in parasitemia level between the groups at time of diagnosis (Supplementary Figure 2B and 2C). The protocol prespecified primary analysis for efficacy was comparison of PMR between the two groups. There was no difference in the mean PMRs between the two groups (Figure 1C). The mean PMR (±SD) for group 1 was 10.32 ± 2.13 (95% confidence interval [CI], 8.97–11.67) and for group 2 was 10.31 ± 2.36 (95% CI, 9.00–11.62; P = .99, by the 2-tailed unpaired t test).
Figure 1.
Blood-stage controlled human malaria infection and parasite multiplication rate (PMR) analysis. Individual quantitative polymerase chain reaction data are shown for the VAC054 phase 2a study, including 12 apical membrane antigen 1 (AMA1) vaccinees (group 1; A) and 15 unvaccinated infectivity controls (group 2; B). The lower limit of quantification is indicated by the dotted line at 20 parasites/mL. C, Primary end point analysis of PMRs, showing data for each individual plus the mean ± SD for each group. Both data sets are normally distributed (as determined by the D'Agostino–Pearson test), with similar variance (P = .74, by the F test).
T-Cell and Antibody Responses in Vaccinees and Controls
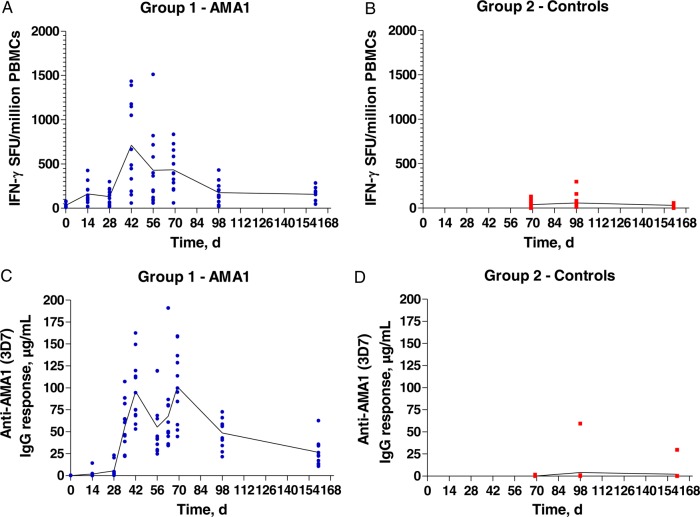
FMP2.1/AS01 elicited T-cell responses as assessed by ex vivo IFN-γ ELISpot, with median responses of 577 and 396 SFU/million PBMCs at d42 and d69/C-1 respectively (Figure 2A and Supplementary Figure 3A). These responses did not boost after the CHMI, with a median of 148 SFU/million PBMCs seen at d98/C+28. Modest responses were induced in the controls (median, 29 SFU/million PBMCs at the same time point), with only 2 volunteers showing responses >150 SFU/million PBMCs (Figure 2B and Supplementary Figure 3B–D).
Figure 2.
T-cell and antibody responses in vaccinees and controls. A and B, T-cell responses were assessed in each group by ex vivo interferon γ (IFN-γ) enzyme-linked immunospot analysis, using fresh peripheral blood mononuclear cells (PBMCs). C and D, Serum anti–apical membrane antigen 1 (AMA1; 3D7) immunoglobulin G (IgG) responses were assessed in Oxford for each group by an enzyme-linked immunosorbent assay. Mean and individual responses are shown over time. Blood-stage controlled human malaria infection took place on day 70. Abbreviation: SFU, spot-forming units.
AMA1-specific serum IgG responses were measured by ELISA in Oxford, with median responses of 85 and 97 µg/mL at d42 and d69/C-1, respectively (Figure 2C and Supplementary Figure 4A). These responses did not boost after CHMI, with a median of 56 µg/mL seen at d98/C+28. Only 1 of 15 controls showed a de novo anti-AMA1 IgG response at d98/C+28 (59 µg/mL; Figure 2D and Supplementary Figure 4B–D). Findings of 2 independent ELISAs (performed at the NIH and WRAIR) correlated with findings of Oxford's ELISA but were not concordant (Supplementary Figure 5A and 5B), with median responses of 114 and 295 µg/mL at d69/C-1, respectively (Supplementary Figure 5C and 5D). The results from WRAIR were significantly lower than those reported by Spring et al for the same vaccine tested in healthy US adults (Supplementary Figure 5E) [8]. The avidity of the anti-AMA1 IgG was similar at d42, d69/C-1, and after CHMI in the vaccinees (Supplementary Figure 6A) and very similar to that observed with other AMA1 vaccines in humans [33, 35]. The response was composed of IgG1, IgG3, immunoglobulin A, and immunoglobulin M, and this profile was not affected by CHMI (Supplementary Figure 6B).
Measures of In Vitro GIA
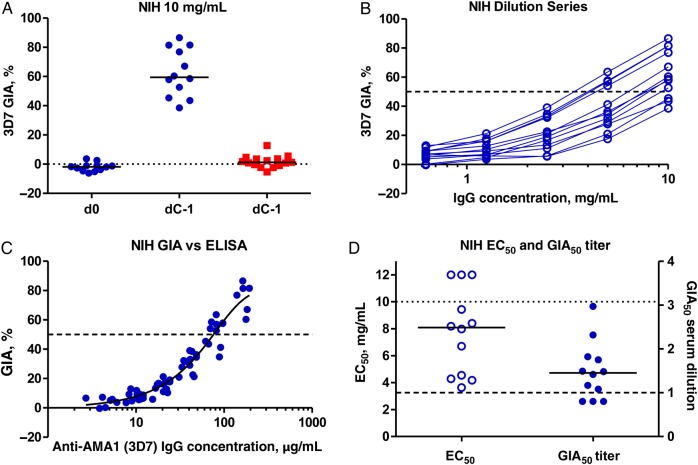
Serum was analyzed at the GIA Reference Center at the NIH, and IgG was purified from each sample. Samples from group 1 volunteers prior to vaccination (d0) and group 2 volunteers prior to CHMI (d69/dC-1) did not demonstrate any GIA above baseline. Samples from group 1 volunteers following 3 vaccinations demonstrated a median in vitro GIA of 59.5% (range, 38.5%–86.5%), with 10 mg/mL purified IgG at dC-1 (Figure 3A). The GIA decreased as purified IgG was diluted (Figure 3B) and was related to the AMA1-specific IgG concentration (Figure 3C), in close agreement with other independent studies [33, 34]. The EC50 was calculated for each purified IgG, with a median of 8.1 mg/mL. To relate these results (using a normalized concentration of purified IgG) back to the original sera, the concentration of IgG in each original serum sample was also measured. This enabled calculation of the GIA50 serum titer, defined previously as the dilution factor of each serum sample required to reach the concentration of purified IgG that gives 50% GIA [36]. The median GIA50 titer for group 1 was 1.5, with the maximum observed being a dilution factor of 3.0 (Figure 3D). Analyses of GIA, using 20% serum, were also conducted at WRAIR. These results were comparable to those observed in the NIH assay at approximately 2 mg/mL purified IgG (equivalent to 20% serum) but significantly lower than those reported by Spring et al for the same vaccine tested in healthy US adults [8] (Supplementary Figure 7).
Figure 3.
Assessment of functional growth inhibitory activity (GIA) induced by FMP2.1/Adjuvant System 01 vaccination. A, In vitro GIA of purified immunoglobulin G (IgG) was assessed at 10 mg/mL against 3D7 clone Plasmodium falciparum parasites at the National Institutes of Health (NIH) GIA Reference Center. Individual data and medians are shown for each group on the day before challenge (dC-1), as well as before vaccination (d0) for group 1. Responses >12% are typically regarded as positive for 3D7. B Dilution series of purified IgG from group 1 samples obtained on dC-1. C, Relationship between GIA and anti-3D7 AMA1 serum IgG concentrations, measured by enzyme-linked immunosorbent assay (ELISA) at the NIH. A nonlinear regression curve is also shown (n = 60). The level of anti-3D7 AMA1 response in this ELISA that gives 50% GIA (GIA50), indicated by the dotted line, was 75.5 µg/mL, (95% confidence interval, 68.3–84.2). D, Individual half maximal effective concentration (EC50) for each purified IgG is shown, as well as the GIA50 titers. Individual data and medians are shown for group 1 at dC-1.
Comparison of Blood-Stage and Mosquito-Bite CHMI
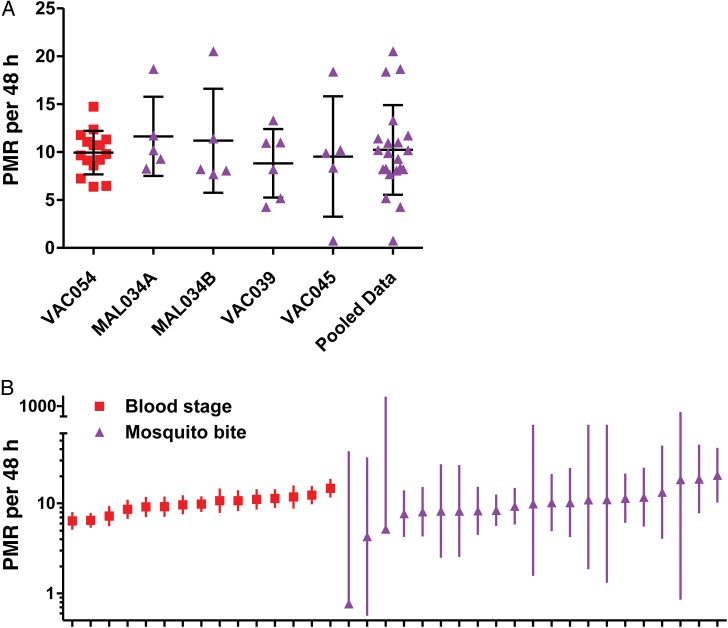
The PMRs in the group 2 infectivity controls were compared to historical data from unvaccinated infectivity control volunteers in 4 previous CHMI trials, in which volunteers were exposed to the bites of 5 mosquitoes infected with the 3D7 clone of P. falciparum [9, 31, 32]. PMRs were modeled from these data sets as described in Supplementary Methods. The pooled data from the 4 trials showed a similar mean PMR of 10.23 per 48 hours (n = 21) to blood-stage CHMI, but a significantly larger spread (SD = 4.67; P = .008, by the F test; Figure 4A). These data indicated that the blood-stage model provides better power to observe partial vaccine efficacy against blood-stage parasite growth rates and, moreover, showed that each individual PMR can be modeled from the qPCR data with greater confidence (Figure 4B).
Figure 4.
Comparison of blood-stage and mosquito-bite controlled human malaria infection (CHMI). A, Parasite multiplication rates (PMRs) were calculated for infectivity control volunteers in 4 historical mosquito-bite CHMI trials (purple). Data are shown for each trial and are also pooled. Individual data points and mean ± SD are shown. B, Individual PMRs and 95% confidence intervals are shown.
DISCUSSION
A suitable means to assess proof-of-concept vaccine efficacy against blood-stage P. falciparum has been widely debated [5]. It has been argued that blood-stage vaccines may prevent disease phenotypes and/or impart immune-mediated protection only at high parasite densities. In these cases, CHMI in healthy malaria-naive adult volunteers would not be suitable because volunteers would require rescue treatment at the time of microscopy-based patency, even when this is prior to the onset of disease symptoms. However, in an era when next-generation vaccines are seeking to build on the success of RTS,S [1, 2], it is vital that a blood-stage vaccine candidate be able to control parasitemia in a malaria-naive individual and prevent onset of disease symptoms. Here we sought to develop the blood-stage CHMI model in healthy United Kingdom adults to address this concept, using the effect on PMR as the primary efficacy end point.
Although AMA1 has long been considered a leading candidate vaccine antigen [25], significant efficacy has not been demonstrated as a primary end point of any phase 2a/b clinical trial [4]. Previous CHMI trials initiated by mosquito bite have suggested that some preerythrocytic immunity can be afforded by vaccines encoding AMA1 alone [8, 9] or in combination with circumsporozoite protein [37] (which associated with CD8+ T-cell responses against AMA1 [38]), but no direct impact on blood-stage parasitemia has been observed [14]. Nevertheless, in a phase 2b field trial in Malian children, FMP2.1/AS02 had an efficacy of 64.3% (hazard ratio, 0.36; 95% CI, .08–.86; P = .03) in a predefined secondary analysis against clinical malaria with 3D7-type parasites (defined by 8 immunologically important AMA1 polymorphisms in the cluster 1 loop of domain I), although the number of cases meeting this definition was small [30, 39]. This allele-specific efficacy, seen in the first malaria season, did not extend into the second season of follow-up [40]. Here we used the blood-stage CHMI model to assess FMP2.1/AS01 against homologous 3D7 clone parasites. In this trial, FMP2.1/AS01 did not demonstrate any efficacy, with no reduction in PMR in vaccinees, compared with the infectivity control group. However, this trial demonstrated the reproducibility of the blood-stage CHMI model, with much larger group sizes than used in previous studies [14, 21, 22]. Moreover, we demonstrate its usefulness for measuring modest reductions in PMR in comparison to mosquito-bite CHMI, where analysis of historical data showed a higher dispersion of data in the infectivity controls.
FMP2.1/AS01 was immunogenic in this trial, eliciting AMA1-specific T-cell and antibody responses. IFN-γ T-cell responses, measured by ELISpot analysis, were higher than those seen with other AMA1 protein-in-adjuvant vaccines tested using the same assay [14]. Purified serum IgG was able to inhibit parasite growth in vitro at high levels at 10 mg/mL, but both the ELISA and functional GIA analysis performed at WRAIR showed the responses to be modestly but significantly lower than those reported in a previous trial of this vaccine in healthy US adults that used AS01 and AS02 [8]. The serum antibody and GIA responses were also comparable to those for another AMA1 vaccine candidate that failed to impact PMR in a much smaller and underpowered blood-stage CHMI trial [14]. The somewhat reduced immunogenicity in this trial may have been related to the age of the FMP2.1 protein, but there had been no measurable change in the protein quality over time, and the vaccine lot passed all rigorous quality control testing prior to use in this study. Nevertheless, the lack of FMP2.1/AS01 efficacy in this trial, in contrast to findings from the phase 2b field study, could be due to a number of possible reasons: reduced vaccine immunogenicity, the use of AS01 instead of AS02, an impact of this vaccine only at high parasite densities, a preerythrocytic effect of the vaccine, or the fact that the Malian children, unlike United Kingdom adults, would have possessed preexisting antimalarial immune responses, including anti-AMA1 IgG, which may have acted in conjunction with the vaccine-induced anti-AMA1 responses.
Irrespective of this result, the immunogenicity analyses from this trial have highlighted important directions for future research. First, there is a need to harmonize immunomonitoring analyses between laboratories, in particular with regard to the reporting of antibody concentrations in µg/mL. Work is currently ongoing between the 3 laboratories to address this issue. More importantly, these data indicate that vaccines developed on the basis of the GIA assay in the future will need to achieve >40% GIA at 2.5 mg/mL purified IgG (in the NIH GIA Reference Center assay) if they are to protect a malaria-naive human. Notably, these trial results are consistent with previously reported data from Aotus monkeys [36, 41], including one trial in which only vaccinated animals that achieved >60% GIA using a purified IgG concentration of 2.5 mg/mL, or a GIA50 titer >5, were protected against blood-stage challenge [36]. These data thus support the clinical development of new vaccines with quantitatively or qualitatively improved antimerozoite antibody responses that may function by GIA or other mechanisms [42, 43]. The blood-stage CHMI model, as reported here, should accelerate proof-of-concept testing of this next generation of blood-stage malaria vaccine candidates and could potentially be used for testing passive immunization regimens that use purified IgG or monoclonal antibodies, as are being developed for human immunodeficiency virus [44].
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank R. Lopez-Ramon, M. Wilkie, M. Smith, A. Minhinnick, P. Marriott, N. Sousa, and M. Patel, for clinical support; N. Lella, J. Quaddy, C. Tyson, F. Martins, D. Kalogiannopoulou, D. Pratt, C. Rubery, A. Bell, H. Richards, M. Gomez, A. Brown, and D. Morelle, for logistical support; D. Muturi, P. Titus, and J. Muita, for microscopy support; S. Gerry, for statistical support; J. Furze, D. Nikolaeva, R. Kandt, L. Stockdale, D. Wright, and M. Wroblewska, for laboratory assistance; R. Sauerwein, G. Bastiaens, and E. Bijker, for sharing supporting controlled human malaria infection data; E. Berrie and M. Breese, for QP support; C. Banner, A. Van Goethem, S. Shanahan, and G. Zollner, for arranging contracts; A. Diouf, for technical support in performing the growth inhibitory activity assays; and all study volunteers.
Disclaimer. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the US Agency for International Development.
Financial support. This work was supported by the PATH Malaria Vaccine Initiative (MVI), through the Infectious Disease Division, Bureau for Global Health, US Agency for International Development (under cooperative agreement GHS-A-00-04-00016-00); by the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; by the United Kingdom National Institute of Health Research (NIHR) infrastructure, through the NIHR Oxford Biomedical Research Centre, the Southampton NIHR Wellcome Trust Clinical Research Facility, and the Imperial College NIHR Wellcome Trust Clinical Research Facility; by the Wellcome Trust (research training fellowships 089455/2/09/Z [to A. D. D.] and 097940/Z/11/Z [to S. H. H.]); by St Catherine's College, Oxford University (junior research fellowship to S. B.) and Nuffield Department of Medicine leadership fellowship (to S. B.); by the Jenner Institute (Jenner Investigator appointments to A. V. S. H. and S. J. D.); by the Lister Institute (research prize fellowship to S. J. D.); and, jointly, by the United Kingdom Medical Research Council (MRC) and Department for International Development (DFID), under the MRC/DFID Concordat agreement, and by the EDCTP2 program, supported by the European Union (MRC career development fellowship G1000527 to S. J. D.).
Potential conflicts of interest. S. D. is named on a patent related to the FMP2.1 vaccine. J. V. is a GlaxoSmithKline (GSK) employee and owns GSK shares. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet 2013; 382:1700–1. [DOI] [PubMed] [Google Scholar]
- 2.Rts SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbroth BR, Draper SJ. Recent developments in malaria vaccinology. Adv Parasitol 2015; 88:1–49. [DOI] [PubMed] [Google Scholar]
- 4.Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol 2010; 104:189–211. [DOI] [PubMed] [Google Scholar]
- 5.Sheehy SH, Douglas AD, Draper SJ. Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum Vaccin Immunother 2013; 9:1831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 2011; 11:57–64. [DOI] [PubMed] [Google Scholar]
- 7.Roestenberg M, O'Hara GA, Duncan CJ et al. Comparison of clinical and parasitological data from controlled human malaria infection trials. PLoS One 2012; 7:e38434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring MD, Cummings JF, Ockenhouse CF et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One 2009; 4:e5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehy SH, Duncan CJ, Elias SC et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther 2012; 20:2355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy JS, Sekuloski S, Griffin PM et al. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 2011; 6:e21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehy SH, Spencer AJ, Douglas AD et al. Optimising controlled human malaria infection studies using cryopreserved parasites administered by needle and syringe. PLoS One 2013; 8:e65960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol 2012; 28:515–21. [DOI] [PubMed] [Google Scholar]
- 13.Duncan CJ, Draper SJ. Controlled human blood stage malaria infection: current status and potential applications. Am J Trop Med Hyg 2012; 86:561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan CJ, Sheehy SH, Ewer KJ et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One 2011; 6:e22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanisic DI, Liu XQ, De SL et al. Development of cultured Plasmodium falciparum blood-stage malaria cell banks for early phase in vivo clinical trial assessment of anti-malaria drugs and vaccines. Malar J 2015; 14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teirlinck AC, Roestenberg M, van de Vegte-Bolmer M et al. NF135.C10: a new Plasmodium falciparum clone for controlled human malaria infections. J Infect Dis 2013; 207:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spring M, Murphy J, Nielsen R et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 2013; 31:4975–83. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson SH, Juma E, Salim A et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 2014; 5:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekalaghe S, Rutaihwa M, Billingsley PF et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 2014; 91:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy JS, Griffin PM, Sekuloski S et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 2013; 208:1688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanderson F, Andrews L, Douglas AD, Hunt-Cooke A, Bejon P, Hill AV. Blood-stage challenge for malaria vaccine efficacy trials: a pilot study with discussion of safety and potential value. Am J Trop Med Hyg 2008; 78:878–83. [PubMed] [Google Scholar]
- 22.Lawrence G, Cheng QQ, Reed C et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 2000; 18:1925–31. [DOI] [PubMed] [Google Scholar]
- 23.Douglas AD, Andrews L, Draper SJ et al. Substantially reduced pre-patent parasite multiplication rates are associated with naturally acquired immunity to Plasmodium falciparum. J Infect Dis 2011; 203:1337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas AD, Edwards NJ, Duncan CJ et al. Comparison of modeling methods to determine liver-to-blood inocula and parasite multiplication rates during controlled human malaria infection. J Infect Dis 2013; 208:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol 2008; 24:74–84. [DOI] [PubMed] [Google Scholar]
- 26.Dutta S, Lalitha PV, Ware LA et al. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun 2002; 70:3101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thera MA, Doumbo OK, Coulibaly D et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS One 2008; 3:e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thera MA, Doumbo OK, Coulibaly D et al. Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS One 2010; 5:e9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polhemus ME, Magill AJ, Cummings JF et al. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 2007; 25:4203–12. [DOI] [PubMed] [Google Scholar]
- 30.Thera MA, Doumbo OK, Coulibaly D et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011; 365:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewer KJ, O'Hara GA, Duncan CJ et al. Protective CD8(+) T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 2013; 4:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgson SH, Ewer KJ, Bliss CM et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 2015; 211:1076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgson SH, Choudhary P, Elias SC et al. Combining viral vectored and protein-in-adjuvant vaccines against the blood-stage malaria antigen AMA1: report on a phase 1a clinical trial. Mol Ther 2014; 22:2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura K, Zhou H, Diouf A et al. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vaccine Immunol 2009; 16:963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswas S, Choudhary P, Elias SC et al. Assessment of humoral immune responses to blood-Stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PLoS One 2014; 9:e107903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglas AD, Baldeviano GC, Lucas CM et al. A PfRH5-Based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe 2015; 17:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang I, Sedegah M, Cicatelli S et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-Mediated immunity. PLoS One 2013; 8:e55571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedegah M, Hollingdale MR, Farooq F et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+ T cells targeting AMA1 class I epitopes. PLoS One 2014; 9:e106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouattara A, Takala-Harrison S, Thera MA et al. Molecular basis of allele-specific efficacy of a blood-stage malaria vaccine: vaccine development implications. J Infect Dis 2013; 207:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurens MB, Thera MA, Coulibaly D et al. Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in Malian children: 24-month follow-up of a randomized, double-blinded phase 2 trial. PLoS One 2013; 8:e79323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta S, Sullivan JS, Grady KK et al. High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model. PLoS One 2009; 4:e8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyle MJ, Reiling L, Feng G et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llewellyn D, Miura K, Fay MP et al. Standardization of the antibody-dependent respiratory burst assay with human neutrophils and Plasmodium falciparum malaria. Sci Rep 2015; 5:14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caskey M, Klein F, Lorenzi JC et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; 522:487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.