Abstract
Purpose:
There are multiple reported risk factors and a wide range of reported blood transfusion rates for total shoulder arthroplasty (TSA). There are no evidence-based guidelines for blood transfusions in TSA patients.
Materials and Methods:
We utilized the Nationwide Inpatient Sample to analyze 51,191 patients undergoing TSA between 1998 and 2011. The purpose was to describe the incidence and identify the preoperative factors that are independently associated with blood transfusion after TSA. In addition, we studied the association of blood transfusions with certain variables such as length of stay (LOS), total charges, and payer status.
Results:
The blood transfusion rate in our study was 6.1%. There was no difference in the rate of blood transfusions over the study period (P < 0.001). In our logistic regression model, significant associations were found with increased age (odds ratio [OR] =1.03), white race (OR = 1.05), higher Charlson-Deyo score (OR = 1.12), presence of ischemic heart disease (OR = 1.24), blood loss anemia (OR = 1.65), female gender (OR = 1.94), presence of coagulation disorders (OR = 2.25), and presence of deficiency anemia (OR = 3.5). Patients receiving a blood transfusion had higher total charges, a longer hospital LOS, and were more likely to be Medicare payers (P < 0.001).
Conclusions:
Our study found five clinically significant risk factors for blood transfusions for TSA: female gender, ischemic heart disease, deficiency anemia, coagulation disorder, and blood loss anemia. Patients with these risk factors should be considered higher risk for requiring a blood transfusion after TSA and counseled appropriately.
Level of Evidence:
Level II, retrospective cohort study, prognostic study.
Keywords: Allogeneic blood transfusion, autologous blood transfusion, complications, total shoulder arthroplasty
INTRODUCTION
Total shoulder arthroplasty (TSA) is a successful treatment option for patients with glenohumeral arthritis that is growing in popularity.[1] TSA is associated with considerable blood loss in some patients. As the frequency of TSA increases; incidence and risk factors for blood transfusion have gained importance.[2,3]
Blood transfusions are not benign interventions with potentially serious adverse effects for recipients. Risks and complications include adverse or allergic reactions, transmission of infectious diseases, and hemodynamic overload.[4] Previously reported rates of transfusion ranges from 7.4%[5] to 43%[6] for shoulder arthroplasty. There is no large nationwide study looking at the incidence and risk factors for blood transfusion after TSA, and there are no evidence-based guidelines for blood transfusions in patients undergoing TSA. The purpose of this study is to describe the incidence of transfusion after shoulder arthroplasty using a large nationwide database and identify which preoperative risk factors are independently associated with blood transfusion after TSA. In addition, we studied the association of postoperative blood transfusion with certain variables such as length of stay (LOS), total charges, and payer status. The goal of this study is to equip the physician and patient with data to assist in patient counseling and preoperative planning.
MATERIALS AND METHODS
The Nationwide Inpatient Sample (NIS) is a survey of hospitals conducted by the federal Healthcare Cost and Utilization Project (HCUP), which has been deemed statistically valid.[1] Based on a random inclusion of 20% of the nation's hospitals, the numbers from these hospitals are then weighted or extrapolated to produce national estimates. We used the NIS to identify 51,191 TSA procedures performed in the United States between 1998 and 2011. All intake and discharge data for these patients was recorded in the database.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes was used to identify the procedure performed as well as the diagnoses of allogeneic blood transfusion (ICD-9-CM 99.04) and autologous blood transfusion (ICD-9-CM 99.02). Risk factors were also identified in this manner in addition to using HCUP codes designated by the NIS database [Table 1]. We identified shoulder arthroplasty cases with use of the procedure codes for TSA (81.80) from the ICD-9-CM. Both conventional TSA and reverse TSA had the same ICD-9-CM code during the period of interest. From the database, the LOS as well as charges as a result of the inpatient stay were calculated and compared. Medical comorbidity was measured using the Charlson–Deyo index, a validated measure of comorbidity which consists of a weighted scale of 17 comorbidities (including myocardial infarction, congestive heart failure [CHF], peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes, diabetes with end-organ damage, hemiplegia, moderate or severe renal disease, tumor without metastasis, leukemia, lymphoma, moderate or severe liver disease, metastatic solid tumor, and AIDS), expressed as a summative score.[7]
Table 1.
List of International Classification of Diseases, Ninth Edition, Clinical Modification and Healthcare Cost and Utilization Project codes used in the present study
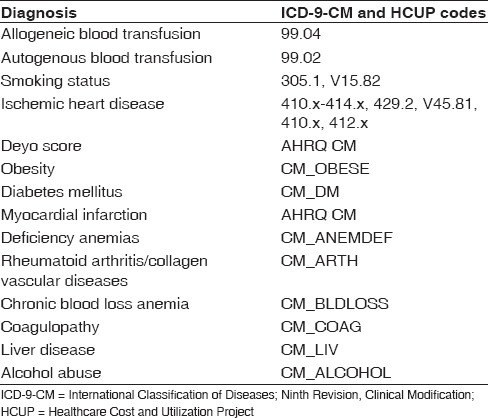
Data analysis was performed using SPSS version 19 (IBM Corporation, Armonk, NY, USA). The databases included in-hospital data only and no preoperative data aside from diagnoses were available. Logarithmic transformation within multivariate analysis using SPSS modeling was used to compare risk-adjusted association between patients undergoing TSA that received blood transfusions versus those who did not. A Chi-square analysis was done for the presence or absence of blood transfusion and multiple risk factors (categorical). In our logistic regression model, we controlled for age, gender, race, payer, Charlson–Deyo score, ischemic heart disease, obesity, hypertension, diabetes, deficiency anemia, coagulation disorders, rheumatoid arthritis (RA), chronic obstructive pulmonary disease (COPD), CHF, depression, blood loss anemia, and reported as an odds ratio (OR) with respect to 95% confidence interval (95% CI). Statistical significance was set at <0.01 level. The P value was set at this level to minimize the chance of a Type I error in this large sample sized database study.
RESULTS
A total of 51,191 TSAs were captured in the NIS database from 1998 to 2011. A steady increase in the number of TSAs was seen from 1998 to 2009, with the frequency noted to plateau between 2009 and 2011 [Figure 1]. There was no significant difference in the rate of postoperative blood transfusions over the study period [Figure 2].
Figure 1.
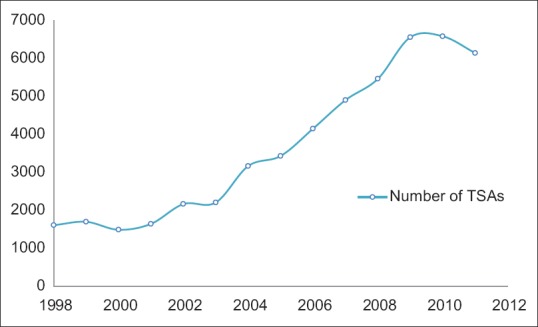
Number of total shoulder arthroplasties performed yearly between 1998 and 2011
Figure 2.
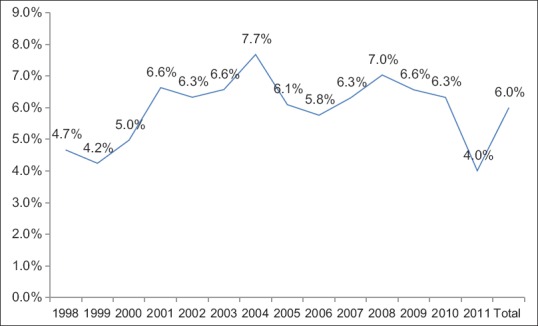
Yearly percentage of total shoulder arthroplasties who had a transfusion
A total of 8% of patients with TSAs were diagnosed with postoperative anemia, but only 6.1% had a blood transfusion. Of these 6.1%, 5.2% had an allogeneic blood transfusion and 0.9% had an autologous blood transfusion [Tables 2 and 3]. Since the percentage of TSA patients requiring autologous blood transfusions was low, we combined the allogeneic and autologous blood transfusion groups into an all-encompassing “any transfusion” group. We then compared that group with those who did not receive any blood transfusion.
Table 2.
Total shoulder arthroplasty patients with postoperative anemia
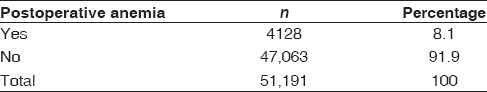
Table 3.
Total shoulder arthroplasty patients receiving a blood transfusion

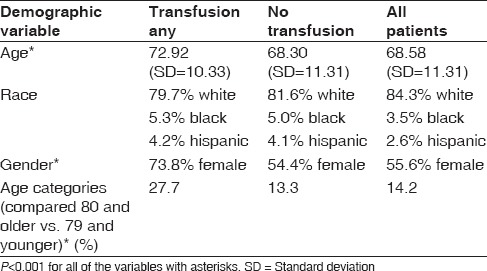
Patients who received a blood transfusion were older than those who did not receive a blood transfusion (72.9 and 68.3-year-old, respectively; [P < 0.001]). Patients over the age of 80 were twice more likely to have a transfusion than those <80 (27.7% vs. 13.3%, respectively; [P < 0.001]). Females were more likely to receive a transfusion than males (73.8% vs. 26.2%, respectively; [P < 0.001]). The rate of transfusions did not differ based on race [Table 4].
Table 4.
Patient demographic risk factors
Patients who received a blood transfusion were also more likely to have a higher number of diagnoses (8.5 vs. 6.0), higher number of procedures (2.9 vs. 1.5), a higher Charlson–Deyo score (0.57 vs. 0.28), diabetes (18.9% vs. 15.5%), ischemic heart disease (21.2% vs. 14.5%), CHF (7.7% vs. 2.5%), COPD (18.3% vs. 15.2%), RA (9.4% vs. 6.7%), and depression (11.5% vs. 9.7%) than those who did not receive a blood transfusion (P < 0.001) [Table 5]. Number of diagnoses simply denotes the medical diagnoses coded in patients' medical charts. For example, a patient with diabetes, hypertension, and cardiac arrhythmia will have a higher number of diagnoses than someone with just hyperlipidemia in their medical chart. While one can deduce why those with medical problems may be at a higher risk of requiring a blood transfusion, the higher incidence of blood transfusions in patients with depression is not clear at this point. One potential explanation is that patients with numerous medical problems may have a higher rate of depression, thus serving as a surrogate marker rather than a true association with blood transfusion risk.
Table 5.
Medical comorbidity risk factors
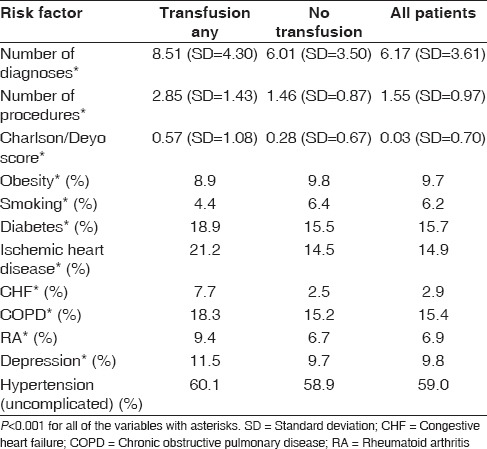
Interestingly, obese patients and smokers had lower rates of transfusion (P < 0.001). Hypertension, while common in patients undergoing TSA, was not found to be different between the groups [Table 5].
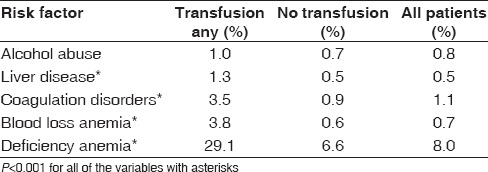
We decided to look at a subset of hematologic risk factors due to their close association with blood transfusions. We found that patients with liver disease (1.3% vs. 0.5%), coagulation disorders (3.5% vs. 0.9%), deficiency anemia (29.1% vs. 6.6%), and blood loss anemia (3.8% vs. 0.6%) had higher transfusion rates (P < 0.001). However, alcohol abuse was not found to be different between the groups [Table 6].
Table 6.
Hematologic risk factors
Patients who received a blood transfusion had a longer hospital LOS (4.1 vs. 2.3 days) [Figure 3] and higher total charges ($50,932 vs. $39,138) [Figure 4] than those without a transfusion (P < 0.001). A higher proportion of patients who received a blood transfusion were Medicare payers as compared to Private payers (P < 0.001) [Figure 5].
Figure 3.

Length of stay in patients with and without a transfusion. Length of stay in patients with a transfusion: 4.05 (standard deviation = 3.80); without transfusion: 2.33 (standard deviation = 1.61); total length of stay in all patients: 2.43 (standard deviation = 1.86)
Figure 4.

Total charges in patients with and without a transfusion. Total charges for transfusion patients: 50,931.82 (standard deviation = 38,651.99); no transfusion: 39,137.89 (standard deviation = 23,933.39); all patients: 39,855.34 (SD = 25234.16)
Figure 5.

Payer status in patients with and without a transfusion. Transfusion patients: 79.7% Medicare, 15.2% Private; no transfusion: 65.5% Medicare, 28.5% Private; total: 66.4% Medicare, 27.7% Private
In our logistic regression model, we controlled for age, gender, race, payer, Charlson–Deyo score, ischemic heart disease, obesity, hypertension, diabetes, deficiency anemia, coagulation disorders, RA, COPD, CHF, depression, and blood loss anemia. Significant ORs were found with increased age (OR = 1.03, 95% CI = 1.02–1.03), female gender (OR = 1.94; 95% CI = 1.75–2.16), white race (OR = 1.05; 95% CI = 1.03–1.07), presence of ischemic heart disease (OR = 1.24; 95% CI = 1.08–1.41), presence of deficiency anemia (OR = 3.52, 95% =3.14–3.96), presence of coagulation disorders (OR = 2.25; 95% CI = 1.69–2.99), blood loss anemia (OR = 1.65; 95% CI = 1.21–2.23), and higher Charlson-Deyo score (OR = 1.12, 95% CI = 1.06–1.20).
DISCUSSION
We utilized the NIS database to determine the incidence and risk factors of blood transfusions after TSA and the association of blood transfusions with certain variables such as LOS, total charges, and payer status. In our study, the total blood transfusion rate after TSA was 6.1%. Interestingly, this value is lower than the rate of blood transfusions reported in the shoulder arthroplasty literature, which ranges from 7.4%[5] to 43%.[8] One potential explanation for this discrepancy is the elective nature of TSA as compared to hemiarthroplasty, which are often done in a trauma setting with multiple coexisting factors that may lead to a requirement for transfusion.
Our logistic regression model identified eight risk factors with significant ORs associated with blood transfusions after TSA. Of these eight statistically significant risk factors, increased age, white race, and higher Charlson–Deyo score were only minimally associated with higher transfusion rates (OR = 1.03, 1.05, 1.12, respectively). The remaining five risk factors were both statistically and clinically significant for blood transfusions after TSA: Female gender, ischemic heart disease, deficiency anemia, coagulation disorder, and blood loss anemia. These risk factors have also been associated with blood transfusions in other shoulder arthroplasty studies.
Hardy et al. reported a 7.4% rate of blood transfusions in their study. Lower preoperative hemoglobin, higher intraoperative blood loss, and humeral cement fixation were predictors of transfusion.[2] Schumer et al. found a transfusion rate of 19.6% in their group of 280 shoulder arthroplasty patients. Preoperative hemoglobin level, age, and number of comorbid conditions were all predictive of transfusion in shoulder arthroplasty.[9] Millett et al.[10] reported transfusion in 25% of patients who underwent primary and revision shoulder arthroplasty in their retrospective study of 124 shoulder arthroplasties. Data from this study showed the preoperative hemoglobin level was the strongest predictor of blood transfusion in this patient population.
Our study also identified the presence of deficiency anemia (the database analog to low preoperative hemoglobin), age, and number of comorbid conditions as risk factors associated with blood transfusions. In our study, patients with deficiency anemia were 3.5 times more likely to require a blood transfusion than those without that diagnosis. Preoperative deficiency anemia had the highest OR of any variable in our study and is supported by a number of other studies in the literature. After controlling for variables in our logistic regression model, age, and number of comorbid conditions did not reach statistical significance.
Gruson et al. found age, preoperative hemoglobin level, female gender, and reverse TSA were independent risk factors for transfusion.[5] Sperling et al.[3] reported an overall transfusion rate of 8.1% in the primary humeral head and TSA. These authors found preoperative hemoglobin, female gender, and arthroplasty for the sequelae of trauma or RA compared with osteoarthritis were significant risk factors for transfusion.
Our study also identified an association between female gender and risk of blood transfusion. Female patients in our study were 1.9 times more likely to require a transfusion than males. This was one of the strongest associations in our study. After controlling for variables in our logistic regression model, the association between RA and risk of blood transfusion did not reach statistical significance. We attempted to study the type of arthritis and its association with blood transfusions, but the ICD-9-CM coding for preoperative diagnosis was not specific enough as the majority of TSAs had a diagnosis of unspecified primary versus secondary arthritis. This study focused on patients undergoing elective TSA. We did not look at patients with hemiarthroplasty to reduce the potential confounder of trauma.
Our study also identified an association between ischemic heart disease and risk of blood transfusion. To our knowledge, this association has not been previously reported in the shoulder arthroplasty literature. There is a debate as to whether patients with ischemic heart disease should have more lax transfusion parameters to lower the likelihood of adverse cardiac events.[7] Most studies looking into this issue are retrospective in nature and more research needs to be done to corroborate this strategy.
Interestingly, obese patients and smokers had lower rates of transfusion in our study. To our knowledge, these associations have not been reported in the shoulder arthroplasty literature. Previous studies have cited a “bleeding obesity paradox” where more obese patients were found to have a lower than average risk of bleeding than normal.[11] This could be a potential explanation for the association between obesity and lower transfusion rate. Numerous studies in the literature have supported the notion that cigarette smoking seems to cause a generalized upward shift of the hemoglobin distribution curve, which reduces the utility of hemoglobin level to detect anemia.[12] This suggests that extra attention should be paid to smokers postoperatively, and clinical symptoms should be scrutinized more carefully to detect postoperative anemia in smokers as they may be at risk of being under-transfused.
Our study found that patients who received a blood transfusion stayed in the hospital an average 1.8 days longer, and their total charges were $11,794 more than those who did not receive a transfusion. These data suggest that the decision to transfuse a patient should be well supported and justified as the immediate effect on charges and LOS is not insignificant. In addition, one should be aware that Medicare payers were more likely to receive a transfusion than Private payers in our study.
A major strength of this study is the large sample size. To our knowledge, this is the largest nationwide database study to look at risk factors associated with blood transfusions after TSA. The large sample size allows us to identify risk factors associated with blood transfusions and find statistically and clinically significant variables that smaller studies may not capture.
Limitations of this study are due in large part to the nature of this nationwide database analysis. The NIS does not take into account other preoperative factors or clinical concerns such as specific preoperative laboratory values and individualized data, the amount of units transfused. Functional outcomes and long-term data are also not available so we cannot comment on how these patients' mid- and long-term outcomes were affected by receiving a blood transfusion. Another limitation is the database does not differentiate between the types of TSAs and the techniques utilized, such as fixation method (cemented vs. uncemented), manufacturer, etc. Since conventional and reverse TSA share the same ICD-9 code, the difference in the risk factors between the two groups for transfusion could not be determined. In addition, database studies rely on clinical coding and entering diagnoses so under-coding or diagnosing may alter results. Another potential limitation is the clinical significance of results that are statistically significant. Future prospective clinical studies may assist in further elucidating these risk factors in more detail in patients undergoing TSA.
CONCLUSIONS
Analysis of the NIS database including more than 50,000 TSA patients over a 13-year period identified a low blood transfusion incidence of 6.1%. In addition, we identified five independent risk factors for postoperative blood transfusion: Females, patients with ischemic heart disease, and those with hematologic risk factors such as coagulation disorders, deficiency anemias, and blood loss anemia. Patients with these characteristics may be at higher risk for developing a blood transfusion after TSA and surgeons may use this information to assist in patient counseling and preoperative planning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–54. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JC, Hung M, Snow BJ, Martin CL, Tashjian RZ, Burks RT, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:233–9. doi: 10.1016/j.jse.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14:599–601. doi: 10.1016/j.jse.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78:1260–70. doi: 10.2106/00004623-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: An analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18:225–30. doi: 10.1016/j.jse.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Gerber DR. Transfusion of packed red blood cells in patients with ischemic heart disease. Crit Care Med. 2008;36:1068–74. doi: 10.1097/CCM.0b013e318169251f. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16:782–3. doi: 10.1016/j.jse.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:91–6. doi: 10.1016/j.jse.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:1223–30. doi: 10.2106/JBJS.E.00706. [DOI] [PubMed] [Google Scholar]
- 11.Bovill DF, Norris TR. The efficacy of intraoperative autologous transfusion in major shoulder surgery. Clin Orthop Relat Res. 1989;240:137–40. [PubMed] [Google Scholar]
- 12.Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264:1556–9. [PubMed] [Google Scholar]


