Abstract
Aim:
We previously proven that carbocisteine, a conventional mucolytic drug, remarkably reduced the rate of acute exacerbations and improved the quality of life in the patients with chronic obstructive pulmonary disease. In this study we investigated the mechanisms underlying the anti-inflammatory effects of carbocisteine in human alveolar epithelial cells in vitro.
Methods:
Human lung adenocarcinoma cell line A549 was treated with TNF-α (10 ng/mL). Carbocisteine was administered either 24 h prior to or after TNF-α exposure. The cytokine release and expression were measured using ELISA and qRT-PCR. Activation of NF-κB was analyzed with Western blotting, immunofluorescence assay and luciferase reporter gene assay. The expression of ERK1/2 MAPK signaling proteins was assessed with Western blotting.
Results:
Carbocisteine (10, 100, 1000 μmol/L), administered either before or after TNF-α exposure, dose-dependently suppressed TNF-α-induced inflammation in A549 cells, as evidenced by diminished release of IL-6 and IL-8, and diminished mRNA expression of IL-6, IL-8, TNF-α, MCP-1 and MIP-1β. Furthermore, pretreatment with carbocisteine significantly decreased TNF-α-induced phosphorylation of NF-κB p65 and ERK1/2 MAPK, and inhibited the nuclear translocation of p65 subunit in A549 cells. In an NF-κB luciferase reporter system, pretreatment with carbocisteine dose-dependently inhibited TNF-α-induced transcriptional activity of NF-κB.
Conclusion:
Carbocisteine effectively suppresses TNF-α-induced inflammation in A549 cells via suppressing NF-κB and ERK1/2 MAPK signaling pathways.
Keywords: carbocisteine, human alveolar epithelial cells, TNF-α, NF-κB, ERK1/2, cytokines, anti-inflammation
Introduction
Carbocisteine (S-carboxymethylcysteine) has been conventionally prescribed as a mucolytic agent because of its capacity to reduce mucus viscosity by modifying mucus's biochemical properties. Consistent with literature reports1,2, we found that carbocisteine was effective in reducing the rate of acute exacerbations and improving quality of life in patients with chronic obstructive pulmonary disease (COPD) in a large-scale multi-center clinical trial3.
Despite the lack of a free thiol group, carbocisteine harbors a thioether group that can be oxidized by reactive oxygen species (ROS)4. Carbocisteine reportedly scavenged H2O2, HOCl, OH* and ONOO− in cell-free medium, inhibited ROS production in rat neutrophils4, promoted GSH and HO-1 expression in cigarette smoke-exposed bronchial epithelial cells5, and ameliorated oxidant-induced 16HBE cell apoptosis6. Furthermore, carbocisteine was capable of attenuating the synthesis of pro-inflammatory cytokines following rhinovirus or respiratory syncytial virus infection7,8. It was speculated that the efficacy of carbocisteine in COPD was more closely associated with its anti-oxidant and anti-inflammatory effects9, which have been scarcely reported previously10, than with mucolytic activity.
Inflammation plays pivotal roles in the pathogenesis and progression of chronic respiratory diseases (particularly in COPD). Exacerbations of COPD have been associated with aggravated inflammatory responses, which increased the risks of morbidity and mortality11,12. Various transcription factors or kinases, including nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs), have been implicated in the pathogenesis of both stable and exacerbations of COPD13.
Tumor necrosis factor-α (TNF-α), a classical pro-inflammatory cytokine, plays an important role in promoting lung inflammation in COPD. The excessive release or spillover of TNF-α has been associated with the maintenance of neutrophilic airway inflammation and systemic manifestations, such as muscle wasting14,15. TNF-α has also been linked to COPD progression because circulating TNF-α levels were found to be inversely correlated with arterial partial pressure of oxygen (PaO2) and increased with dyspnea severity16. Mechanistically, TNF-α is a major inflammatory mediator of the lung and induces NF-κB and MAPK activation in different respiratory cell lines17,18. Of these, alveolar epithelial cells serve as an important dynamic barrier against environmental stimuli, sepsis, and other illnesses affecting the lung. Alveolar epithelial cells may amplify inflammatory signals via inflammatory cell chemotaxis and inflammatory mediator release19. Exploration of the effects on injured alveolar epithelial cells may provide greater insights into the mechanisms of action of medications (ie, mucolytics) in patients with COPD. We have previously revealed that carbocisteine could inhibit the inflammation induced by hydrogen peroxide in human lung alveolar type II epithelial cells (A549 cells)10. However, limited information is available about the effects as well as molecular mechanisms of carbocisteine against TNF-α-induced inflammation.
Therefore, we sought to explore the impact of carbocisteine on the synthesis and release of inflammatory cytokines and the signaling pathways in cultured A549 cells and HEK293 cells. Our findings will offer new insight into clarifying the mechanisms responsible for the effects of carbocisteine and lend support to its clinical application.
Materials and methods
Materials
Fetal bovine serum and RPMI-1640 medium were purchased from Gibco BRI (Grand Island, NY, USA), and the Trizol and PrimeScript RT Master Mix (Perfect Real Time) kits were from Takara (Dalian, China). Primers and probes of tested genes were synthesized by Invitrogen Trading Co, Ltd (Shanghai, China). Human IL-6 and IL-8 ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA), the BCA Protein Assay Kit and Super-Signal West Pico Trial Kit were from Thermo Scientific (Rockford, IL, USA), rabbit anti-pNF-κB p65, anti-NF-κB p65, anti-pIκB-α, anti-IκB-α, anti-pERK1/2, anti-ERK1/2 and anti-GAPDH monoclonal antibodies were from Cell Signaling Technology Inc (Beverly, MA, USA), HRP-conjugated goat anti-rabbit antibodies were from EarthOx Life Science (Millbrae, CA, USA), and the Bright-GloTM Luciferase assay system was from Promega Corporation (Madison, WI, USA). All other materials were purchased from Sigma Chemical Inc (St Louis, MO, USA).
Cell culture
Cells of the human lung adenocarcinoma cell line A549 were incubated in vitro in RPMI-1640 medium containing penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% (v/v) fetal bovine serum (FBS). Human embryonic kidney (HEK-293) cells were co-transfected with pNF-κB-TATA-F-LUCI (4.5 μg) and pQCXIP-eGFP (0.5 μg) plasmid (9:1) in a 60-mm dish and selected with 1 μg/mL puromycin for one week. The cells expressing eGFP with NF-κB reporter luciferase activity were cultured in vitro in DMEM medium supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% (v/v) FBS. All cells were incubated at 37 °C in a humidified incubator with 5% CO2.
When the confluence reached 70%–80%, A549 cells and HEK293 cells were treated with carbocisteine with/without TNF-α for various time periods with various doses as follows. To examine the protective effect of carbocisteine on cytokine expression and release, cells were pre-treated with 10, 100, and 1000 μmol/L carbocisteine for 24 h prior to or after exposure to TNF-α (10 ng/mL for 24 h). To determine intracellular signaling, A549 cells and HEK293 cells were pre-treated with 10, 100, and 1000 μmol/L carbocisteine for 24 h before exposure to TNF-α (10 ng/mL for 30 min). The cells and cell culture medium were harvested for subsequent measurements.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was extracted from cultured A549 cells using Trizol reagent by following the manufacturer's protocol. cDNA synthesis and quantitative PCR were performed using standard procedures as described previously10. Primers and probes of tested genes are listed in Table 1. Amplification was performed under the following conditions: 10 s at 95 °C, followed by 40 cycles with 5 s at 95 °C and 40 s at 60 °C. Cycle threshold (Ct) values were determined by the fit point method from the exponential phase of each amplification. The difference in Ct values was calculated as follows: ΔCt=Ct (test gene)−Ct (GAPDH), ΔΔCt=ΔCt (treated sample)−ΔCt (untreated control). The relative expression of tested genes was calculated according to the formula: Relative expression=2−ΔΔCt 20.
Table 1. Specific primers of tested genes were used for qRT-PCR.
| Gene | Primer/probe sequences |
|---|---|
| IL-6 | Forward primer 5′-CGGGAACGAAAGAGAAGCTCTA-3′ |
| Reverse primer 5′-CGCTTGTGGAGAAGGAGTTCA-3′ | |
| Probe 5′-FAM-TCCCCTCCAGGAGCCCAGCT-3′-TAMRA | |
| IL-8 | Forward primer 5′-TTGGCAGCCTTCCTGATTTC-3′ |
| Reverse primer 5′-TATGCACTGACATCTAAGTTCTTTAGCA-3′ | |
| Probe 5′-FAM-CCTTGGCAAAACTGCACCTTCACACA-3′-TAMRA | |
| TNF-α | Forward primer 5′-AACATCCAACCTTCCCAAACG-3′ |
| Reverse primer 5′-GACCCTAAGCCCCCAATTCTC-3′ | |
| Probe 5′-FAM-CCCCCTCCTTCAGACACCCTCAACC-3′-TAMRA | |
| MCP-1 | Forward primer 5′-CAAGCAGAAGTGGGTTCAGGAT-3′ |
| Reverse primer 5′-AGTGAGTGTTCAAGTCTTCGGAGTT-3′ | |
| Probe 5′-FAM-CATGGACCACCTGGACAAGCAAACC-3′-TAMRA | |
| IP-10 (forward) | Forward primer 5′-GAAATTATTCCTGCAAGCCAATTT-3′ |
| Reverse primer 5′-TCACCCTTCTTTTTCATTGTAGCA-3′ | |
| Probe 5′-FAM-TCCACGTGTTGAGATCA-3′-MGB | |
| MIP-1α | Forward primer 5′-CTGCATCACTTGCTGCTGACA-3′ |
| Reverse primer 5′-CACTGGCTGCTCGTCTCAAAG-3′ | |
| Probe 5′-FAM-TTCAGCTACACCTCCCGGCAGATTCC-3′-TAMRA | |
| MIP-1β | Forward primer 5′-AAAACCTCTTTGCCACCAATACC-3′ |
| Reverse primer 5′-GAGAGCAGAAGGCAGCTACTAG-3′ | |
| Probe 5′-FAM-TGAAGCTCTGCGTGACTGTCCTGTCT-3′-TAMRA | |
| GAPDH | Forward primer 5′-CAAGCAGAAGTGGGTTCAGGAT-3′ |
| Reverse primer 5′-AGTGAGTGTTCAAGTCTTCGGAGTT-3′ | |
| Probe 5′-FAM-CAAGCTTCCCGTTCTCAGCC-3′-TAMRA |
Enzyme-linked immunosorbent assay (ELISA)
After treatment with carbocisteine, interleukin-6 (IL-6) and interleukin-8 (IL-8) in the cell culture supernatant were measured by ELISA according to the manufacturer's instructions.
Western blotting
Cellular proteins of A549 cells were extracted from treated A549 cells. An aliquot of proteins was electrophoresed on SDS-PAGE gel and transferred to polyvinylidene difluoride membranes. Membranes were blocked and subsequently incubated with antibodies against pNF-κB p65 (Ser536), total NF-κB p65, pIκB-α (S32), total IκB-α, pERK1/2 (T202/Y204), total ERK1/2 and GAPDH (1:1000). Following washing, the bound antibody was detected using horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000), and the bound complexes were detected using electrochemiluminescence (ECL). The relative intensities of bands were analyzed using an image analysis software program (Image J 1.44p, Wayne Rasband, National Institutes of Health, USA).
Immunofluorescence
Following treatment with carbocisteine alone, TNF-α alone, or carbocisteine plus TNF-α, A549 cells were washed with PBS, fixed with 4% paraformaldehyde solution for 30 min, permeabilized with 0.5% Triton X-100 for 15 min and blocked with 5% BSA for 20 min. This was followed by cell incubation with a rabbit monoclonal antibody against pNF-κB p65 (1:100) overnight at 4 °C. After conjugation with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:150) and 4′,6-diamidino-2-phenylindole (DAPI) (10 μmol/L), fluorescence was visualized with a Zeiss Axiovert 135 fluorescence microscope (Jena, Germany).
Luciferase assay of NF-κB in HEK-293 cells
HEK293 cells, which were stably co-transfected with a NF-kB-responsive luciferase reporter and constitutively expressed GFP as an internal control, were grown in 96-well plates and treated with carbocisteine (24 h) followed by TNF-α (10 ng/mL, 30 min). Luciferase activity was determined using the Luciferase Assay System according to the manufacturer's instructions and normalized against the levels of GFP expression21.
Statistical analysis
Continuous data were expressed as the means±standard deviation from the indicated number of studies (n) and compared by using Student's t-test or one-way ANOVA analysis if appropriate. Comparisons of data with P<0.05 were considered statistically significant.
Results
Carbocisteine ameliorated TNF-α-induced IL-6, IL-8, TNF-α, MCP-1 and MIP-1β expression in A549 cells
There is a wide array of cytokines and chemokines that have been implicated in various facets of the pathogenesis of COPD. IL-6 contributes to the pathogenesis of the autoimmune response observed in the lungs of the patients with more severe COPD; IL-8 plays a major role in neutrophil chemotaxis caused by alveolar macrophage-derived conditioned media of COPD; TNF-α is an important chemotactic protein for neutrophils22; IP-10 is a chemokine strongly associated with viral infection of COPD23; MCP-1 is involved in the migration of macrophages into the small airways, thus contributing to the inflammatory load associated with COPD24; neutrophil-derived MCP-1, MIP-1α, and MIP-1β are involved in macrophage recruitment into inflamed tissue during COPD25. MIP-1β contributes substantially to the development of lung inflammation following exposure to tobacco smoke26. The levels of IL-6, IL-8, TNF-α, IP-10, MCP-1, MIP-1α and MIP-1β were increased in the sputum and/or serum of patients with stable COPD or during exacerbation compared with controls23,24,25. Therefore, these cytokines are cardinal markers of airway inflammation in COPD patients.
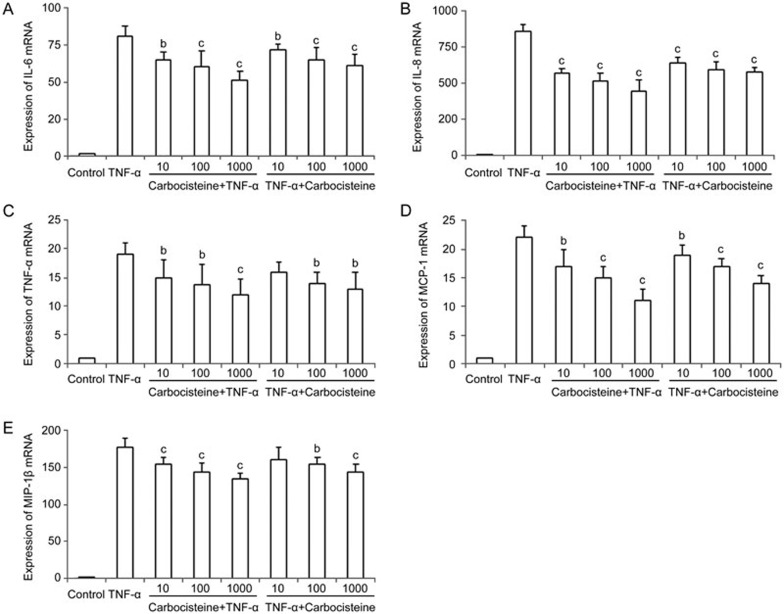
To investigate the protective effect of carbocisteine against TNF-α-induced inflammatory responses, we compared the relative abundance of all of these cytokines by using qRT-PCR. Treatment with TNF-α markedly up-regulated IL-6, IL-8, TNF-α, IP-10, MCP-1, MIP-1α and MIP-1β mRNA expression in A549 cells, whereas treatment with carbocisteine (before or after TNF-α stimulation) led to decreased IL-6, IL-8, TNF-α, MCP-1 and MIP-1β mRNA expression in a dose-dependent manner (Figure 1). However, IP-10 and MIP-1α expression levels remained relatively unchanged (data not shown).
Figure 1.
Effects of carbocisteine on the TNF-α-induced expression of the IL-6, IL-8, TNF-α, MCP-1 and MIP-1β genes in A549 cells. Control group: same amount of vehicle (PBS); TNF-α group: 10 ng/mL; Carbocisteine+TNF-α group: pre-treated with 10, 100, and 1000 μmol/L carbocisteine for 24 h, then exposure to 10 ng/mL TNF-α for 24 h; TNF-α+Carbocisteine group: pre-treated with 10 ng/mL TNF-α for 24 h, then exposure to 10, 100, and 1000 μmol/L carbocisteine for 24 h. (A) IL-6, (B) IL-8, (C) TNF-α, (D) MCP-1, (E) MIP-1β. Mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α group.
Carbocisteine attenuated TNF-α-induced IL-6 and IL-8 production in A549 cells
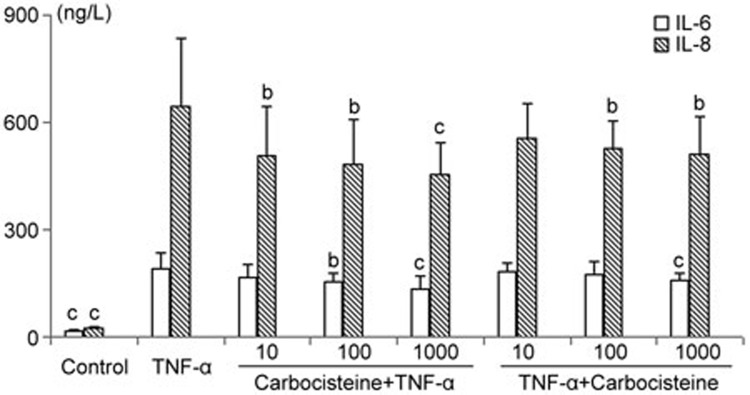
Next, we quantified IL-6 and IL-8 release from A549 cells using ELISA assay. As a potent pleiotropic proinflammatory cytokine, TNF-α elicited a pronounced increase in the release of IL-6 and IL-8 in A549 cells, which could be markedly inhibited by carbocisteine (before or after TNF-α stimulation) in a dose-dependent manner (particularly for IL-8) (Figure 2).
Figure 2.
Effects of carbocisteine on TNF-α-induced IL-6 and IL-8 release in A549 cells. Control group: same amount of vehicle (PBS); TNF-α group: 10 ng/mL; Carbocisteine+TNF-α group: pre-treated with 10, 100, or 1000 μmol/L carbocisteine for 24 h, then exposure to 10 ng/mL TNF-α for 24 h; TNF-α+Carbocisteine group: pre-treated with 10 ng/mL TNF-α for 24 h, then exposure to 10, 100, or 1000 μmol/L carbocisteine for 24 h. Mean±SD. n=6. *P<0.05, **P<0.01 compared with the TNF-α group.
Carbocisteine inhibited TNF-α-induced NF-κB activation and IκB-α degradation in A549 cells
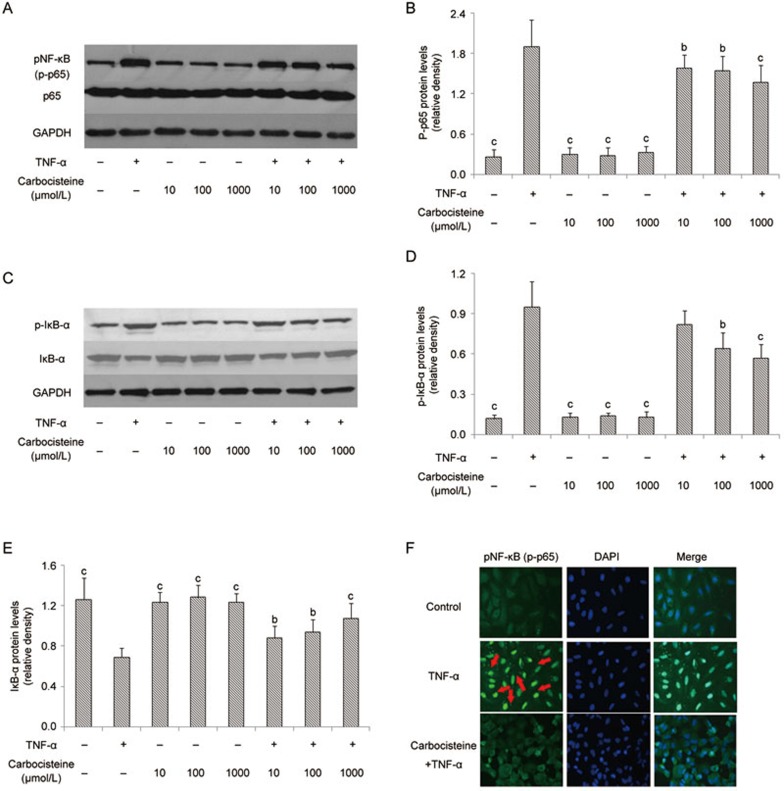
Carbocisteine reportedly inhibited type A seasonal influenza virus-induced NF-κB activation in human tracheal epithelial cells27. To examine whether this inhibitory effect of carbocisteine also applied to A549 cells, we pre-treated A549 cells with three doses of carbocisteine (10, 100 and 1000 μmol/L) for 24 h before exposure to TNF-α. Western blotting revealed that TNF-α significantly promoted NF-κB p65 and IκB-α phosphorylation (Figure 3A–3D) accompanied by IκB-α degradation (Figure 3C, Figure 3E), which was in accordance with the translocation of the NF-κB p65 subunit from the cytosol to the nucleus (Figure 3F). Pre-treatment with carbocisteine significantly reduced the levels of phosphorylated NF-κB, nuclear NF-κB protein and IκB-α degradation, whilst the carbocisteine alone showed no effects on the phosphorylation of NF-κB or IκB-α degradation (Figure 3).
Figure 3.
Carbocisteine inhibited TNF-α-induced NF-κB activation, including p65, IκB-α phosphorylation and IκB-α degradation, in A549 cells. (A) Representative Western blots of p-p65, total p65 and GAPDH. (B) Histograms represented means and SD of the ratio of p-p65 protein bands normalized to the GAPDH protein. (C) Representative Western blots of p-IκB-α, total IκB-α and GAPDH. (D) Histograms represent means and SD of the ratio of p-IκB-α protein bands normalized to the GAPDH protein. (E) Histograms represent means and SD of the ratio of IκB-α protein bands normalized to the GAPDH protein. (F) Immunofluorescence analysis was performed for the identification of p65 translocation (red arrows show the translocation of the NF-κB p65 subunit from the cytosol to the nucleus). Mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α group.
Carbocisteine inhibited TNF-α-induced NF-κB activation in HEK 293 cells
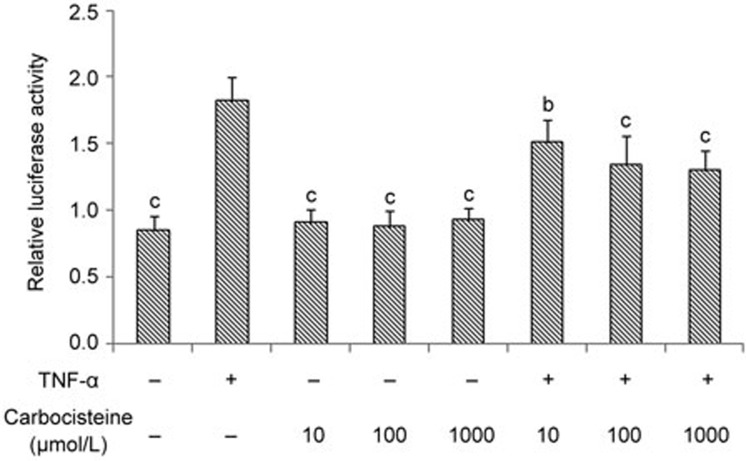
As a potent activator, TNF-α could directly activate NF-κB signaling pathways in many cells, including HEK 293 cells28. Consequently, we determined whether TNF-α-induced NF-κB activation could also be inhibited by carbocisteine in HEK 293 cells. As shown in a luciferase reporter assay, carbocisteine alone had no effects on the phosphorylation of NF-κB, and the TNF-α-induced activation of NF-κB was dose-dependently ameliorated by pre-treatment with carbocisteine (Figure 4). These findings were consistent with those of the A549 cell line.
Figure 4.
Carbocisteine attenuated TNF-α-induced NF-κB activation in HEK293 cells. Mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α group.
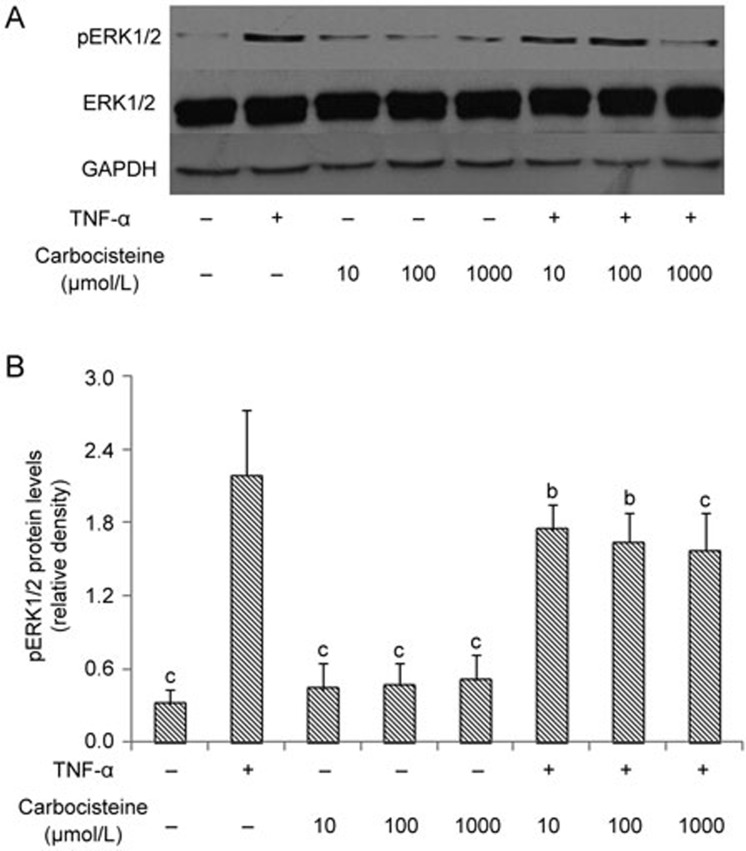
Carbocisteine inhibited TNF-α-induced ERK1/2 MAPK activation in A549 cells
It has been reported that TNF-α could activate the ERK1/2 MAPK signaling pathway in A549 cells29. As demonstrated in Figure 5, ERK1/2 MAPK phosphorylation was observed at 30 min in response to TNF-α induction. Pre-treatment with carbocisteine significantly abolished ERK1/2 MAPK activation in a dose-dependent fashion in A549 cells compared with TNF-α alone.
Figure 5.
Carbocisteine inhibited TNF-α-induced ERK1/2 activation in A549 cells. (A) Representative Western blots of pERK1/2, total ERK1/2 and GAPDH. (B) Histograms represent the means and SD of the ratio of pERK1/2 protein bands normalized to the GAPDH protein. Mean±SD. n=3. *P<0.05, **P<0.01 compared with the TNF-α group.
Discussion
Pro-inflammatory cytokines (ie, TNF-α) play pivotal roles in triggering and amplifying the inflammatory response leading to lung tissue injury in COPD. In this study, we investigated the anti-inflammatory effects of carbocisteine using TNF-α-induced A549 cell and HEK 293 cell models. Carbocisteine significantly reduced IL-6 and IL-8 production, and attenuated IL-6, IL-8, TNF-α, IP-10 and MIP-1β mRNA expression, which was accompanied by suppressed NF-κB and ERK1/2 MAPK phosphorylation in TNF-α-stimulated A549 cells. In addition, by using an NF-κB luciferase reporter system consisting of the stably transfected cell line HEK293/NF-κB-luc, we also found that carbocisteine inhibited the TNF-α-induced transcriptional activity of NF-κB.
TNF-α aggravates airway inflammation via the activation of inflammatory signaling pathways, such as NF-κB and MAPKs. It has been demonstrated that various pathogens and cytokines activate NF-κB signaling, and multiple inflammatory cytokines and chemokines are target genes of NF-κB30. MAPKs are evolutionarily conserved mediators in signaling pathways, which modulate embryogenesis, gene expression and cell functions. MAPKs also mediate pro-inflammatory gene transcription via phosphorylation31. TNF-α plays pivotal role in triggering down-stream airway inflammation in many respiratory diseases, including COPD; therefore, the attenuation of TNF-α-mediated inflammatory responses might achieve considerable therapeutic outcomes.
Apart from the anti-inflammatory capacity reported previously10, carbocisteine could suppress IL-6 and IL-8 release and inhibit IL-6, IL-8, TNF-α, IP-10 and MIP-1β mRNA expression after TNF-α stimulation. Our findings were consistent with a previous report which documented that carbocisteine reduced the levels of IL-1β, IL-6, and IL-8 at 5 days following FluA virus infection and diminished baseline concentrations of these cytokines prior to infection in human airway epithelial cells27. Similar findings have also been reported in human tracheal epithelial cells following rhinovirus or respiratory syncytial virus infection7,8. Furthermore, Nogawa et al showed that carbocisteine inhibited IL-1β-induced IL-6 and IL-8 release in NCI-292 cells, as did N-acetylcysteine (NAC)4. These findings further supported the cytoprotective effects of carbocisteine against airway inflammation in COPD. Because the exacerbation of COPD has been associated with a substantial release of pro-inflammatory cytokines and chemokines32,33, the inhibitory effects of carbocisteine, as confirmed by previous studies and our team, may lend support to interpreting the reduced frequency of exacerbations in COPD.
We evaluated the roles of the NF-κB and MAPK signaling pathways to explore the mechanisms by which carbocisteine modulates TNF-α-induced inflammation. Our results showed that the inhibitory effects of carbocisteine were mainly attributed to the inactivation of NF-κB (by inhibiting NF-κB translocation from cytoplasm to nucleus and attenuating NF-κB phosphorylation) and ERK1/2 MAPK (by attenuating ERK1/2 phosphorylation) following TNF-α stimulation. This was in line with the findings of Yamaya and colleagues, who also reported that carbocisteine inhibited FluA virus-induced inflammation partially by decreasing the activation of NF-κB DNA-binding activity27. In a pilot study that investigated the effects of carbocisteine on TNF-α-induced phosphorylation of p38 MAPK and JNK in A549 cells, we showed that carbocisteine conferred no effects on these signaling pathways. Furthermore, we also noted a dose-response inhibitory effect of carbocisteine on airway inflammation in this study. This suggested that greater doses of carbocisteine or other thiol-containing mucolytics would be needed to achieve better therapeutic outcomes, such as a reduction in the future risk of COPD exacerbation. This was consistent with the findings of the PANTHEON34 and HIACE35 studies, which utilized high-dose drugs, whilst administration of the relatively low-dose N-acetylcysteine failed to produce a favorable response36. Further studies on different cell lines are needed to determine if longer durations of treatment with carbocisteine would lead to more favorable protective effects on airway inflammation and oxidative stress.
There are limitations that should be addressed. It has also been reported that carbocisteine harbors other anti-inflammatory targets, such as nuclear factor-erythroid 2-related factor (Nrf2), which is a redox-sensitive transcription factor in COPD37,38 that can be activated by TNF-α in certain cell lines39. Via the activation of Nrf2, carbocisteine attenuated inflammation in vivo after exposure to cigarette smoke following influenza virus infection40 and alleviated cigarette smoke extract (CSE)-induced oxidative stress in vitro5. It remains to be further revealed whether Nrf2 is involved in the regulation of TNF-α-stimulated inflammation by carbocisteine. Our principal findings have not been replicated in animal (in vivo) studies. Furthermore, we could not verify the effects of prolonged use of carbocisteine on reducing COPD exacerbation because there has not been consensus on the duration of administration for in vivo studies. Further studies are warranted to confirm whether carbocisteine would antagonize inflammatory mediators other than TNF-α, the principal biomarker contributing to the amplification of airway inflammation.
In conclusion, we have demonstrated that carbocisteine was effective in suppressing inflammation in human alveolar epithelial cells upon stimulation with TNF-α in vitro. These effects are intimately associated with the inhibition of the NF-κB and ERK1/2 MAPK signaling pathways, suggesting that carbocisteine harbors considerable therapeutic potential for the prevention and management of airway inflammation in COPD.
Author contribution
Wei WANG and Jin-ping ZHENG designed research; Wei WANG, Wei-jie GUAN, Jin-ping ZHENG, Shao-xuan ZHU, Mao CHEN, and Nan-shan ZHONG contributed to study conception; Wei WANG, Rong-quan HUANG, and Yan-qing XIE performed research; Wei WANG performed statistical analyses; Wei WANG and Wei-jie GUAN drafted the manuscript.
Acknowledgments
We thank Dr Chu-fang LI (State Key Laboratory of Respiratory Disease, Guangzhou, China) for donating HEK293/NF-κB-luc cells.
This study was supported by the grants from the Guangdong Provincial Project for Great New Drug Research and Development (No 2012A080201003), the National Key Technology R&D Program (No 2012BAI05B01, 2013BAI09B09), and the National Natural Science Foundation of China (No 81470234).
References
- Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Tomita N, et al. Carbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2006; 54: 378–80. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Fukuchi Y. Carbocisteine improves quality of life in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2007; 55: 1884–6. [DOI] [PubMed] [Google Scholar]
- Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet 2008; 371: 2013–8. [DOI] [PubMed] [Google Scholar]
- Nogawa H, Ishibashi Y, Ogawa A, Masuda K, Tsubuki T, Kameda T, et al. Carbocisteine can scavenge reactive oxygen species in vitro. Respirology 2009; 14: 53–9. [DOI] [PubMed] [Google Scholar]
- Pace E, Ferraro M, Di Vincenzo S, Cipollina C, Gerbino S, Cigna D, et al. Comparative cytoprotective effects of carbocysteine and fluticasone propionate in cigarette smoke extract-stimulated bronchial epithelial cells. Cell Stress Chaperones 2013; 18: 733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Nakayama K, Yasuda H, Kubo H, Kuwano K, Arai H, et al. Carbocisteine inhibits oxidant-induced apoptosis in cultured human airway epithelial cells. Respirology 2009; 14: 1027–34. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Yamada M, et al. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. Eur Respir J 2006; 28: 51–8. [DOI] [PubMed] [Google Scholar]
- Asada M, Yoshida M, Hatachi Y, Sasaki T, Yasuda H, Deng X, et al. l-carbocisteine inhibits respiratory syncytial virus infection in human tracheal epithelial cells. Respir Physiol Neurobiol 2012; 180: 112–8. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol 2012; 12: 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zheng JP, Zhu SX, Guan WJ, Chen M, Zhong NS. Carbocisteine attenuates hydrogen peroxide-induced inflammatory injury in A549 cells via NF-kappaB and ERK1/2 MAPK pathways. Int Immunopharmacol 2015; 24: 306–13. [DOI] [PubMed] [Google Scholar]
- Vitacca M. Exacerbations of COPD: predictive factors, treatment and outcome. Monaldi Arch Chest Dis 2001; 56: 137–43. [PubMed] [Google Scholar]
- Wedzicha JA. Exacerbations: etiology and pathophysiologic mechanisms. Chest 2002; 121: 136S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer DM, Kidney JC, Ennis M, Elborn JS. Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers. Eur Respir J 2013; 41: 1058–67. [DOI] [PubMed] [Google Scholar]
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994; 150: 1453–5. [DOI] [PubMed] [Google Scholar]
- Antoniu SA, Mihaltan F, Ulmeanu R. Anti-TNF-alpha therapies in chronic obstructive pulmonary diseases. Expert Opin Investig Drugs 2008; 17: 1203–11. [DOI] [PubMed] [Google Scholar]
- Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1179–84. [DOI] [PubMed] [Google Scholar]
- Knobloch J, Lin Y, Konradi J, Jungck D, Behr J, Strauch J, et al. Inflammatory responses of airway smooth muscle cells and effects of endothelin receptor antagonism. Am J Respir Cell Mol Biol 2013; 49: 114–27. [DOI] [PubMed] [Google Scholar]
- Chunlian W, Heyong W, Jia X, Jie H, Xi C, Gentao L. Magnolol inhibits tumor necrosis factor-alpha-induced ICAM-1 expression via suppressing NF-kappaB and MAPK signaling pathways in human lung epithelial cells. Inflammation 2014; 37: 1957–67. [DOI] [PubMed] [Google Scholar]
- Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2004; 286: L741–9. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–8. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Finkelstein D, Li X, Wu D, Shi DL, Zheng JJ. Identification of transmembrane protein 88 (TMEM88) as a dishevelled-binding protein. J Biol Chem 2010; 285: 41549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 2014; 9: 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA, et al. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest 2010; 137: 812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Di Stefano A, Casolari P, Kirkham PA, Padovani A, Chung KF, et al. Chemokines and chemokine receptors blockers as new drugs for the treatment of chronic obstructive pulmonary disease. Curr Med Chem 2013; 20: 4317–49. [DOI] [PubMed] [Google Scholar]
- Blidberg K, Palmberg L, Dahlen B, Lantz AS, Larsson K. Chemokine release by neutrophils in chronic obstructive pulmonary disease. Innate Immun 2012; 18: 503–10. [DOI] [PubMed] [Google Scholar]
- Ma B, Kang MJ, Lee CG, Chapoval S, Liu W, Chen Q, et al. Role of CCR5 in IFN-gamma-induced and cigarette smoke-induced emphysema. J Clin Invest 2005; 115: 3460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M, Nishimura H, Shinya K, Hatachi Y, Sasaki T, Yasuda H, et al. Inhibitory effects of carbocisteine on type A seasonal influenza virus infection in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2010; 299: L160–8. [DOI] [PubMed] [Google Scholar]
- Roussel RR, Barchowsky A. Arsenic inhibits NF-kappaB-mediated gene transcription by blocking IkappaB kinase activity and IkappaBalpha phosphorylation and degradation. Arch Biochem Biophys 2000; 377: 204–12. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Devillier P, Xu GN, Qi H, Zhu L, Zhou W, et al. TNF-alpha-induced CXCL8 production by A549 cells: involvement of the non-neuronal cholinergic system. Pharmacol Res 2013; 68: 16–23. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18: 6853–66. [DOI] [PubMed] [Google Scholar]
- Guan KL. The mitogen activated protein kinase signal transduction pathway: from the cell surface to the nucleus. Cell Signal 1994; 6: 581–9. [DOI] [PubMed] [Google Scholar]
- Pinto-Plata VM, Livnat G, Girish M, Cabral H, Masdin P, Linacre P, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest 2007; 131: 37–43. [DOI] [PubMed] [Google Scholar]
- Kersul AL, Iglesias A, Rios A, Noguera A, Forteza A, Serra E, et al. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 2011; 47: 176–83. [DOI] [PubMed] [Google Scholar]
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2: 187–94. [DOI] [PubMed] [Google Scholar]
- Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144: 106–18. [DOI] [PubMed] [Google Scholar]
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365: 1552–60. [DOI] [PubMed] [Google Scholar]
- Biswal S, Thimmulappa RK, Harvey CJ. Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proc Am Thorac Soc 2012; 9: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med 2011; 17: 363–71. [DOI] [PubMed] [Google Scholar]
- Zhang HS, Wang SQ. Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem Pharmacol 2007; 73: 1358–66. [DOI] [PubMed] [Google Scholar]
- Yageta Y, Ishii Y, Morishima Y, Ano S, Ohtsuka S, Matsuyama M, et al. Carbocisteine reduces virus-induced pulmonary inflammation in mice exposed to cigarette smoke. Am J Respir Cell Mol Biol 2014; 50: 963–73. [DOI] [PubMed] [Google Scholar]