Abstract
Aim:
To investigate the effects of ROS scavenger N-acetylcysteine (NAC) on angiotensin II (Ang II)-mediated renal fibrosis in vivo and in vitro.
Methods:
Mice were subjected to unilateral ureteral obstruction (UUO), and then treated with vehicle or NAC (250 mg/kg, ip) for 7 days. Histological changes of the obstructed kidneys were observed with Masson's trichrome staining. ROS levels were detected with DHE staining. The expression of relevant proteins in the obstructed kidneys was assessed using Western blotting assays. Cultured rat renal fibroblast NRK-49F cells were used for in vitro experiments.
Results:
In the obstructed kidneys, Ang II levels were significantly elevated, and collagen I was accumulated in the interstitial spaces. Furthermore, ROS production and the expression of p47 (a key subunit of NADPH oxidase complexes) were increased in a time-dependent manner; the expression of fibronectin, α-SMA and TGF-β were upregulated. Administration of NAC significantly alleviated the fibrotic responses in the obstructed kidneys. In cultured NRK-49F cells, treatment with Ang II (0.001–10 μmol/L) increased the expression of fibronectin, collagen I, α-SMA and TGF-β in dose-dependent and time-dependent manners. Ang II also increased ROS production and the phosphorylation of Smad3. Pretreatment with NAC (5 μmol/L) blocked Ang II-induced oxidative stress and ECM production in the cells.
Conclusion:
In mouse obstructed kidneys, the fibrotic responses result from Ang II upregulation can be alleviated by the ROS scavenger N-acetylcysteine.
Keywords: chronic kidney disease, renal fibrosis, unilateral ureteral obstruction, angiotensin II, ROS, oxidative stress, N-acetylcysteine, rat renal fibroblast NRK-49F cells
Introduction
Renal fibrosis is the final common pathway of chronic kidney disease (CKD). It is characterized by the accumulation of extracellular matrix (ECM) proteins in both the glomeruli and tubulointerstitial spaces. Renal fibrosis is an important predictor of clinical prognosis and a determinant of renal insufficiency1. Renal fibrosis can be initiated by many pathological factors including toxicity, ischemia, pathogenic microorganism infections, paraneoplastic syndromes, congenital abnormalities, inherited defects, and endocrine and immunological diseases2. Eventually, progression of renal fibrosis leads to end-stage renal failure, and patients require lifelong dialysis or kidney transplantation. Although CKD is widespread and the incidence is increasing, no effective medication is currently available to manage the progression of renal fibrosis. Understanding the molecular and cellular mechanisms is necessary for identifying and developing an effective therapy for renal fibrosis.
Many studies have shown that overactivation of the intrarenal renin-angiotensin system (RAS) plays an essential role in the pathogenesis of CKD3,4,5. Overactivation of RAS stimulates the proliferation of renal mesangial cells and interstitial fibroblasts, increases the synthesis and secretion of inflammatory factors by renal inherent cells, and promotes the morphological and functional remodeling of cells6,7,8,9,10,11. Because angiotensin II (Ang II) is the major effector in RAS, angiotensin-converting enzyme inhibitors (ACEI) and Ang II type I receptor blockers (ARB) are currently recommended as the first-line medications for the treatment of CKD.
Reactive oxygen species (ROS) include the superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·). ROS were once recognized as endogenous pathogenic molecules in cellular injury due to the damage they inflict on lipids, proteins, and DNA in cells12,13. However, ROS have now been widely accepted as important signaling molecules that play multiple roles in regulating normal cellular functions14,15. Only under pathological conditions, for various reasons, are the formation and elimination of ROS disturbed and excessive ROS generated; the elevated ROS levels induce a condition called oxidative stress and inflict cell and tissue damage.
Oxidative stress is associated with multiple factors that induce the development of CKD16,17,18,19, and eliminating oxidative stress is an alternative strategy for the treatment of CKD. In this study, we aimed to clarify the relationship between renal RAS activation and oxidative stress, and we investigated the effect of ROS elimination on RAS activation-induced renal fibrosis in the unilateral ureteral obstructive (UUO) model and on cultured renal fibroblasts.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), recombinant human angiotensin II, N-acetylcysteine (NAC), and dihydroethidium (DHE) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Bovine calf serum (BCS) was obtained from Gibco (Grand Island, NY, USA). The BCA Protein Assay Kit was obtained from Shenergy Biocolor BioScience and Technology (Shanghai, China). Anti-fibronectin antibody was obtained from Sigma-Aldrich (Saint Louis, MO, USA). Anti-collagen I antibody was obtained from Abcam (Cambridge, MA, USA), and anti-p47 antibody was obtained from Santa Cruz Biotechnologies, Inc (Santa Cruz, CA, USA). Anti-GAPDH antibody, an enhanced chemiluminescence (ECL) detection kit and hydrogen peroxide assay kits were purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Ang II ELISA kit was obtained from Westang Biotech (Shanghai, China). Polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Billerica, MA, USA). Proteinase inhibitor and phosphatase inhibitor were obtained from Roche (Mannheim, Germany). All other chemicals and reagents used were of analytical grade.
UUO animal model
Male C57BL/6J mice (20–25 g) were purchased from Shanghai SLAC Laboratory Animal Co Ltd (Shanghai, China). All animal experiments were performed according to the Criteria of the Medical Laboratory Animal Administrative Committee of Shanghai and the Guide for Care and Use of Laboratory Animals of Fudan University, and the protocols were approved by the Ethics Committee for Experimental Research, Shanghai Medical College, Fudan University. The mice were anesthetized with 10% chloral hydrate via intraperitoneal injection. The left ureter was visualized by a flank incision and ligated with 4–0 silk. The sham group underwent the same surgery except for left ureter ligation. Over the course of the study, six mice were included in each group and were euthanized 1, 3, or 7 days after surgery. Mice that underwent the UUO surgery were randomly divided into two groups: UUO with vehicle (0.9% saline) and UUO with NAC (250 mg/kg body weight) administered by intraperitoneal injection at 0.1 mL per day for 7 days. The kidneys were then harvested, quick-frozen by liquid nitrogen, and stored at −80°C until use.
Cell culture
Normal rat kidney fibroblasts (NRK-49F) were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). Cells were cultured in DMEM containing 10% BCS, in 5% CO2–95% air at 37 °C. Cells were deprived of serum for 16 h when they reached approximately 80% confluence. Cells were pretreated with either 5 μmol/L NAC or vehicle for 30 min followed by the administration of 1×10−6 mol/L Ang II.
Western blotting
Protein was isolated from the homogenized frozen kidneys or cell lysate homogenates as previously described20. Protein concentrations were determined using a BCA Protein Assay Kit according to the manufacturer's instructions. An equal amount (30 μg per sample) of total protein was loaded and separated by electrophoresis on a 10% SDS-PAGE gel and then transferred onto a PVDF membrane. The membrane was then blocked with 5% skim milk for 1 h at room temperature with gentle shaking. The membranes were then incubated with primary antibody overnight at 4 °C [anti-fibronectin antibody, 1:10000; anti-collagen I antibody, 1:500; anti-α-smooth muscle actin (anti-α-SMA) antibody, 1:3000 anti-phospho-smad3 antibody, 1:1000; anti-smad3 antibody, 1:1000; anti-p47 phox antibody, 1:500; and anti-GAPDH antibody, 1:3000]. The membranes were then washed 3 times with Tris-buffered saline with 0.1% Tween and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After another 3 washes with TBS/Tween, the hybridizing bands were developed using the ECL detection kit according to the manufacturer's instructions. Protein bands were visualized by GE chemiluminescence system for the required time. Bands were normalized using GAPDH or smad3.
Measurement of kidney content of Ang II
The concentration of Ang II was measured using commercially available enzyme immunoassay kits (Westang Biotech, Shanghai, China). The procedures were performed according to the manufacturer's instructions. The Ang II concentration was calculated from a standard curve constructed with standard solutions.
Histological examination
Kidneys were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were collected and prepared accordingly. Slides of paraffinized tissue sections were deparaffinized and then rehydrated. Sections were stained with Masson's trichrome. Histological changes were observed at 400× optical magnification.
DHE staining
The production of superoxide in oxidative stress was measured in the frozen kidneys using the oxidative fluorescent dye DHE. DHE (2 μmol/L) was applied to 10 μm kidney sections, and the slides were then incubated in a light-protected humidified chamber at 37 °C for 30 min. In the presence of superoxide, DHE is oxidized to fluorescent ethidium, which is trapped by intercalation with DNA. Ethidium is excited at 518 nm with an emission wavelength of 605 nm. The intensity of fluorescence was observed at ×400 optical magnification.
Hydrogen peroxide assay
The reactions were performed with a hydrogen peroxide assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). In this assay, Fe2+ is oxidized to Fe3+ by H2O2. The Fe3+ then forms a purple complex with xylenol orange (3,3′-bis [N, N-di(carboxymethyl)-aminomethyl]-o-cresolsulfone-phthalein, disodium salt and xylenol orange), which is measurable with a microplate reader at a wavelength of 560 nm21. The level of H2O2 in the kidney tissues was calculated according to the manufacturer's instructions.
Statistical analyses
Data are presented as the mean±SEM with statistical analysis performed using one-way analysis of variance with post hoc analysis using Tukey's multiple comparison test. The differences between the two groups were compared using Student's t test. P<0.05 was considered to indicate a statistically significant difference between mean values.
Results
Renin-angiotensin system was activated in UUO mice
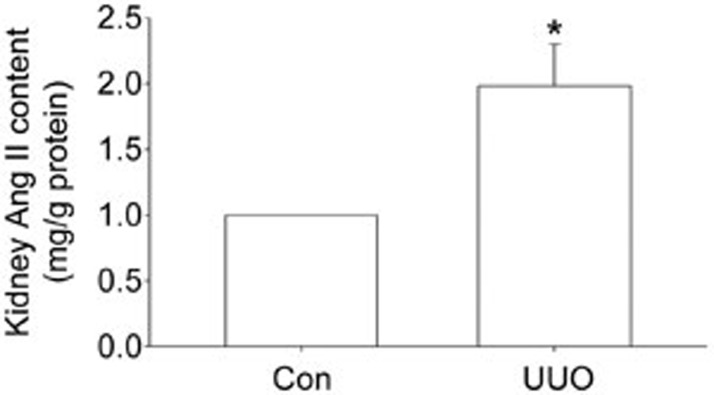
The ELISA result showed that the level of Ang II was elevated in obstructed kidney tissues, indicating the activation of RAS (Figure 1).
Figure 1.
Unilateral ureteral obstruction (UUO)-induced activation of the renin-angiotensin system in the kidney. The level of Ang II was detected by ELISA kit. Data are the mean±SEM of 6 animals. *P<0.05 compared with Con.
Oxidative stress was induced in the UUO model
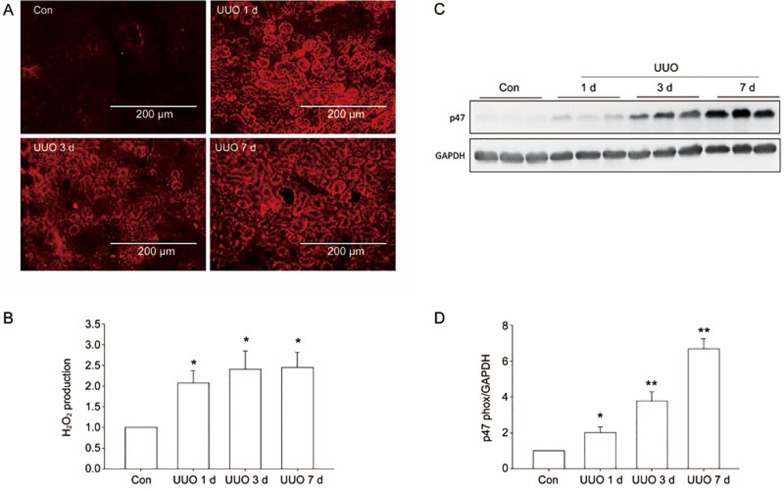
The DHE staining results indicated that ROS increased 1 day after the UUO surgery and persisted to the end of the experiment (7 days) (Figure 2A). The level of H2O2 increased significantly in the kidney tissues (Figure 2B). Western blot analysis showed that the protein level of p47 phox, a key subunit of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complexes, increased significantly in the UUO model (Figure 2C–D). These results indicated that oxidative stress was induced in the obstructed kidneys.
Figure 2.
Unilateral ureteral obstruction (UUO)-induced oxidative stress in the kidney. (A) The level of ROS was detected by DHE staining after UUO for 1, 3, and 7 days. (B) The level of H2O2 in kidney tissue was analyzed by hydrogen peroxide assay. (C, D) Western blotting visualized p47 in a time course of UUO mice. Data are the mean±SEM of 6 animals. *P<0.05, **P<0.01 compared with Con.
An ROS scavenger attenuated fibrosis in obstructed kidneys
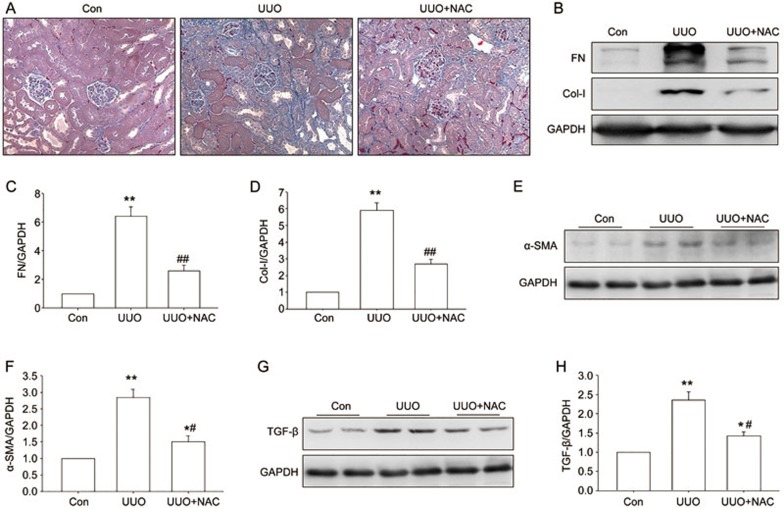
To determine whether ROS is involved in the progression of renal fibrosis, an ROS scavenger, NAC, was administered by intraperitoneal injection in the UUO mice. Masson trichrome staining indicated that NAC treatment significantly attenuated UUO-induced collagen deposition (Figure 3A). The Western blot results indicated that NAC also inhibited UUO-induced upregulation of fibronectin, collagen I, and α-SMA protein expression in the obstructed kidney (Figure 3B–F). The UUO-induced increase in TGF-β was also suppressed by NAC treatment (Figure 3G–H). These results indicate that ROS scavenger NAC did inhibit the production and accumulation of ECM protein in the obstructed kidneys; thus, ROS was involved in the progression of renal fibrosis.
Figure 3.
Effect of NAC on tubulointerstitial fibrosis in the UUO kidney. Control and UUO mice received vehicle or NAC (200 mg/kg body weight) by intraperitoneal injection at 0.1 mL per day for 7 days. (A) Representative sections of Masson's trichrome-stained kidneys. Collagen was stained blue. Original magnifications ×400. (B–F) Western blotting visualized fibronectin, collagen I and α-SMA. (G–H) TGF-β were assayed by Western blot analysis. Data are the mean±SEM of 6 animals. *P<0.05, **P<0.01 compared with Con. ##P<0.01 compared with UUO treated with vehicle.
Ang II induced profibrotic responses in NRK-49F cells
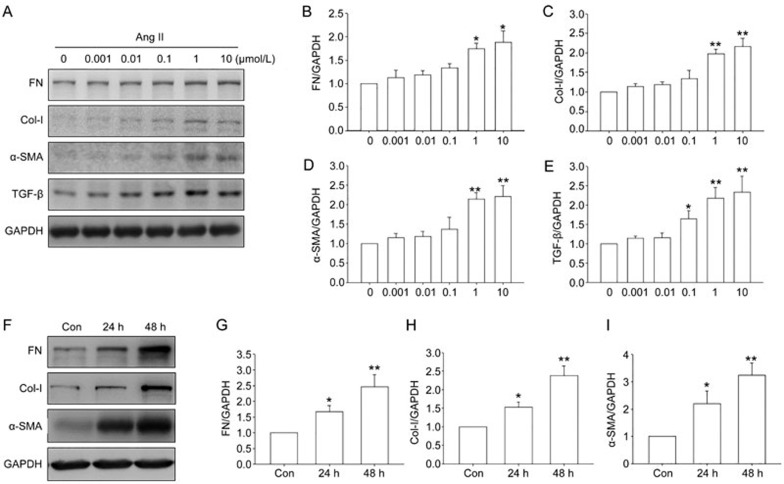
To determine whether elevated ROS was involved in Ang II-induced profibrotic effects, in vitro studies were performed in renal fibroblast cells. Western blot results showed that Ang II treatment increased fibronectin, collagen I, and α-SMA protein levels in NRK-49F cells in a dose-dependent and time-dependent manner (Figure 4), confirming the profibrotic effects of Ang II.
Figure 4.
Effect of angiotensin II (Ang II) on expression of profibrotic genes in NRK-49F cells. (A–E) Cells were treated with Ang II at the indicated concentrations for 48 h. Fibronectin, collagen I, α-smooth muscle actin (α-SMA), and TGF-β were analyzed by Western blot analysis. (F–I) Cells were stimulated with 1 μmol/L Ang II for 24 or 48 h. Fibronectin, collagen I, and α-SMA were assayed by Western blot analysis. Data are the mean±SEM of 3 experiments. *P<0.05, **P<0.01 compared with Con.
Ang II induced oxidative stress in NRK-49F cells
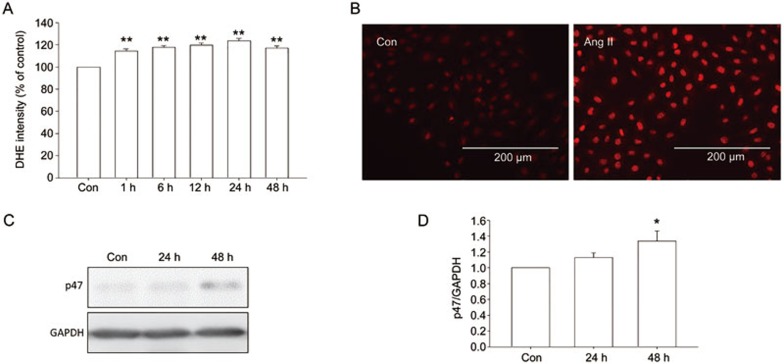
To determine whether Ang II treatment could induce the upregulation of ROS in NRK-49F, the level of ROS was assayed. The results showed that the increase in ROS levels was statistically significant beginning 1 h after Ang II treatment and persisted to the end of the experiment (Figure 5A). The DHE fluorescence result showed that Ang II treatment induced a significant increase in ROS production in NRK-49F cells (Figure 5B). Western blot results showed that p47 protein level increased after Ang II treatment (Figure 5C–D).
Figure 5.
Effect of Ang II on the level of ROS in NRK-49F cells. (A) Cells were treated with Ang II for indicated times. DHE assay was used to detect the level of ROS in NRK-49F cells. (B) Cells were treated with Ang II for 24 h. The level of ROS was visualized by DHE fluorescence images. (C, D) P47 was assayed by Western blot. Data are the mean±SEM of 3 experiments. *P<0.05, **P<0.01 compared with control.
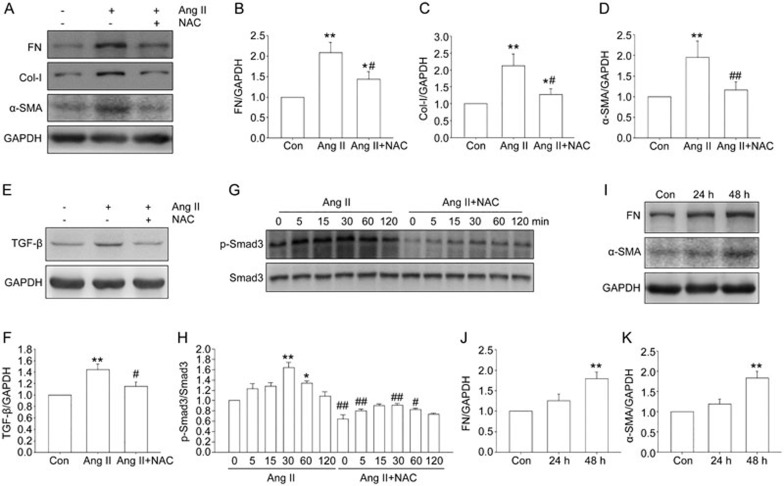
NAC inhibited Ang II-induced expression of profibrotic genes in NRK-49F cells
To determine whether ROS contributed to the profibrotic effects of Ang II, the ROS scavenger NAC was employed. Western blot analysis showed that pretreatment with NAC for 1 h abolished the Ang II-stimulated upregulation of fibronectin, collagen I, and α-SMA (Figure 6A–D). The upregulation of TGF-β induced by Ang II was inhibited by NAC (Figure 6E–F). Smad3 activation was also suppressed by NAC (Figure 6G–H), suggesting the inhibition of TGF-β signaling. Furthermore, H2O2 treatment was sufficient to induce the upregulation of fibronectin and α-SMA in NRK-49F cells (Figure 6I–K).
Figure 6.
Effect of NAC on Ang II-induced profibrotic responses in NRK-49F cells. Cells were treated with Ang II in the presence or absence of NAC. (A–F) The cells were pretreated without or with NAC for 1 h, then treated with Ang II for 48 h, fibronectin, collagen I, α-SMA and TGF-β were assayed by Western blot analysis. (G–H) After Ang II treatment for indicated time, with or without 1 h NAC (5 μmol/L) pretreatment, p-Smad3 was assayed by Western blot analysis. (I–K) After 48 h of H2O2 treatment, fibronectin and α-SMA were assayed by Western blot analysis. Data are the mean±SEM of 3 experiments. *P<0.05, **P<0.01 compared with control. #P<0.05, ##P<0.01 compared with Ang II only.
Discussion
Renal fibrosis is a major health problem affecting millions of people worldwide and is the final common pathway of CKD in the progression to end-stage renal disease. It can be considered a sustained scarring process in response to pathogenic factors, during which an excessive amount of ECM is deposited in interstitial spaces and causes irreversible tissue damage and impairment of renal function.
The overactivation of RAS is known to be involved in renal injury induced by multiple factors. Ang II, one of the major components of RAS, has emerged as an important profibrotic cytokine in renal fibrosis. Due to the specific role of RAS in renal fibrosis, ACEI inhibitors and ARB blockers have been recommended as first-line therapy in the treatment of CKD. However, the results of both clinical and laboratory studies have shown that these agents can only slow but not halt the progression of renal disease. In recent years, with the identification of the ACE-2/Ang (1–7)/Mas receptor pathway and the prorenin–renin/prorenin receptor, RAS has proved to be far more complex than originally recognized. The newer understanding of RAS explains why ARB or ACEI agents fail to halt the progression of CKD and also suggests that new targets and strategies are needed to improve treatment outcomes.
ROS are critical intermediates in both normal physiological and pathological conditions of kidney cells. Oxidative stress refers to the aberrantly high levels of intracellular ROS that cause damage to proteins, lipids, and DNA. Oxidative stress is the result of the disrupted balance between ROS generation and antioxidants and is linked to a variety of human diseases, including organ fibrosis in the liver and kidney. ROS act as mediators of Ang II-induced epithelial–mesenchymal transition (EMT) and apoptosis. The use of antioxidant therapy for the treatment of renal fibrosis has attracted wide interest in recent years22. Some reports have shown an interaction between ROS and RAS, but the relationship is still unclear.
Our results showed that interstitial fibrosis was induced in the UUO animals, and the concentration of Ang II in renal tissue was significantly elevated. These results suggested that activation of RAS was involved in the UUO-induced renal fibrosis. Both DHE staining and hydrogen peroxide analysis showed that ROS levels were increased in the obstructed kidney in UUO model mice; this finding indicated that oxidative stress was present in the UUO kidney. P47 phox, an organizer subunit for NADPH oxidase 1/2, was also elevated in the UUO kidney. To determine whether ROS was involved in the progression of renal fibrosis, the ROS scavenger NAC was administered to the UUO mice. NAC is a metabolite of the sulfur-containing amino acid cysteine and has been used in clinical practice for several decades23. Currently NAC is used as an antioxidant and a mucolytic agent. The therapeutic potential of NAC has been investigated for the treatment of numerous disorders such as ischemia–reperfusion cardiac injury, acute respiratory distress syndrome, chemotherapy-induced toxicity, radio-contrast-induced nephropathy, heavy metal toxicity, and psychiatric disorders24,25,26,27. Several studies have shown that NAC could be used for the treatment of pulmonary fibrosis, liver fibrosis, and skin fibrosis28,29,30,31.
In the present study, NAC inhibited the production of ECM proteins and the activation of TGF-β signaling pathway in the UUO kidneys. The histology results showed that NAC also ameliorated interstitial fibrosis, indicating that ROS elimination effectively slowed the progression of renal fibrosis in UUO model mice. These results suggest that ROS plays a crucial role in renal fibrosis.
Resident renal fibroblasts are the most important source of scar-producing myofibroblasts32. The activation and proliferation of resident fibroblasts in the kidney results in enhanced production and excessive deposition of ECM. To determine whether the elevation of ROS was involved in the Ang II-induced profibrotic effects, the renal fibroblast cells were used for in vitro studies. The results showed that the Ang II induced elevation in ROS in the cultured NRK-49F renal fibroblast cells, suggesting that ROS is a downstream molecule of RAS activation. Treatment with Ang II induced significant profibrotic effects including Smad3 signaling activation and excessive production of ECM proteins. The elimination of ROS by NAC treatment in vivo reversed the Ang II-induced renal fibroblast activation and ECM overproduction, suggesting that blocking ROS production is an effective means to attenuate the profibrotic effects of RAS activation.
In summary, Ang II stimulates ROS generation, and overproduction of ROS is indispensable for Ang II-induced fibrogenesis in the kidney. The newer understanding of RAS is much more complex than recognized previously; it is demonstrated that ACEI and ARB inhibitor therapy can only partially reverse the RAS activation-induced progression of CKD. If ROS acts downstream of Ang II to mediate the fibrotic effect, then inhibition of ROS production may be a feasible therapeutic strategy to address CKD-associated fibrosis. Whether ROS scavenging or ACEI/ARB inhibition is a more effective treatment strategy awaits extensive investigation in both laboratory and clinical studies. The efficacy of NAC treatment on patients with renal fibrosis has not been reported. Our study showed that NAC attenuated renal fibrosis effectively both in vitro and in vivo. Given its high safety, we propose that NAC could be used clinically to treat renal fibrosis.
Author contribution
Wei ZHANG and Li-min LU designed the research project; Yang SHEN and Nai-jun MIAO performed the experiments; Jin-lan XU and Xin-xin GAN analyzed the data; Dan XU contributed the reagents and materials; Yang SHEN, Li ZHOU and Hong XUE wrote the manuscript.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No 81470591, 81170636) to Li-min LU, and (No 81100531) to Wei ZHANG.
References
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011; 7: 684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JJ, Foley RN, Collins AJ. Prevalence of CKD in the United States: a sensitivity analysis using the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 2009; 53: 218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 2012; 318: 1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucero AC, Goncalves S, Benito-Martin A, Santamaria B, Ramos AM, Berzal S, et al. Obstructive renal injury: from fluid mechanics to molecular cell biology. Open Access J Urol 2010; 2: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos P, Krieger JE, Pereira AC. Renin angiotensin system, hypertension; and chronic kidney disease: pharmacogenetic implications. J Pharmacol Sci 2012; 120: 77–88. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Miyajima A, Kikuchi E, Kozakai N, Kosaka T, Ieda M, et al. Renal damage inhibited in mice lacking angiotensinogen gene subjected to unilateral ureteral obstruction. Urology 2009; 74: 938–43. [DOI] [PubMed] [Google Scholar]
- Jung GS, Jeon JH, Jung YA, Choi YK, Kim HS, Kim JG, et al. Clusterin/apolipoprotein J attenuates angiotensin II-induced renal fibrosis. PLoS One 2014; 9: e105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YF, Jin XG, Yan JY, Entman ML, Wang YL. CXCR6 plays a critical role in angiotensin II-induced renal injury and fibrosis. Arterioscler Thromb Vasc Biol 2014; 34: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, et al. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 2014; 86: 75–85. [DOI] [PubMed] [Google Scholar]
- Ruster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 2011; 22: 1189–99. [DOI] [PubMed] [Google Scholar]
- Kagami S. Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury. Clin Exp Nephrol 2012; 16: 214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, Del Razo LM, Quintanilla-Vega B, et al. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Sign 2014; 21: 66–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. Redox-mediated signal transduction by cardiovascular Nox NADPH oxidases. J Mol Cell Cardiol 2014; 73: 70–9. [DOI] [PubMed] [Google Scholar]
- Rhee SG. H2O2, a necessary evil for cell signaling. Science 2006; 312: 1882–3. [DOI] [PubMed] [Google Scholar]
- Okamura DM, Pennathur S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol 2015; 6: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, Nasrallah R, Touyz RM, Hebert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 2013; 24: 1512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdo S, Zhang SL, Chan JS. Reactive oxygen species and nuclear factor erythroid 2-related factor 2 activation in diabetic nephropathy: a hidden target. J Diabetes Metab 2015; 6. doi: 10.4172/2155-6156.100547. [DOI] [PMC free article] [PubMed]
- Hirata Y, Yamamoto E, Tokitsu T, Fujisue K, Kurokawa H, Sugamura K, et al. The pivotal role of a novel biomarker of reactive oxygen species in chronic kidney disease. Medicine (Baltimore) 2015; 94: e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Shen Y, Wang Z, Shao DC, Liu J, Kong YL, et al. P300-dependent STAT3 acetylation is necessary for angiotensin II-induced pro-fibrotic responses in renal tubular epithelial cells. Acta Pharmacol Sin 2014; 35: 1157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana L, Carru C, Pes G, Tadolini B. Spectrophotometric measurement of hydroperoxides at increased sensitivity by oxidation of Fe2+ in the presence of xylenol orange. Free Radic Res 1999; 31: 237–44. [DOI] [PubMed] [Google Scholar]
- Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 2012; 17: 311–21. [DOI] [PubMed] [Google Scholar]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther 2008; 8: 1955–62. [DOI] [PubMed] [Google Scholar]
- Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis. Eur J Cardiothorac Surg 2009; 35: 521–7. [DOI] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 2013; 34: 167–77. [DOI] [PubMed] [Google Scholar]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther 2008; 8: 1955–62. [DOI] [PubMed] [Google Scholar]
- Hsu TF, Huang MK, Yu SH, Yen DH, Kao WF, Chen YC, et al. N-acetylcysteine for the prevention of contrast-induced nephropathy in the emergency department. Intern Med 2012; 51: 2709–14. [DOI] [PubMed] [Google Scholar]
- Demiroren K, Dogan Y, Kocamaz H, Ozercan IH, Ilhan S, Ustundag B, et al. Protective effects of L-carnitine, N-acetylcysteine and genistein in an experimental model of liver fibrosis. Clin Res Hepatol Gastroenterol 2014; 38: 63–72. [DOI] [PubMed] [Google Scholar]
- Lee JS, McLaughlin S, Collard HR. Comprehensive care of the patient with idiopathic pulmonary fibrosis. Curr Opin Pulm Med 2011; 17: 348–54. [DOI] [PubMed] [Google Scholar]
- Zhang L, He YL, Li QZ, Hao XH, Zhang ZF, Yuan JX, et al. N-acetylcysteine alleviated silica-induced lung fibrosis in rats by down-regulation of ROS and mitochondrial apoptosis signaling. Toxicol Mech Methods 2014; 24: 212–9. [DOI] [PubMed] [Google Scholar]
- Zhou CF, Yu JF, Zhang JX, Jiang T, Xu SH, Yu QY, et al. N-acetylcysteine attenuates subcutaneous administration of bleomycin-induced skin fibrosis and oxidative stress in a mouse model of scleroderma. Clin Exp Dermatol 2013; 38: 403–9. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 2013; 19: 1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]