Abstract
Aim:
The inhaled anesthetic sevoflurane may induce cognitive impairment in both animals and humans. Previous study has shown that sevoflurane triggers ER stress and may lead to apoptosis in rat hippocampal neurons. In this study, we examined whether sevoflurane caused autophagy and its contributions to sevoflurane induced neuronal cell injury.
Methods:
H4 human neuroglioma cells were exposed to 4.1% sevoflurane for 6 h. Cell viability and apoptosis ratio were assessed using a CCK8 kit and flow cytometry, respectively. Autophagosomes in the cells were detected using GFP-LC3 plasmid transfection or transmission electronic microscopy. The expression of LC3B, p62/SQSTM, C/EBP homologous protein (CHOP) and glucose-related protein 78 (GRP78) was assessed with Western blotting.
Results:
Sevoflurane treatment induced apoptosis and markedly increased the LC3-II level and GFP-LC3 puncta number, decreased p62 expression in H4 cells. Activation of autophagy by rapamycin (1 μmol/L) significantly reduced sevoflurane-induced apoptosis and increased cell viability, whereas inhibition of autophagy with 3-MA (5 mmol/L) caused the opposite effects. Furthermore, sevoflurane treatment markedly increased the expression of CHOP and GRP78, two hallmark proteins of ER stress. Inhibition of ER stress by 4-phenylbutyrate (500 μmol/L) abrogated sevoflurane-induced autophagy and apoptosis, and improved the viability. Moreover, sevoflurane-stimulated expression of CHOP and GRP78 was inhibited by rapamycin, but further enhanced by 3-MA.
Conclusion:
Sevoflurane treatment induces ER stress and activates autophagy, which antagonizes sevoflurane-induced apoptosis in H4 human neuroglioma cells. The results suggest that autophagy may be a potential therapeutic target in preventing sevoflurane-induced neurotoxicity.
Keywords: sevoflurane, inhalation anesthetics, H4 human neuroglioma cells, autophagy, apoptosis, ER stress, rapamycin, 3-MA, 4-phenylbutyrate, neuroprotection
Introduction
Sevoflurane is the most widely used halogenated inhaled anesthetic in clinical practice, characterized by low pungency, non-irritating odor and a low blood/gas partition coefficient1. However, increasing evidence has suggested that sevoflurane may induce cognitive impairment in both animals and humans2,3,4. Previous investigations found that neural apoptosis, neuroinflammation and abnormal protein deposition in the neurons may contribute to the cognitive impairment5,6,7,8. Consistently, our previous investigation also found that sevoflurane triggers endoplasmic reticulum (ER) stress and may lead to neuronal cell apoptosis9. Although the mechanism is not fully understood, it seems that the elderly are more vulnerable to sevoflurane-induced cognitive deficit10.
Autophagy, an evolutionarily conserved process of self-digestion, plays an important role in maintaining cellular homeostasis from yeast to mammals11. In the central nervous system, autophagy plays a critical role because the post-mitogenesis nature of neurons makes it difficult for them to dilute aging proteins and impaired organelles in daughter cells. Certain lines of evidence have indicated that autophagy declines along with aging, suggesting a close association of autophagy deficiency with age-related neurological disorders12,13,14. Indeed, recent investigations have suggested that insufficient autophagy may be responsible for cognitive decline and neurodegeneration in the aging process15. Therefore, autophagy deficiency may be one of the underlying reasons for sevoflurane-induced cognitive impairment in older people. However, it is still not clear whether and how sevoflurane regulates the autophagy process in neuronal cells.
H4 human neuroglioma cells have been used as an alternative to primary neurons in several previous studies, despite their immortal nature16,17. In this study, we employed H4 human neuroglioma cells to investigate whether sevoflurane causes autophagy and its contributions to sevoflurane-induced cell injury.
Materials and methods
Materials
The following materials were used: bafilomycin A1 (Sigma-Aldrich, B1793, St Louis, MO, USA), rapamycin (Sigma-Aldrich, R0395), 3-MA (Sigma-Aldrich, M9281), 4-phenylbutyrate (4-PBA, Sigma-Aldrich, SML0309), anti-C/EBP homologous protein (CHOP) antibody (Cell Signaling Technology, 2895, Danvers, MA, USA), anti-β-actin antibody (Cell Signaling Technology, 4970), anti-SQSTM1/p62 antibody (Medical and Biological Laboratories, PM045, Tokyo, Japan), anti-LC3 antibody (Novus Biologicals, NB100-2220, Littleton, CO, USA), anti-glucose-related protein 78 (GRP78) antibody (Santa Cruz Biotechnology, sc-1050, Santa Cruz, CA, USA), anti-cleaved caspase 3 antibody (Cell Signaling Technology, 9664), HRP-conjugated goat anti-rabbit secondary antibody (Pierce, 31460), and HRP-conjugated goat anti-mouse secondary antibody (Pierce, 31430).
Cell culture
H4 human neuroglioma cells purchased from the China Center for Type Culture Collection were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L glutamine (all from Gibco, Grand Island, NY, USA) at 37 °C with 5% CO2 in a humidified incubator.
Exposure to sevoflurane
Culture plates were put into an airtight plastic chamber with inlet and outlet connectors. The inlet port of the chamber was connected to a sevoflurane vaporizer to adjust the concentration of sevoflurane. The chamber was then gassed with 0%, 4.1% or 8% sevoflurane in the carrier gas (95% air/5% CO2) for 15 min. The concentration of sevoflurane in the chamber was monitored at the chamber outlet port by a gas monitor (PM 8060, Drager, Lübeck, Germany) until the target concentration was reached. The chamber was then sealed and incubated at 37 °C for 6 h. The gas in the chamber was renewed every 3 h, and the target concentration of sevoflurane was confirmed at the end of the incubation by gas monitor. The control cells underwent the same procedure but with 5% CO2 air.
Cell viability analysis
Cell viability was measured by the CCK8 assay (cell counting kit-8, 7 sea Molecular Technologies, Shanghai, China) according to the manufacturer's instructions. Cells were seeded in 96-well cell culture plates at a cellular density of 5×103 cells/well. After exposure to sevoflurane, the cell monolayer was rinsed with phosphate-buffered saline (PBS) three times, and then 1:10 diluted CCK8 solution in DMEM was added to the cells and incubated for 2 h at 37 °C. The absorbance was measured by a microplate reader at 450 nm and expressed as percentages of the control values.
Apoptotic cell analysis
Comparative levels of apoptotic cells were determined by flow cytometry. After exposure to sevoflurane, the cells were harvested and washed twice with cold PBS, resuspended in 1×Annexin V binding buffer and then stained with PI and annexin V using the Annexin V-FITC apoptosis detection kit (Becton–Dickinson, San Jose, USA) according to the manufacturer's protocol. The apoptosis ratio was measured by BD FACS Accuri C6 (Becton-Dickinson, San Jose, USA).
Western blotting analysis
The treated cells were collected and washed twice with cold PBS, then lysed in an appropriate amount of cell lysis buffer for Western and IP (Beyotime, Shanghai, China) with 1× protease inhibitor cocktail (Merck, Darmstadt, Germany) on ice for 15 min. The supernatant was collected by centrifugation at 16 200×g for 10 min, and the protein concentration was determined using a bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). Equal amounts of the proteins were subjected to 13.5% SDS-PAGE and then transferred to a nitrocellulose filter membrane (Whatman, Dassel, Germany) after separation by electrophoresis. After blocking with 5% skim milk at room temperature for 1 h, the membrane was incubated with primary antibody overnight at 4 °C. The membranes were then washed 5 times for 3 min with TBST and incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. Bands were visualized using the ECL plus Western blotting detection system (PerkinElmer, USA), and the membranes were revealed in a C-DiGit Blot Scanner (Li-cor Bioscience, Lincoln, NE, USA). The signals were quantified using Image Studio Digits Vers 3.1.
Transmission electron microscopy
After treatment, cells were fixed with 2.5% glutaraldehyde in PBS (pH 7.4) at 4 °C for 2 h and then post-fixed in 1% osmium tetroxide in water for 1 h. After several washes in distilled water, the samples were dehydrated by a graded ethanol series and embedded in resin. Thin sections (0.1 μm) were cut and stained with 2% uranyl acetate and lead citrate in the dark. The autophagic vacuoles and dilated endoplasmic reticulum were detected using a Zeiss EM900 transmission electron microscope (Carl Zeiss, Oberkochen, Germany).
LC3 puncta analysis
The GFP-LC3 plasmid was a generous gift from Dr Zhao (School of Public Health, Zhejiang University, Hangzhou, China). The transfection of GFP-LC3 plasmid into H4 cells was performed using Lipofectamine 3000 according to the manufacturer's protocol. Forty-eight hours after transfection, cells were exposed to 0% or 4.1% sevoflurane for 6 h, and then the percentage of GFP-LC3 puncta-positive cells and the number of green puncta in each cell were observed and recorded under a Zeiss LSM 510 confocal microscope (Carl Zeiss, Germany). For each section, at least five random fields were included, and at least 20 cells were counted for each group.
Statistical analysis
Representative results from at least three independent experiments are shown. The differences among groups were analyzed by one-way analysis of variance (ANOVA), and the means of two groups were compared using Student's t-test with GraphPad Prism 6. P<0.05 was considered to be statistically significant. The data were presented as mean±SEM of three replications.
Results
Sevoflurane induces autophagy in H4 cells
To determine whether sevoflurane can induce autophagy, we first examined the level of autophagic mark protein MAP1LC3 (LC3) in H4 cells when the cells were exposed to sevoflurane (0%, 4.1% or 8%) for 6 h. The soluble form of LC3 (LC3-I) converts to the autophagosome-associated form (LC3-II) during the process of autophagosome formation18. The results of Western blotting analysis showed that sevoflurane increased the level of LC3-II in H4 cells in a concentration-dependent manner (Figure 1A).
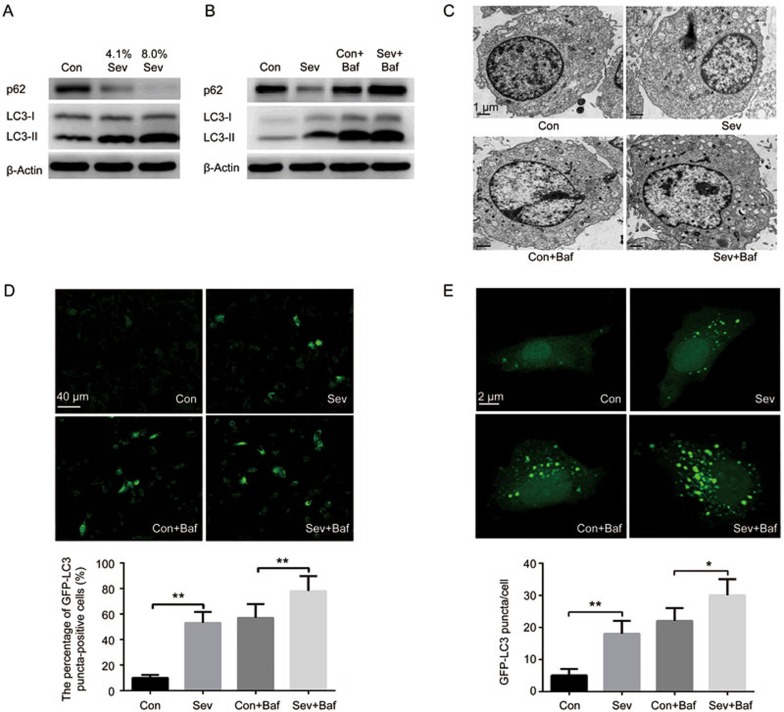
Figure 1.
Sevoflurane induced autophagy in H4 cells. (A) Western blot analysis of LC3-II and p62 in H4 cells exposed to 0%, 4.1% or 8% sevoflurane for 6 h. (B) Western blot analysis of LC3-II and p62 in H4 cells exposed to 0% or 4.1% sevoflurane with/without bafilomycin A1 (Baf, 10 nmol/L) for 6 h. (C) H4 cells were exposed to 0% or 4.1% sevoflurane with/without bafilomycin A1 for 6 h, followed by the assessment of autophagic vacuoles (black arrow head) by transmission electronic microscopy. Representative results are shown. (D) Effect of sevoflurane on the percentage of GFP-LC3 puncta-positive cells in H4 cells exposed to 0% or 4.1% sevoflurane with/without bafilomycin A1 (Baf, 10 nmol/L) for 6 h. (E) Effect of sevoflurane on the formation of GFP-LC3 puncta in H4 cells exposed to 0% or 4.1% sevoflurane with/without bafilomycin A1 (Baf, 10 nmol/L) for 6 h. The data represent the mean±SEM of three replications. *P<0.05, **P<0.01.
However, an increased level of LC3-II may result from either the increased formation of autophagosomes or the decreased degradation of autophagosomes. To discriminate between these two possibilities, we detected the expression of p62, which is degraded by the autophagosome-lysosome pathway19. Our results showed that sevoflurane decreased the p62 level, suggesting that autophagy flux was not blocked by sevoflurane (Figure 1A). Moreover, we used bafilomycin A1 (Baf, 10 nmol/L), an inhibitor of autophagosome and lysosome fusion, to block the autophagic flux when cells were exposed to a clinically relevant concentration (4.1%) of sevoflurane for 6 h. Our results showed that bafilomycin A1 further increased the sevoflurane-induced accumulation of LC3-II (Figure 1B).
Moreover, the results of transmission electronic microscopy showed that the exposure of H4 cells to 4.1% sevoflurane for 6 h led to the pronounced formation of autophagosomes and autophagic vacuoles in H4 cells, while autophagosome-like vacuoles were hardly seen in untreated cells, and bafilomycin A1 further increased the pronounced formation of autophagosomes and autophagic vacuoles induced by sevoflurane (Figure 1C).
In addition, to further confirm that sevoflurane increased autophagic flux, we transfected H4 cells with GFP-LC3 plasmid and detected the percentage of GFP-LC3 puncta-positive cells and the number of GFP-LC3 puncta in the H4 cells. Our results showed that sevoflurane increased the percentage of GFP-LC3 puncta-positive cells and the number of GFP-LC3 puncta in H4 cells, and these changes were significantly enhanced by bafilomycin A1 (Figure 1D and 1E).
Overall, these results demonstrate that sevoflurane activates autophagy in H4 cells.
Autophagy protects against sevoflurane-induced apoptosis in H4 cells
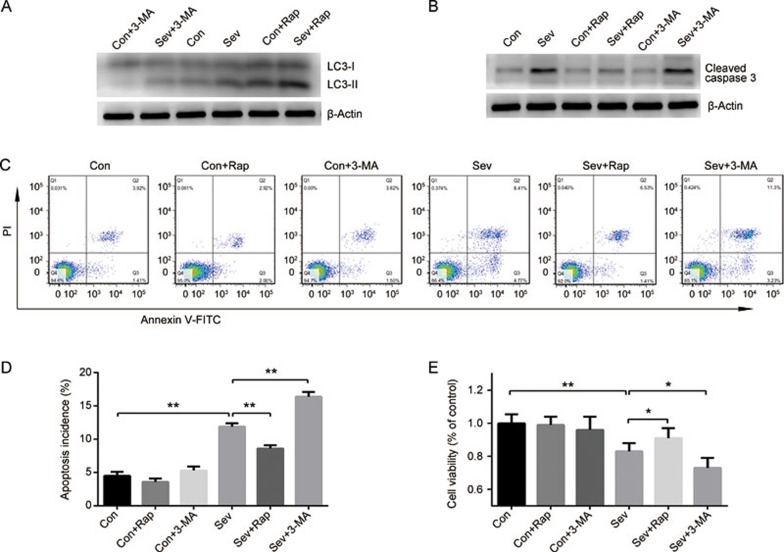
Sevoflurane has been reported to induce apoptosis in H4 cells20, and autophagy has been suggested to modulate apoptosis21. To determine the role of autophagy in sevoflurane-induced apoptosis, cells were exposed to sevoflurane (0% or 4.1%) with or without rapamycin (Rap, an autophagy inducer, 1 μmol/L) or 3-MA (an autophagy inhibitor, 5 mmol/L) for 6 h22. As shown in Figure 2A, the level of LC3-II was increased by rapamycin and decreased by 3-MA, suggesting that autophagy in H4 cells was enhanced by rapamycin and inhibited by 3-MA. In line with previous findings, our results showed that exposure to 4.1% sevoflurane for 6 h significantly decreased cell viability and increased the expression of cleaved caspase 3 and the apoptosis rate of H4 cells (Figure 2B–2E). Rapamycin improved the viability of sevoflurane-treated cells and attenuated the apoptosis induced by sevoflurane (Figure 2B–2E). Conversely, 3-MA further decreased the cell viability after sevoflurane treatment and promoted cell apoptosis (Figure 2B–2E). Neither rapamycin nor 3-MA, used alone, has a significant effect on cell viability and apoptosis (Figure 2B–2E). Therefore, these results suggest that the activation of autophagy prevents sevoflurane-induced apoptosis in H4 cells.
Figure 2.
Activation of autophagy prevents sevoflurane-induced apoptosis in H4 cells. H4 cells were exposed to 0% or 4.1% sevoflurane with/without rapamycin (Rap, 1 μmol/L) or 3-MA (5 mmol/L) for 6 h. (A) Expression of LC3 detected by Western blotting. (B) Expression of cleaved caspase 3 detected by Western blotting. (C) Apoptosis rate of H4 cells determined by Annexin V-FITC/PI staining and flow cytometry analysis. (D) Histogram shows the effect of sevoflurane, rapamycin and 3-MA on cell apoptosis determined by flow cytometry. (E) Effects of sevoflurane, rapamycin and 3-MA on cell viability were measured by CCK8 kit. The data represent the mean±SEM of three replications. *P<0.05, **P<0.01.
Sevoflurane-induced autophagy is mediated by ER stress in H4 cells
The above results suggest that autophagy plays an important role in sevoflurane-induced cell apoptosis. We then investigated the mechanism underlying sevoflurane-induced autophagy.
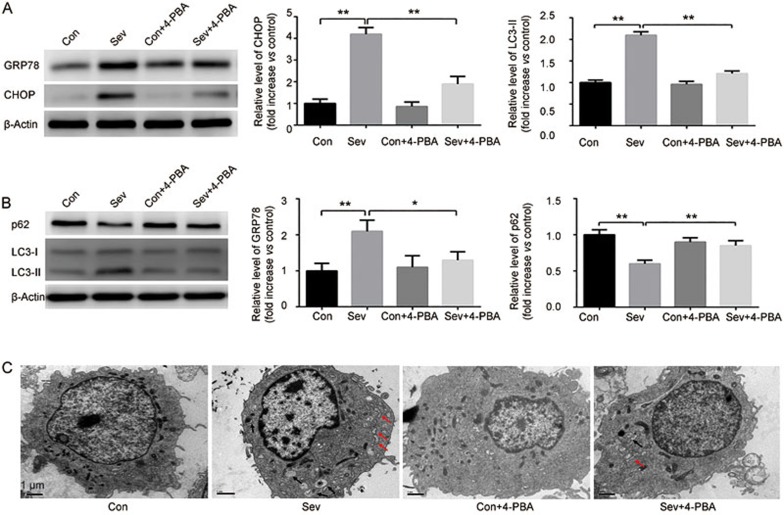
Previous studies suggested that sevoflurane induces neuron apoptosis by activating ER stress, which has been reported to be a potent trigger of autophagy23,24. To investigate the role of ER stress in sevoflurane-induced autophagy, we analyzed two hallmark proteins of ER stress, CHOP and GRP78. Our results showed that 4.1% sevoflurane exposure for 6 h significantly increased the expression levels of CHOP and GRP78, indicating that sevoflurane induced ER stress in H4 cells (Figure 3A). To verify this result, 4-PBA, an chemical chaperone that enhances the ER's capacity to cope with misfolded protein in cells25, was employed as an ER stress inhibitor. Our results suggested that 4-PBA (500 μmol/L) not only inhibited the increased expression of CHOP and GRP78 but also suppressed the increase of LC3-II and induced p62 accumulation in H4 cells exposed to sevoflurane (Figure 3A and 3B). There were no significant alterations to the levels of CHOP, GRP78, LC3-II and p62 by 4-PBA alone. Moreover, transmission electronic microscopy observation showed that the exposure of H4 cells to 4.1% sevoflurane for 6 h led to the pronounced formation of autophagic vacuoles and dilated endoplasmic reticulum, while 4-PBA significantly inhibited the formation of autophagic vacuoles and dilated endoplasmic reticulum induced by sevoflurane (Figure 3C). Altogether, these results indicate that ER stress mediates the sevoflurane-induced autophagy in H4 cells.
Figure 3.
Sevoflurane-induced autophagy is mediated by ER stress in H4 cells. H4 cells were exposed to sevoflurane (0% or 4.1%) with/without 4-PBA (500 μmol/L), an inhibitor of ER stress for 6 h. (A) The effect of sevoflurane and 4-PBA on the expression of CHOP and GRP78 were determined by Western blot analysis. (B) Effect of sevoflurane and 4-PBA on the expression of p62 and LC3 were determined by Western blot analysis. (C) Autophagic vacuoles (black arrow head) and dilated endoplasmic reticulum (red arrow head) observed under the transmission electronic microscopy. The data represent the mean±SEM of three replications. *P<0.05, **P<0.01.
ER stress is involved in sevoflurane-induced apoptosis in H4 cells
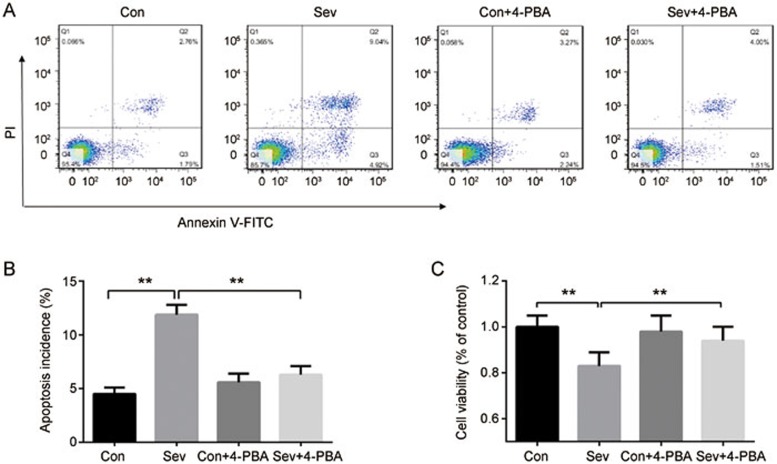
In addition to inducing autophagy, ER stress has been reported to mediate cell apoptosis in neural cells9. We then explored the role of ER stress in sevoflurane-induced apoptosis in H4 cells. We found that the inhibition of ER stress by 4-PBA (500 μmol/L) abrogated cell apoptosis and reversed the reduction in cell viability in H4 cells exposed to 4.1% sevoflurane for 6 h. No significant effect on cell viability was produced by 4-PBA alone (Figure 4A–4C). These results suggest that ER stress is involved in sevoflurane-induced apoptosis.
Figure 4.
ER stress is involved in sevoflurane-induced apoptosis in H4 cells. H4 cells were exposed to 0% or 4.1% sevoflurane with/without 4-PBA (500 μmol/L) for 6 h. (A) Apoptosis rate of H4 cells determined by Annexin V-FITC/PI staining and flow cytometry analysis. (B) Histogram shows the effect of sevoflurane and 4-PBA on cell apoptosis determined by flow cytometry. (C) Effect of sevoflurane and 4-PBA on cell viability were measured by CCK8 kit. The data represent the mean±SEM of three replications. **P<0.01.
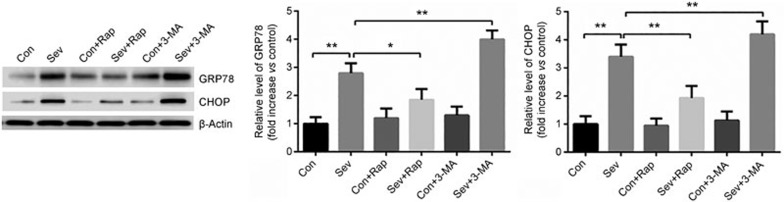
Activation of autophagy reduces ER stress in sevoflurane-treated H4 cells
Having demonstrated that the activation of autophagy prevents sevoflurane-induced apoptosis and that sevoflurane-induced apoptosis involves ER stress in H4 cells, we then hypothesized that the activation of autophagy in turn regulates ER stress in H4 cells. As shown in Figure 5, the activation of autophagy by rapamycin (1 μmol/L) significantly reduced the high expression of CHOP and GRP78 induced by sevoflurane; conversely, the inhibition of autophagy by 3-MA (5 mmol/L) further increased the sevoflurane-upregulated levels of CHOP and GRP78 (Figure 5). These results suggested that the activation of autophagy reduces ER stress in H4 cells exposed to sevoflurane.
Figure 5.
H4 cells were exposed to sevoflurane (0% or 4.1%) with/without rapamycin (Rap, 1 μmol/L) or 3-MA (5 mmol/L) for 6 h. The expression of CHOP and GRP78 was detected by Western blot analysis. The data represent the mean±SEM of three replications. *P<0.05, **P<0.01.
Discussion
In this study, we found that sevoflurane induced autophagy in H4 cells. Moreover, we found that the inhibition of autophagy by 3-MA sensitized the cells to sevoflurane-induced cell injury, while the activation of autophagy by rapamycin ameliorated the effect, suggesting that autophagy acts as a pro-survival mechanism in cells exposed to sevoflurane. The inhibition of ER stress by 4-PBA blocked sevoflurane-induced autophagy and cell demise, suggesting that ER stress may contribute to the neuroprotection conferred by autophagy. Our results suggested that autophagy may be a potential therapeutic target in prevention of sevoflurane-induced neurotoxicity.
Autophagy is an important homeostatic mechanism that can be induced by multiple forms of cellular stresses, including nutrient or growth factor deprivation, hypoxia, ER stress, protein aggregates and intracellular pathogens26. Recently, it has been proposed that the activation of autophagy might be a potential way to prevent the neurotoxicity of general anesthetics, including sevoflurane, which is the most widely used inhalation anesthetic23,27. However, the effect of sevoflurane on autophagy remains unknown. Previous studies have demonstrated that sevoflurane generates reactive oxygen species and induces ER stress in neural cells9,23,28. Moreover, at the molecular level, sevoflurane has been suggested to increase neuron apoptosis by inhibiting phosphoinositide-3-kinase activity, which may induce autophagy via the PI3K-I/AKT/TSC/mTOR pathway29. However, direct evidence of sevoflurane's effect on autophagy activation in neural cells remains lacking. In this study, we provided the first evidence that sevoflurane activates autophagy in H4 human neuroglioma cells.
Autophagy plays a double pro-survival or pro-death role in cells, which is dependent on various conditions30. On one hand, autophagy may act as an alternative pathway to induce cell demise or act together with apoptosis as a combined mechanism for cell death31. On the other hand, autophagy has been viewed as a cellular self-defense response, particularly in ischemic heart disease and neurodegenerative diseases11. Recent studies have suggested that the activation of autophagy mediates the cardioprotection of both preconditioning and delayed preconditioning by sevoflurane in myocardial ischemia–reperfusion injury32,33,34. Increasing evidence has shown that autophagy plays a protective role in various chronic and acute neurodegenerative diseases due to its role in eliminating 'toxic assets' and promoting cell viability35,36. Here, we found that sevoflurane induced apoptosis in H4 cells, which is consistent with previous studies20,28. To investigate the effect of autophagy on sevoflurane-induced injury in H4 cells, we exposed H4 cells to sevoflurane combined with 3-MA or rapamycin. Our results showed that 3-MA further decreased the viability of sevoflurane-treated H4 cells and enhanced sevoflurane-induced apoptosis; conversely, rapamycin significantly increased the viability of sevoflurane-treated H4 cells and ameliorated apoptosis. Although the most effective method for autophagy intervention is the modulation of autophagy-related gene expression, our results are sufficient to indicate that the autophagy induced by sevoflurane is a protective response in H4 cells.
We found that sevoflurane increased the expression of GRP78 and CHOP, suggesting that sevoflurane induced ER stress in H4 cells. This result is consistent with our previous finding in rat hippocampal neurons9. ER stress may be induced by many factors37. The exact mechanisms underlying sevoflurane-induced ER stress remain unclear. Both intracellular calcium disturbance and oxidative stress induced by volatile anesthetics may contribute28,38,39,40. Unfolded protein response (UPR) and autophagy are two compensatory mechanisms during ER stress. Many studies have demonstrated that UPR helps to attenuate ER stress; however, prolonged UPR may lead to cell apoptosis41,42. In this study, we demonstrated that ER stress is involved in sevoflurane-induced apoptosis. Autophagy is an important compensatory mechanism for ER stress and promotes cell survival by eliminating the unfolded protein and the damaged organelles that accumulate in the cell43. We found that the inhibition of ER stress abrogated sevoflurane-induced autophagy, and the activation of autophagy attenuated the sevoflurane-induced ER stress, suggesting that autophagy is a protective response to sevoflurane-induced ER stress. However, the precise mechanisms underlying ER stress-mediated autophagy induced by sevoflurane need to be further elucidated. Moreover, because ER stress mediates sevoflurane-induced apoptosis and autophagy is a protective response to sevoflurane-induced ER stress, cells may possess different degrees of autophagy activity at different stages of apoptosis. However, such correlations should be further investigated.
In conclusion, this study indicated that sevoflurane activates autophagy, which protects against sevoflurane-induced apoptosis in H4 cells. These findings suggest that it is worth conducting further research to investigate the potential importance of autophagy in protection from sevoflurane-induced neuronal injury.
Author contribution
Gang CHEN, You-fa ZHOU, Qing-xia WANG, and Hai-yan ZHOU were participated in conceiving and designing the experiments; You-fa ZHOU and Qing-xia WANG conducted the experiments; Hai-yan ZHOU and Gang CHEN performed the data analysis; You-fa ZHOU and Qing-xia WANG wrote the paper; and Gang CHEN provided supervision and modified the paper.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 81371214) and Zhejiang Provincial Natural Science Foundation of China (No LY12H09005).
References
- Ghatge S, Lee J, Smith I. Sevoflurane: an ideal agent for adult day-case anesthesia? Acta Anaesthesiol Scand 2003; 47: 917–31. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Gruver R, Miller J, McReynolds JR, Hahn EL, Cahill L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc Natl Acad Sci U S A 2008; 105: 1722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, Eriksson LI. Object memory in young and aged mice after sevoflurane anaesthesia. Neuroreport 2009; 20: 1419–23. [DOI] [PubMed] [Google Scholar]
- Rohan D, Buggy DJ, Crowley S, Ling FK, Gallagher H, Regan C, et al. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anaesth 2005; 52: 137–42. [DOI] [PubMed] [Google Scholar]
- Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav 2015; 151: 16–23. [DOI] [PubMed] [Google Scholar]
- Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011; 61: 1354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 2013; 118: 502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz A, Perneczky R. Neurobiology of cognitive disorders. Curr Opin Psychiatry 2009; 22: 546–51. [DOI] [PubMed] [Google Scholar]
- Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS One 2013; 8: e57870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bo L, Wang J, Zhao Z, Xu Z, Deng X, et al. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg 2013; 8: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010; 221: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini E. Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med 2006; 27: 403–10. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet 2008; 24: 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem 2000; 275: 31505–13. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 2015; 84: 39–49. [DOI] [PubMed] [Google Scholar]
- Cheng B, Zhang Y, Wang A, Dong Y, Xie Z. Vitamin C attenuates isoflurane-induced caspase-3 activation and cognitive impairment. Mol Neurobiol 2015; 52: 1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang Y, Cheng B, Dong Y, Pan C, Li T, et al. Glucose may attenuate isoflurane-induced caspase-3 activation in H4 human neuroglioma cells. Anesth Analg 2014; 119: 1373–80. [DOI] [PubMed] [Google Scholar]
- Kimura S, Fujita N, Noda T, Yoshimori T. Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol 2009; 452: 1–12. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 2005; 171: 603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol 2009; 66: 620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti V, Torriglia A, Scovassi AI. Conversation between apoptosis and autophagy: “Is it your turn or mine?”. Apoptosis 2011; 16: 321–33. [DOI] [PubMed] [Google Scholar]
- Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, Xiong KP, et al. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin 2013; 34: 625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komita M, Jin H, Aoe T. The effect of endoplasmic reticulum stress on neurotoxicity caused by inhaled anesthetics. Anesth Analg 2013; 117: 1197–204. [DOI] [PubMed] [Google Scholar]
- Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, et al. ER stress inhibits neuronal death by promoting autophagy. Autophagy 2012; 8: 915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem 2007; 282: 27905–12. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010; 40: 280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yu B. Elevation of protective autophagy as a potential way for preventing developmental neurotoxicity of general anesthetics. Med Hypotheses 2014; 82: 177–80. [DOI] [PubMed] [Google Scholar]
- Tian Y, Guo S, Guo Y, Jian L. Anesthetic propofol attenuates apoptosis, Abeta accumulation, and inflammation induced by sevoflurane through NF-kappaB pathway in human neuroglioma cells. Cell Mol Neurobiol 2015; 35: 891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Wang M, Xie K, Wang H, Wang C, Wang C, et al. Internalization of GluA2 and the underlying mechanisms of cognitive decline in aged rats following surgery and prolonged exposure to sevoflurane. Neurotoxicology 2015; 49: 94–103. [DOI] [PubMed] [Google Scholar]
- Xu M, Zhang HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin 2011; 32: 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med 2008; 8: 78–91. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Miyamae M, Takemura G, Kaneda K, Inamura Y, Onishi A, et al. Sevoflurane induces cardioprotection through reactive oxygen species-mediated upregulation of autophagy in isolated guinea pig hearts. J Anesth 2014; 28: 593–600. [DOI] [PubMed] [Google Scholar]
- Qiao S, Xie H, Wang C, Wu X, Liu H, Liu C. Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-kappaB and upregulation of autophagy. J Anesth 2013; 27: 251–60. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Yao YT, Fang NX, Zhou CH, Gong JS, Li LH. Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats. Acta Pharmacol Sin 2014; 35: 758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 2008; 32: 329–39. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M. Autophagy in neurodegenerative diseases: From pathogenic dysfunction to therapeutic modulation. Semin Cell Dev Biol 2015; 40: 115–26. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 2006; 66: S102–9. [DOI] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008; 109: 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra FJ, do Vale NB, Macedo Bde O, Rezende AA, Almeida M. Evaluation of antioxidant parameters in rats treated with sevoflurane. Rev Bras Anestesiol 2010; 60: 162–9, 93–7. [DOI] [PubMed] [Google Scholar]
- Wong CH, Liu TZ, Chye SM, Lu FJ, Liu YC, Lin ZC, et al. Sevoflurane-induced oxidative stress and cellular injury in human peripheral polymorphonuclear neutrophils. Food Chem Toxicol 2006; 44: 1399–407. [DOI] [PubMed] [Google Scholar]
- Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic Biol Med 2015; 78: 30–41. [DOI] [PubMed] [Google Scholar]
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833: 3460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 2007; 14: 1576–82. [DOI] [PubMed] [Google Scholar]