Abstract
The thyroid metastasis from any primary is rare, and usually is a late event and presents as a thyroid swelling. Further, the diagnosis of a metastatic lesion in a patient with no antecedent history of any malignancy can be very challenging. Recently, a patient presented to us with a history of diagnostic evaluation suggesting a primary thyroid malignancy with a synchronous lung primary. After surgery for the thyroid swelling, final histopathology revealed a metastatic lesion from a lung primary. Here, we discuss this rare case of isolated synchronous thyroid metastasis from a lung primary and review the relevant literature.
KEY WORDS: Metastatic lung cancer, nonsmall cell lung cancer, papillary thyroid cancer, thyroid metastasis
INTRODUCTION
The reported incidence of metastases to the thyroid gland in the living is 2–3% of all the thyroid malignancies as opposed to the postmortem of about 1–24%.[1] The most common primaries spreading to the thyroid gland by order are lung, breast, and kidney.[2] Most of the ante-mortem secondaries being metachronous, appearing several years after the detection of the primary. Further, in most of the cases, by the time any primary cancer spreads to the thyroid gland, it would have been a disseminated disease with multiple sites of metastasis. Isolated metastasis to thyroid gland from any cancer is rare despite its rich vascular supply.[3] Here, we report one such rare case of an isolated synchronous metastasis to thyroid from a nonsmall cell lung cancer presenting as a primary thyroid cancer.
CASE REPORT
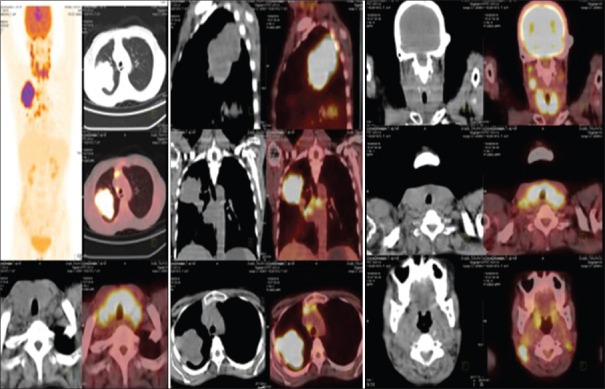
A 42-year-old postmenopausal woman presented initially to endocrinology division with a 1-month history of swelling in front of the neck and throat pain. She did not give any history of dysphagia, voice change, or dyspnea. There was no history suggestive of either hypo- or hyper-thyroidism. On examination, the patient was poorly built and nourished with an eastern cooperative oncology group (ECOG) performance scale of 1. The patient had 5 cm × 4 cm thyroid swelling and multiple lymph nodes on the right side of the neck levels 2 and 3, largest measuring 3 cm × 2 cm [Figure 1]. An ultrasonography (US) of the neck, fine needle aspiration cytology (FNAC) of the thyroid lesion and cervical node, and serum thyroid stimulating hormone (TSH) were requested. On review visit, US revealed enlarged thyroid gland (right lobe 4.9 cm × 1.9 cm × 2.2 cm, left lobe 3.5 mm × 1.6 mm × 1.5, and isthmus 6.5 mm) being bulky and heterogenous with enlarged bilateral cervical lymph nodes. The serum TSH was within the normal reference range. The FNAC from the thyroid gland was called out as features suggestive of papillary carcinoma [Figure 2], FNAC from the largest cervical node was reported as reactive hyperplasia, and the patient was referred to surgical oncology (SO) division for further management. At the SO OPD, on detailed enquiry, the patient gave a history of nonproductive cough from the past 1 month, in addition to all that was previously documented. She also gave a history of episodic fever and shortness of breath on exertion. There was no history of hemoptysis or chest pain, no loss of appetite, or weight. Apart from the details of local examination mentioned above, respiratory system examination revealed diminished breath sounds on the right upper part of the chest. A chest radiograph was ordered, which showed a homogenous opacity in the upper zone of the right lung with well-defined superior and medial borders and ill-defined inferior and lateral border [Figure 3], and she was advised a contrast-enhanced computerized tomography (CECT) chest. The CECT chest revealed a heterogeneously enhancing mass lesion of 6.5 cm × 5 cm in the apicoposterior segment of the right upper lobe [Figure 4]. Further, considering the possibilities of either a synchronous new primary cancer or a metastatic focus in the lung or thyroid lesion being metastatic, a computerized tomography (CT) guided biopsy of the lung lesion was done which was called out as features suggestive of nonsmall cell lung carcinoma (NSCLC). On immunohistochemistry (IHC), thyroid transcription factor-1 (TTF 1) was intensely nuclear positive, cytokeratin (CK) 7 was diffusely cytoplasmic positive, and CK 20 was negative [Figure 5]. A whole body 18F fluorodeoxyglucose positron emission tomography/CT was ordered which revealed metabolically active lesion in entire thyroid (maximum standardized uptake value [SUVmax] 9.0) with high uptake suggestive of poorly differentiated thyroid carcinoma with metabolically active bilateral cervical lymph nodes (SUVmax 6) at multiple levels. There was a metabolically active irregular soft tissue density lesion in the upper lobe of the right lung (SUVmax 15, 5.4 cm × 5.6 cm × 8 cm) with nodule in middle lobe (SUVmax 4.6) and metabolically active ipsilateral paratracheal (SUVmax 5), precarinal (SUVmax 5.2), subcarinal (SUVmax 7), prevascular (SUVmax 8), and right hilar lymph nodes (SUVmax 4) [Figure 6]. A multidisciplinary discussion was initiated with the departments of pathology, nuclear medicine radiology, medical oncology, and thoracic surgeons, and a provisional diagnosis of synchronous dual malignancy was made. As the lung mass was deemed inoperable and advised chemotherapy by the thoracic surgeons, the patient was planned for thyroidectomy with neck dissection followed by chemotherapy for lung lesion. At surgery, thyroid was enlarged and densely adherent to strap muscles, lymph nodes were adherent to internal jugular vein on the right side. Total thyroidectomy with bilateral selective (levels 2–5) lymph node dissection and central compartment dissection were done. Postoperatively, the patient developed symptoms of hypocalcemia which was treated appropriately, and the patient was discharged in stable condition.
Figure 1.
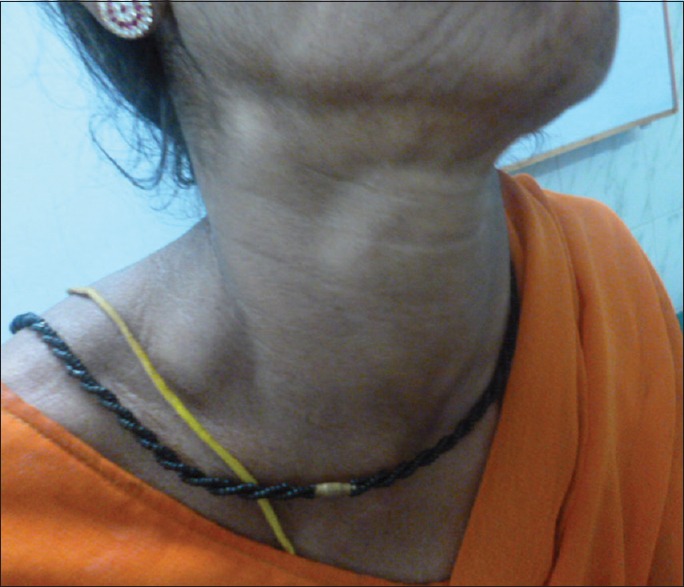
Clinical photograph showing thyroid swelling
Figure 2.
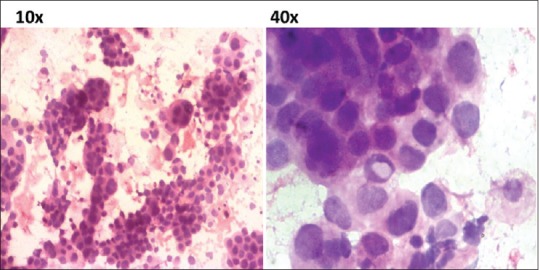
Photomicrograph of fine needle aspiration cytology from thyroid showing rich cellularity with tumor cells in papillary arrangement (H and E, ×10) and occasional intranuclear inclusions (×40)
Figure 3.
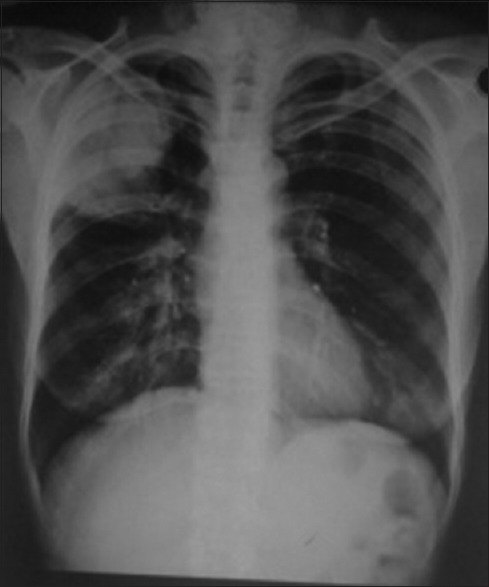
Chest radiograph showing the right upper lobe mass
Figure 4.
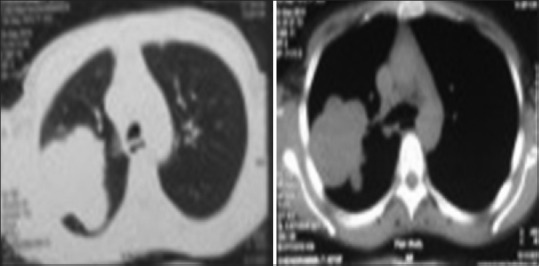
Contrast-enhanced computerized tomography chest showing the lung mass
Figure 5.
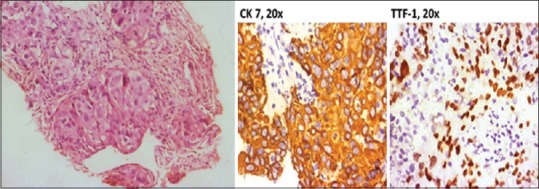
Photomicrograph from computerized tomography-guided right lung biopsy showing solid nests and ill-defined acinar formation of round to polygonal neoplastic cells and immunohistochemistry staining
Figure 6.
Whole body positron emission tomography/computerized tomography images showing lung mass and thyroid lesion with increased fluorodeoxyglucose uptake
The postoperative histopathology of the thyroidectomy specimen revealed NSCLC with perivascular infiltration and perineural spread with 34 of the 54 lymph nodes showing the presence of tumor [Figure 5]. On IHC, there was intense cytoplasmic positivity for CK 7 and nuclear positivity for TTF 1 in tumor cells. The tumor cells were negative for thyroglobulin and CK 20 markers, confirming a final diagnosis of adenocarcinomatous deposits from an NSCLC [Figure 7]. She was started on once daily dose of tablet thyroxine 50 mcg and was referred to medical oncology services for chemotherapy. At the time of reporting, she had completed 4 cycles of pemetrexed and cisplatin chemotherapy without any major complications or interruptions and has been placed on tablet erlotinib on once a day regimen.
Figure 7.
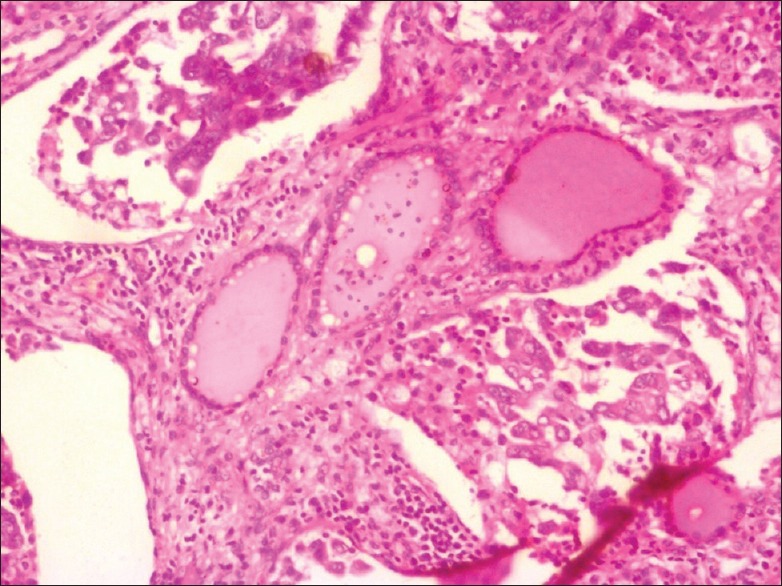
Photomicrograph from total thyroidectomy specimen showing normal thyroid follicles filled with colloid and intervening tumor component in cribriform and pseudopapillary arrangement (H and E, ×10)
DISCUSSION
Whenever a patient with FNAC diagnosis of papillary thyroid carcinoma is diagnosed with a lung lesion, the common possibilities that strike to a clinician's mind are either a secondary in the lung or a second primary cancer in the lung as the accuracy of the FNAC for papillary neoplasm is pretty high.[4] This may not always be the case as in the case reported above highlights the third possibility of the thyroid being a metastatic site for a lung primary cancer.
In spite of its rich vascularity, the thyroid gland is relatively protected from the development of metastasis due to various reasons such as most of the malignant cells entering venous circulation from the primary cancer are being filtered initially by the lung, those cells which succeed in reaching the thyroid bed being washed away due to high velocity intraglandular blood flow, and the tumoricidal effect of high intraglandular oxygen and iodine content.[5]
The metastases to the thyroid are rare comprising 4% of all the thyroid neoplasms.[6,7] The most common primary cancer site being breast followed by lung and kidney in the majority of the Western series, whereas lung, breast, and stomach are the most common primary cancer sites (in decreasing order) reported in the majority of the oriental studies.[3,8] In patients with lung cancers metastasizing to the thyroid, adenocarcinoma is the most common histology followed by squamous cell, small cell, and large cell.[8] The thyroid metastasis can be synchronous or metachronous, the latter being more common. A history of malignancy often alerts the clinician regarding the possibility of thyroid swelling being metastatic in the metachronous setting. Sometimes, when the thyroid metastasis precedes the diagnosis of another primary cancer or presents synchronously, the diagnosis would be difficult.[9] The clinical presentation of thyroid metastasis often mimics the primary thyroid neoplasm, especially when it is isolated and synchronous. The diagnosis will be challenging to the clinician when the primary cancer is clinically occult.[10]
One of the important diagnostic tools in the evaluation of thyroid enlargement is FNAC. The diagnosis of metastatic involvement of the thyroid gland in the absence of an antecedent history of another primary cancer is difficult as it was in our case.[11] The accuracy of FNAC in the diagnosis of primary thyroid cancer is very high with a false negativity rate of <1% and a false positivity rate of 2–3%. The accuracy being influenced by the sampling as well as the interpretation issues.[4] In our case, the chest radiograph showed a lung lesion, which was later evaluated with CECT and reported as a probable metastasis from the thyroid primary. Considering the other possibilities of a synchronous dual malignancy or thyroid lesion being the metastasis from the lung primary cancer, a CT-guided biopsy was done from the lung lesion. The IHC studies selected in our case indicated a possibility of another primary cancer in the lung. We did not do any immunocytochemical studies on thyroid aspirate as the diagnostic accuracy of FNAC is very high for papillary thyroid cancer. On reviewing the literature, we could find that whenever a follicular tumor is diagnosed on FNAC in a patient with prior history of malignancy, a metastatic possibility is to be considered.[11] Sometimes, a papillary adenocarcinoma of the lung can mimic papillary thyroid carcinoma on histopathological sections.[12] The role of several immunocyto/histochemical markers in the determination of lung origin in metastatic adenocarcinoma has been debated. Some of these include TTF 1, monoclonal, and polyclonal napsin A. With regards to thyroid metastasis of lung origin, TTF 1 may not be contributory as it can be positive in both the conditions.[13,14,15]
To conclude, the possibility of a thyroid swelling being a secondary rather than primary should be kept in mind in the evaluation of the thyroid nodule. The preoperative diagnosis of such a condition is often challenging. The appropriate use of immunocyto/histochemistry may aid the diagnosis and may avoid unnecessary surgery for the thyroid gland.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zhou XC, Hu KQ, Zhang XF, Ye YH, Jiang Y, Pan F, et al. Breast cancer metastatic to the bilateral thyroid: A case report and literature review. Head Neck Oncol. 2012;4:51. [Google Scholar]
- 2.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–8. doi: 10.1002/(sici)1097-0142(19970201)79:3<574::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Lam KY, Lo CY. Metastatic tumors of the thyroid gland: A study of 79 cases in Chinese patients. Arch Pathol Lab Med. 1998;122:37–41. [PubMed] [Google Scholar]
- 4.Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. A review of 6226 cases and correlation with surgical or clinical outcome. Arch Pathol Lab Med. 2001;125:484–8. doi: 10.5858/2001-125-0484-AOFNAO. [DOI] [PubMed] [Google Scholar]
- 5.Kim AY, Park SB, Choi HS, Hwang JC. Isolated thyroid metastasis from renal cell carcinoma. J Ultrasound Med. 2007;26:1799–802. doi: 10.7863/jum.2007.26.12.1799. [DOI] [PubMed] [Google Scholar]
- 6.Berge T, Lundberg S. Cancer in Malmö 1958-1969. An autopsy study. Acta Pathol Microbiol Scand Suppl. 1977;260:1–235. [PubMed] [Google Scholar]
- 7.Silverberg SG, Vindone RA. Metastatictumours in the thyroid. Pac Med Surg. 1966;74:175–80. [Google Scholar]
- 8.Watanabe I, Tsuchiya A. Secondary carcinoma of the thyroid gland. Jpn J Surg. 1980;10:130–6. doi: 10.1007/BF02468677. [DOI] [PubMed] [Google Scholar]
- 9.Feldman ER, Eagan RT, Schaid DJ. Metastatic bronchioloalveolar carcinoma and metastatic adenocarcinoma of the lung: Comparison of clinical manifestations, chemotherapeutic responses, and prognosis. Mayo Clin Proc. 1992;67:27–32. doi: 10.1016/s0025-6196(12)60273-0. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Wang W, Zhang C. Thyroid gland metastasis arising from breast cancer: A case report. Oncol Lett. 2013;5:1836–1838. doi: 10.3892/ol.2013.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bula G, Waler J, Niemiec A, Koziolek H, Bichalski W, Gawrychowski J. Diagnosis of metastatic tumours to the thyroid gland by fine needle aspiration biopsy. Endokrynol Pol. 2010;61:427–9. [PubMed] [Google Scholar]
- 12.Zhu YZ, Li WP, Wang ZY, Yang HF, He QL, Zhu HG, et al. Primary pulmonary adenocarcinoma mimicking papillary thyroid carcinoma. J Cardiothorac Surg. 2013;8:131. doi: 10.1186/1749-8090-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med. 2008;132:384–96. doi: 10.5858/2008-132-384-AOITTD. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, Katzenstein AL. Comparison of monoclonal napsin A, polyclonal napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomas. Am J Clin Pathol. 2012;138:703–11. doi: 10.1309/AJCPKVBXTI9O3TEM. [DOI] [PubMed] [Google Scholar]
- 15.Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: Evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163–71. doi: 10.5858/arpa.2011-0320-OA. [DOI] [PubMed] [Google Scholar]