Abstract
Gabapentin, a drug used in the treatment of epileptic seizures and neuropathic pain, has shown efficacy in the treatment of alcohol dependence. Moreover, given that gabapentin is used in the general population (e.g., non-dependent individuals, social drinkers), we sought to utilize preclinical assessments to examine the effects of gabapentin on sensitivity to moderate alcohol doses and alcohol self-administration in rats with a history of moderate drinking. To this end, we assessed whether gabapentin (0, 10, 30, 120 mg/kg, IG) pretreatment alters sensitivity to experimenter- and self-administered alcohol, and whether gabapentin alone has alcohol-like discriminative stimulus effects in rats trained to discriminate a moderate alcohol dose (1 g/kg, IG) vs. water. Second, we assessed whether gabapentin (0, 10, 30, 60 mg/kg, IG) would alter alcohol self-administration in rats with a history of moderate alcohol consumption. Gabapentin pretreatment potentiated the interoceptive effects of both experimenter-administered and self-administered alcohol in discrimination-trained rats. Additionally, the highest gabapentin doses tested (30 and 120 mg/kg) were found to have partial alcohol-like discriminative stimulus effects when administered alone (e.g., without alcohol). In the self-administration trained rats, gabapentin pretreatment (60 mg/kg) resulted in an escalation in alcohol self-administration. Given the importance of interoceptive drug cues in priming and maintaining self-administration, these data define a specific behavioral mechanism (i.e., potentiation of alcohol effects) by which gabapentin may increase alcohol self-administration in non-dependent populations.
Keywords: drug discrimination, self-administration, gabapentin, alcohol
1.1 Introduction
Alcohol use disorders (AUDs) continue to be a significant health and societal burden. As such, there is continued interest in novel pharmacological treatment approaches for AUDs (Muller et al., 2014). Moreover, pharmacological compounds that are already FDA approved for other uses are especially attractive targets. To this end, there has been growing interest in gabapentin (Neurontin), a drug currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of epileptic seizures and neuropathic pain. Gabapentin has been used for the treatment of alcohol withdrawal in alcoholics (Mirijello et al., 2015) and initial studies showed efficacy in the maintenance of abstinence and sleep disturbances in alcohol-dependent individuals (Furieri and Nakamura-Palacios, 2007, Brower et al., 2008, Karam-Hage and Brower, 2000, Anton et al., 2011). Furthermore, in a recent double-blind controlled randomized clinical trial gabapentin treatment resulted in increased rates of abstinence and number of no heavy drinking days, and had positive effects on craving, sleep, and mood (Mason et al., 2014).
Gabapentin is a GABA analog that is structurally similar to GABA, however, it does not bind to GABA receptors and does not affect GABA uptake or degradation (Taylor et al., 1998, Sills, 2006). Gabapentin has been shown to bind with high affinity to α2δ-1 subunit of voltage-gated calcium channels (VGCCs; (Brown and Randall, 2005, Uchitel et al., 2010) and consequently gabapentin actions at presynaptic α2δ-1-containing VGCCs result in its ability to reduce postsynaptic excitability and decrease the release of excitatory neurotransmitters (Cunningham et al., 2004, Dooley et al., 2002, Fehrenbacher et al., 2003). Interestingly, gabapentin has a different electrophysiological and behavioral profile under alcohol-dependent and non-dependent conditions (Roberto et al., 2008), which emphasizes the relevance and importance of alcohol dependence-induced neuroadaptations on gabapentin efficacy. Additionally, this suggests that in non-dependent populations (e.g., social drinkers) gabapentin may have different behavioral consequences, which is important given that gabapentin is commonly prescribed. Accordingly, one of the goals of the present work was to examine the effects of gabapentin on sensitivity to moderate alcohol doses and alcohol self-administration in rats with a history of moderate alcohol drinking.
Interestingly, to date, characterization of the effects of gabapentin on the discriminative stimulus (i.e., interoceptive) effects of alcohol has not been reported. Such characterization is highly relevant given that the interoceptive drug cues can impact drug taking, seeking and relapse-like behaviors (Paulus and Stewart, 2014, Verdejo-Garcia et al., 2012, Wise et al., 2008, Stolerman, 1992). Therefore, in male Long Evan rats trained to discriminate a moderate dose of alcohol (1 g/kg, IG) from water (IG) using well-characterized drug discrimination methods, the present study sought to determine whether gabapentin would alter the discriminative stimulus effects of alcohol, have alcohol-like effects, and whether sensitivity to the discriminative stimulus of self-administered alcohol would be altered.
Additionally, surprisingly few preclinical studies have examined the effects of gabapentin on alcohol self-administration. In one study, gabapentin was found to reduce alcohol self-administration in alcohol-dependent rats, with no effect in non-dependent control rats (Roberto et al., 2008). Pregabalin (Lyrica), a successor to gabapentin with a similar basic chemical structure and therapeutic profile, was found to reduce alcohol self-administration in alcohol-preferring Marchigian Sardinian (msP) rats (Stopponi et al., 2012). Therefore, in parallel to the discrimination studies, we sought to determine whether gabapentin would alter maintenance of ongoing alcohol self-administration, in male Long Evans rats with a history of moderate alcohol consumption. Based on the mechanism of action of gabapentin, we hypothesized that the compound would have alcohol-like effects and further, that self-administration would be reduced by gabapentin pretreatment. Together, the goal of this experimental approach was that examination of the effects of gabapentin on the discriminative stimulus of alcohol may provide a behavioral mechanism to inform and expand our understanding of potential gabapentin-induced changes in alcohol self-administration in rats with a history of moderate alcohol consumption.
1.2 Methods
1.2.1 Animals
Male Long Evans rats (Harlan Sprague–Dawley, Indianapolis, IN) were single (discrimination studies) or double (self-administration studies) housed in ventilated cages. Food intake was regulated to maintain body weight between 325 and 340 g for all the discrimination studies. Water was available ad libitum in the home cage. The colony room was maintained on a 12-h light/dark cycle, with lights on at 07:00. All experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines. As described below, several strategies (within subject testing; used in multiple experiments) were taken throughout this work to reduce the number of animals needed for the conduct of the studies.
1.2.2 Behavioral Training Procedures
Alcohol discrimination training
Rats were trained to discriminate a moderate dose of alcohol (1 g/kg, IG) from water using a standard two-lever operant procedure as previously described (Randall et al., 2015, Besheer et al., 2014, Jaramillo et al., 2015). Briefly, alcohol (1 g/kg) or water was administered IG and immediately afterwards rats were placed in the chambers. Following a 10-min delay, both levers were introduced into the chamber and the house light was illuminated, signaling the start of a 15-min session. Training days varied on a double alternation schedule (alcohol, alcohol, water, water, …). During an alcohol session, completion of a fixed ratio 10 (FR10) on the alcohol-appropriate lever (e.g., right lever) resulted in the delivery of a sucrose reinforcer (10% w/v; 0.1 ml) and during a water session, completion of an FR10 on the water-appropriate lever (e.g., left lever) resulted in reinforcement delivery. The following accuracy criteria were met prior to testing: the percentage of appropriate lever responses before the first reinforcer, and during the entire session was >80% for at least 8 out of 10 consecutive days.
Testing
Test sessions were similar to training sessions except that they were 2 minutes in duration (after the 10-min delay), and an FR10 on either lever resulted in sucrose delivery. Sucrose reinforcement was delivered to examine the effects of gabapentin treatment on overall response rates. For testing, cumulative dosing or single dose procedures were used as indicated in the Experiments. Cumulative alcohol dose testing procedures (Schechter, 1997) were similar to those we previously describe (Besheer et al., 2014, Besheer et al., 2012, Cannady et al., 2011). Briefly, to determine the alcohol curve (0.1, 0.3, 1.0, 1.7 g/kg, IG), rats initially received 0.1 g/kg alcohol and were placed in the chamber for the 10-min pre-session delay and 2 min test session. At the conclusion of the session, the rats received a subsequent alcohol administration of 0.2 g/kg and another test session. This procedure was repeated with two subsequent administrations of 0.7 g/kg alcohol, which are additive to produce the stated dose range. Thus, testing of the alcohol dose curve was completed in ~48 min. Test sessions were interspersed with training sessions and only occurred when performance during 3 out of 4 consecutive training sessions met accuracy criteria. No more than two test sessions were conducted per week.
Alcohol self-administration training
Rats were trained using the same two lever behavioral chambers and procedures as in previous studies from this laboratory (Randall et al., 2015, Besheer et al., 2013), with the exception that rats had an alcohol lever and an inactive lever. Locomotor activity was measured during the self-administration sessions by infrared photobeams that divided the behavioral chamber into 4 parallel zones. Self-administration sessions (30 min) took place 5 days/week (M–F) with the alcohol lever on a fixed ratio 2 (FR2) schedule of reinforcement such that every second response on the lever resulted in delivery of alcohol (0.1 ml) into a liquid receptacle. Responses on the inactive lever were recorded, but produced no programmed consequences. A sucrose fading procedure was used in which alcohol was gradually added to a 10% (w/v) sucrose solution. The exact order of exposure was as follows: 10 % sucrose (w/v)/2 % (v/v) alcohol (10S/2A), 10S/5A, 10S/10A, 5S/10A, 5S/15A, 2S/15A. There were two sessions at each concentration. Following sucrose fading, sweetened alcohol (2S/15A) continued as the reinforcer for the remainder of the study. Based on our previous findings using similar self-administration procedures, we typically observe moderate daily alcohol intake ranging from 0.5–0.8 g/kg (Randall et al., 2015, Besheer et al., 2013) and corresponding to approximately 40 mg/dl when blood alcohol concentration is measured immediately after the 30 min session (Besheer et al., 2013).
1.2.3 Experiments
Experiment 1. Effects of gabapentin on the discriminative stimulus effects of experimenter-administered alcohol
The goal of this experiment was to determine whether gabapentin would alter the discriminative stimulus effects of alcohol in discrimination-trained rats (n=6). All rats used in this experiment were also used in Experiment 2. Gabapentin (0, 10, 30, 120 mg/kg, IG) was administered 1 hour prior to the start of a cumulative alcohol dose response curve (0.1, 0.3, 1.0, 1.7 g/kg, IG). A repeated measures design was utilized such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
Experiment 2. Discriminative stimulus effects of gabapentin alone
To determine whether gabapentin has alcohol-like discriminative stimulus effects in discrimination-trained rats (n=6). Gabapentin (0, 10, 30, 120 mg/kg, IG) was administered 1 hour prior to Water (1 g/kg, IG), after which rats were placed in the chambers for a test session. A repeated measures design was utilized such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
Experiment 3. Effects of gabapentin on the discriminative stimulus effects of self-administered alcohol
Sweetened alcohol reinforcer
To determine whether gabapentin would alter the discriminative stimulus effects of self-administered alcohol, a discrimination/self-administration (Discrim/SA) test session was conducted as detailed in (Besheer et al., 2012, Besheer et al., 2006). These test sessions differed in duration (30 min) and reinforcer (sweetened alcohol solution; 10%, w/v sucrose+10%, v/v alcohol) from the standard test sessions; however, as in the standard test session behavior was free to vary between the two levers since completion of an FR10 on either lever resulted in reinforcer presentation. Briefly, in these sessions, following water (IG) administration, rats begin the session responding predominantly on the water-appropriate lever; as the session continues and rats have consumed significant amounts of the sweetened alcohol reinforcer, responding shifts to the alcohol-appropriate lever, indicating that the interoceptive effects of the consumed alcohol are detected by the animal (i.e., behavior under discriminative stimulus control of alcohol). For testing, gabapentin (0, 10, 30, 120 mg/kg, IG; n=8) was administered 1 hour prior to the start of a Discrim/SA session. Water (IG) was administered prior to placement in the chambers. Some (n=5) of the rats used in Experiments 1 and 2 were also used in this experiment. A repeated measures design was utilized such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
Sucrose reinforcer
To assess gabapentin effects alone under the same testing conditions, the same group of rats (n=8) was tested using the Discrim/SA procedure detailed above with the exception that the standard sucrose (10% w/v) reinforcer was used (i.e., no alcohol was added). Rats received gabapentin (0, 10, 30, 120 mg/kg, IG) 1 hour prior to the start the Discrim/SA test session. Water (IG) was administered prior to placement in the chambers. A repeated measures design was utilized such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
Experiment 4. Effects of gabapentin on maintenance of alcohol self-administration
Prior to initiation of testing, rats had 5 months of self-administration training. Baseline alcohol intake (mean±S.E.M. of 2 sessions prior to initiation of testing) was 0.83±0.04 g/kg. To assess the effects of gabapentin on the maintenance of alcohol self-administration, on test days, rats received gabapentin (0, 10, 30, 60 mg/kg, IG; n=8) 1 h prior to a self-administration session. Doses were assigned in a repeated measures design with each rat receiving all treatments in a randomized order, with at least two baseline sessions between testing days.
1.2.4 Drugs and Dosing
For the discrimination experiments, alcohol (95 %, w/v, Pharmaco-AAPER, Shelbyville, KY) was diluted in distilled water to a concentration of 20% (v/v) and administered IG, with volumes varied to obtain the desired dose. For the self-administration experiment, alcohol and sucrose were diluted with distilled water to the appropriate concentrations. Gabapentin was dissolved in distilled water and administered IG at a volume of 1 ml/kg with a 1 h pretreatment interval. Gabapentin dose ranges and pretreatment intervals were selected based on previous work (Filip et al., 2007, Roberto et al., 2008, McDonald et al., 2008) and pilot experiments. In pilot experiments 240 mg/kg gabapentin produced significant response rate reductions in discrimination-trained rats and as such, 120 mg/kg was the highest dose selected.
1.2.5 Data analysis
For the discrimination experiments (Experiments 1 and 2), response accuracy was expressed as the percentage of alcohol-appropriate responses upon delivery of the first reinforcer. For the Discrim/SA tests (Experiments 3), response accuracy was expressed as percentage of alcohol-appropriate lever presses across the session, and alcohol (g/kg) and sucrose (ml) intake was estimated from the number of delivered reinforcers. For all discrimination experiments, response rate (responses/min) was analyzed for the entire session and served as an index of motor activity. Complete expression of the discriminative stimulus effects of alcohol (i.e., full substitution) was defined as >80% choice of the alcohol lever upon completion of the first FR10 during test sessions. Partial substitution for the alcohol training dose was defined as >40% and <80% alcohol-appropriate responses (Glennon and Young, 2011, Solinas et al., 2006). For the self-administration experiment (Experiment 4), alcohol intake (g/kg) was approximated based on body weight and number of reinforcements delivered. For all studies, one- or two-way repeated measures analysis of variance (RM ANOVA) were used to analyze data as appropriate. Post hoc analysis (Tukey) was used to determine differences between specific treatment conditions. Statistical significance was declared at P≤0.05.
1.3 Results
Experiment 1. Effects of gabapentin on the discriminative stimulus effects of experimenter-administered alcohol
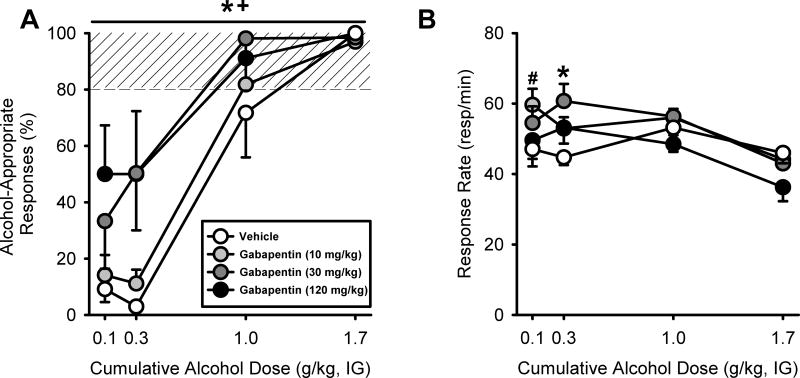
Gabapentin pretreatment increased sensitivity to the discriminative stimulus effects of alcohol (Figure 1A), as confirmed by a two-way RM ANOVA that showed a significant gabapentin-induced potentiation of alcohol-appropriate responses [F(3,15]=3.44, p<0.04) with increased alcohol-appropriate responses following the highest gabapentin doses (30 and 120 mg/kg). Partial substitution for the alcohol training dose was observed at the low alcohol doses (0.1 and 0.3 mg/kg) following the highest gabapentin dose (120 mg/kg), and at the 0.3 mg/kg alcohol dose following 30 mg/kg gabapentin pretreatment. Additionally, confirming alcohol discriminative stimulus control, a dose-dependent increase in alcohol-appropriate responses was observed [F(3,15)=45.61, p <0.001]. No significant interaction was observed. Response rate was affected by gabapentin pretreatment as confirmed by a two-way RM ANOVA (Figure 1B). A significant main effect of gabapentin dose [F(3,15)=4.08, p<0.03], alcohol dose [F(3,15)=5.11, p<0.01], and a significant interaction were observed [F(9,45)=2.71, p<0.01]. Gabapentin pretreatment (10 and 30 mg/kg) increased response rates at the lowest alcohol doses (0.1 and 0.3 g/kg), respectively relative to vehicle (p<0.02). Additionally, there was a trend for a response rate reduction at the highest alcohol dose (1.7 g/kg) following pretreatment with the highest gabapentin dose (120 mg/kg; p<0.08). Together, these results show that gabapentin pretreatment potentiates the discriminative stimulus effects of low alcohol doses.
Figure 1.
Discriminative stimulus effects of alcohol following gabapentin pretreatment. Mean (±SEM) alcohol-appropriate responses (A) and response rate (B) following gabapentin pretreatment prior to testing a cumulative alcohol dose range (0.1 – 1.7 g/kg, IG) in rats trained to discriminate alcohol (1 g/kg, IG) vs. water (n=6). Partial substitution (>40% alcohol-appropriate responses) for the alcohol training dose was observed at the low alcohol doses (0.1–0.3 g/kg, IG) following gabapentin pretreatment (120 mg/kg, IP), and at 0.3 g/kg following 30 mg/kg gabapentin pretreatment. Gabapentin pretreatment (10 and 30 mg/kg) increased response rates at the lowest alcohol doses (0.1 and 0.3 g/kg), respectively relative to vehicle (p<0.02). Shaded area (>80%) represents full expression of the discriminative stimulus effects of alcohol. *p<0.05 (30 mg/kg gabapentin vs. vehicle); +p<0.05 (120 mg/kg gabapentin vs. vehicle); #p<0.05 (10 mg/kg gabapentin vs. vehicle).
Experiment 2. Discriminative stimulus effects of gabapentin alone
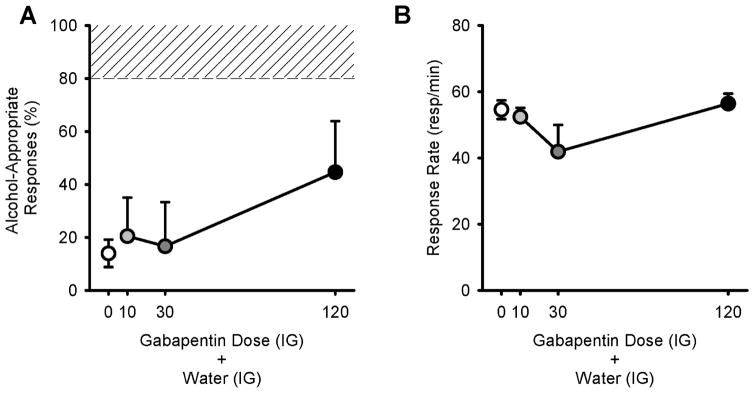
Gabapentin administered alone did not have full alcohol-like discriminative stimulus effects, as alcohol-appropriate responses were below the substitution threshold (i.e., < 80% alcohol-appropriate responses; Figure 2A). Additionally, the one-way RM ANOVA showed no significant gabapentin effects on alcohol-appropriate responses. However, partial substitution for the alcohol training dose was observed at the highest gabapentin dose (120 mg/kg) as alcohol-appropriate responses were 44.7±19.2%. Responses rates were not significantly affected by gabapentin pretreatment (Figure 2B).
Figure 2.
Discriminative stimulus effects of gabapentin alone. Mean (±SEM) alcohol-appropriate responses (A) and response rate (B) following gabapentin administration in rats trained to discriminate alcohol (1 g/kg, IG) vs. water (n=6). Gabapentin alone did not have an overall effect on the discriminative stimulus effects of alcohol or response rate; however, partial substitution for the alcohol training dose was observed at the highest gabapentin dose (120 mg/kg, IP). Shaded area (>80%) represents full expression of the discriminative stimulus effects of alcohol.
Experiment 3. Effects of gabapentin on the discriminative stimulus effects of self-administered alcohol
Sweetened alcohol reinforcer
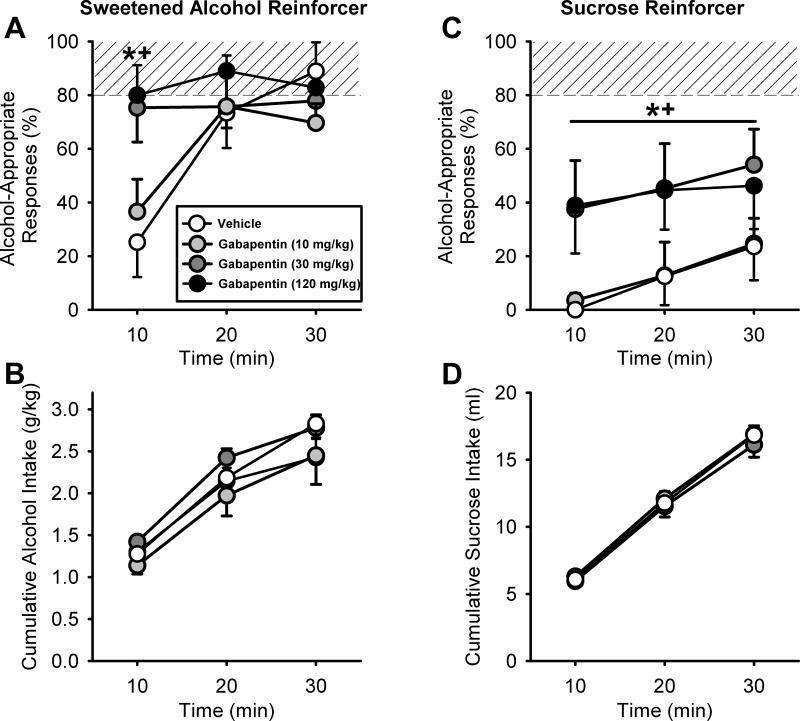
Addition of alcohol (10%, v/v) to the sucrose (10%, w/v) reinforcer resulted in an increase in alcohol-appropriate responding across the session, indicating that behavior was under discriminative stimulus control of the consumed alcohol. In the vehicle controls and the lowest gabapentin dose (10 mg/kg), almost full substitution for the alcohol training dose was observed 20 min into the session. In contrast, following the highest gabapentin doses (30 and 120 mg/kg) almost full substitution was observed 10 min into the session. This was confirmed by a significant main effect of time [F(2,14)=6.65, p<0.008], and a significant interaction [F(6,36)=2.61, p<0.03]. Gabapentin pretreatment (30 and 120 mg/kg) significantly increased alcohol-appropriate responses during the first 10 min (p<0.05), indicating potentiated sensitivity to alcohol early in the session. Importantly, gabapentin pretreatment did not affect alcohol intake (g/kg; Figure 3B). Alcohol intake (g/kg) increased across time [F(2,14)=87.40, p<0.001]. Thus, the potentiation of the discriminative stimulus effects of alcohol within the first 10 min of the session following gabapentin pretreatment (Figure 3A), was directly related to increased sensitivity to the discriminative stimulus effects of the consumed alcohol and not to differences in the alcohol dose consumed. These findings support and extend the findings in Experiment 1 with experimenter-administered alcohol, to show that gabapentin pretreatment also potentiates sensitivity to the discriminative stimulus effects of consumed/self-administered alcohol.
Figure 3.
Discriminative stimulus effects of self-administered alcohol following gabapentin pretreatment. Mean (±SEM) alcohol-appropriate responses (A) and cumulative alcohol intake (g/kg; B) in 10-min intervals for the sweetened alcohol (10% w/v sucrose/10% v/v alcohol) test session (i.e., sweetened alcohol reinforcer). Following pretreatment with the highest gabapentin doses (30 and 120 mg/kg, IP) a significant increase in alcohol-appropriate responses was observed during the first 10 min, indicating potentiation of sensitivity to the discriminative stimulus effects of the consumed alcohol. Mean (±SEM) alcohol-appropriate responses (C) and cumulative sucrose intake (ml; D) in 10-min intervals for the sucrose (10% w/v) test session (i.e., sucrose reinforcer). Overall alcohol-appropriate responses were significantly increased following gabapentin pretreatment (30 and 120 mg/kg, IP) indicating partial alcohol-like effects in the absence of alcohol. Rats were used in both assessments and were trained to discriminate alcohol (1 g/kg, IG) vs. water (n=8). Shaded area (>80%) represents full expression of the discriminative stimulus effects of alcohol. *p<0.05 (30 mg/kg gabapentin vs. vehicle); +p<0.05 (120 mg/kg gabapentin vs. vehicle).
Sucrose reinforcer
As shown in Figure 3C, following vehicle pretreatment alcohol-appropriate responding remained low throughout the session given that sucrose (10% w/v) was the reinforcer (i.e., no alcohol was present in the solution). Interestingly, gabapentin pretreatment potentiated alcohol-appropriate responding (i.e., partial substitution) as confirmed by the two-way RM ANOVA showing a significant main effect of gabapentin dose [F(3,21)=4.87, p=0.01], with overall greater alcohol-appropriate responses at the two highest gabapentin doses (30 and 120 mg/kg; p<0.05). As shown in Figure 3D, sucrose intake (ml) increased across the session [F(2,14)=510.63, p<0.001], with no significant main effect of gabapentin dose. These results are consistent with the findings of Experiment 1 showing that in the absence of alcohol, gabapentin alone (specifically, 120 mg/kg) has some partial alcohol-like effects.
Experiment 4. Effects of gabapentin on maintenance of alcohol self-administration
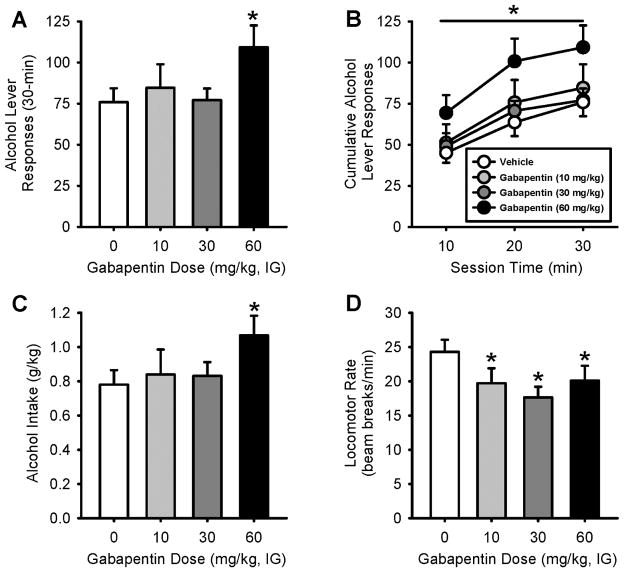
Gabapentin pretreatment significantly increased alcohol self-administration (i.e., alcohol lever responses) as confirmed by a RM ANOVA [F(3,21)=3.74, p=0.03; Figure 4A], with significantly greater alcohol lever responses at the highest gabapentin dose (60 mg/kg) relative to vehicle (p<0.05). A corresponding increase in alcohol intake (g/kg) was also observed [F(3,21)=2.94, p=0.05; Figure 4C]. Analysis of cumulative alcohol responses across the session showed a significant main effect of gabapentin dose [F(3,42)=3.83, p<0.05, Figure 4B], with significantly greater alcohol lever responses at the highest gabapentin dose (60 mg/kg) compared to vehicle. A significant main effect of session time was also observed [F(2,42)=66.108, p < 0.001]. There was no significant interaction. Inactive lever responses were not affected by gabapentin pretreatment [mean ± S.E.M. 0: 1.0 ± 0.26; 10: 2.12 ± 0.85; 30: 1.0 ± 0.38; 60: 1.37 ± 0.53]. Interestingly, the increase in alcohol lever responses occurred despite a significant gabapentin-induced decrease in locomotor rate [F(3,21)=8.47, p<0.001, Figure 4D]. Post-hoc analysis showed that locomotor rate was decreased by all doses of gabapentin compared to vehicle (p<0.05).
Figure 4.
Effects of gabapentin on alcohol self-administration. Mean (±SEM) alcohol lever responses (A), alcohol lever responses across the 30-min session (B), alcohol intake (g/kg; C), and locomotor rate (D) following gabapentin pretreatment (n=8). *p<0.05 different from vehicle. A significant increase in alcohol lever responses and alcohol intake (g/kg) was observed following gabapentin pretreatment (60 mg/kg, IG). The increase in alcohol lever responses was evident throughout the 30-min session. Locomotor rate during the self-administration sessions was significantly reduced at each gabapentin dose tested. *p<0.05 (vs. vehicle).
1.4 Discussion
The goal of the present work was to characterize the effects of gabapentin on the discriminative stimulus effects of alcohol and alcohol self-administration. In rats trained to discriminate alcohol (1 g/kg) vs. water, gabapentin pretreatment potentiated the discriminative stimulus effects of low alcohol doses, in two separate assessments (experimenter- and self-administered alcohol, Experiments 1 and 3, respectively). Moreover, gabapentin was found to have partial alcohol-like effects when administered alone (e.g., without alcohol; Experiments 2 and 3). Interestingly, in separate rats with a history of alcohol self-administration, gabapentin pretreatment resulted in an escalation in alcohol self-administration.
Gabapentin (30 and 120 mg/kg) administered prior to experimenter-administered alcohol potentiated sensitivity to low alcohol doses (0.1 and 0.3 g/kg, IG; Figure 1A), with rats showing partial substitution for the alcohol training dose (1 g/kg). Given that gabapentin (specifically 120 mg/kg) produced partial substitution when administered alone (in the absence of alcohol – Experiment 2), the gabapentin-induced potentiation of alcohol effects is likely driven by a pharmacological interaction between alcohol and gabapentin. Indeed, increased sensitivity to the effects of self-administered alcohol was observed following gabapentin pretreatment (30 and 120 mg/kg) when alcohol (10% v/v) was added to the standard sucrose reinforcer (Figure 3A). That is, by 10 min into the test session, at which point estimated alcohol intake for all treatment conditions was in the 1–1.5 g/kg range (Figure 3B), almost full substitution for the training dose was observed in rats pretreated with the highest gabapentin doses (30, 120 mg/kg). The advantage of this assessment is that animals consume pharmacologically relevant levels of alcohol rapidly. In doing so, the interoceptive effects of the consumed alcohol drive a shift in lever selection, which is a strong demonstration of discriminative stimulus control and consistent with prior work (Besheer et al., 2006, Besheer et al., 2012, Hodge et al., 2001). Importantly, alcohol intake (directly estimated from response rate), did not differ between the gabapentin doses (Figure 3B). Therefore, the gabapentin-induced potentiation of alcohol-appropriate responding was not due to a direct increase in the consumed dose of alcohol, which would, by definition, result in greater or more detectable interoceptive effects. Rather, gabapentin potentiated sensitivity to the interoceptive effects of the consumed alcohol. Interestingly, in the absence of alcohol in the reinforcer (i.e., sucrose reinforcer – Figure 3C), partial substitution for the alcohol training dose was observed at those same gabapentin doses (30 and 120 mg/kg). This data pattern lends further support to the premise that the partial alcohol-like effects of gabapentin contribute to the observed potentiation of sensitivity to alcohol when alcohol is administered (experimenter- or self-administered).
Interestingly, in Experiments 2 and 3 (both under sucrose reinforcer conditions), gabapentin (120 mg/kg) administered alone resulted in partial substitution for alcohol. Additionally, partial substitution for alcohol was also observed at 30 mg/kg gabapentin in Experiment 3 (Figure 3C), but not Experiment 2 (Figure 2A). The difference in substitution patterns can likely be accounted for by the slightly different manner in which the dependent variables are generated across the two assessments. That is, during the standard substitution test (Figure 2A), alcohol-appropriate responses are determined prior to delivery of the first reinforcer. In contrast, given the nature of the sweetened alcohol reinforcer assessment (Figure 3C) in which dynamic behavioral patterns are monitored to capture the change in the discriminative stimulus effects of the consumed alcohol, alcohol-appropriate responses reflect ongoing behavior throughout the entire session.
To our knowledge, the discriminative stimulus effects of gabapentin have not been previously characterized in regards to alcohol stimulus effects. The present findings of potentiation of low alcohol doses, suggest in part, some pharmacological overlap with alcohol. The therapeutic mechanism of action for gabapentin is likely through binding at the α2δ subunit of VGCC (Gee et al., 1996), which are present in L-type channels and can regulate presynaptic-release in brain N- and P/Q-type channels (Catterall et al., 2005, Dolphin, 2013). Functional studies have demonstrated gabapentin-induced inhibition of presynaptic VGCCs and attenuation of neurotransmitter release, including glutamate (Meder and Dooley, 2000, Dooley et al., 2002, van Hooft et al., 2002, Fink et al., 2000, Quintero et al., 2011). Therefore, it is not entirely surprising that gabapentin-induced CNS inhibition can potentiate alcohol-like effects and have partial alcohol-like effects alone. That is, even though the alcohol discriminative stimulus is a multi-component cue modulated by a variety of different receptor systems, pharmacological manipulations that, in general, promote neural inhibition or reduce neuronal excitation tend to have alcohol-like effects or can potentiate the discriminative stimulus effects of alcohol (Kostowski and Bienkowski, 1999, Grant, 1999). Interestingly, examination of the contribution of L-type VGCC to alcohol discriminative stimulus effects has found that antagonists of L-type VGCC tend not to have alcohol-like discriminative stimulus effects (De Beun et al., 1996, Green and Grant, 1999). However, they can modulate aspects of the discriminative stimulus effects of alcohol (Colombo et al., 1994, Green and Grant, 1999, Green-Jordan and Grant, 2000) and this interaction can be related to the components of the alcohol cue (GABAA or NMDA receptors), and alcohol training doses (Green-Jordan and Grant, 2000). Together, the present work showing that gabapentin has alcohol-like effects and potentiates the alcohol-like effects of low alcohol doses, suggests that the α2δ subunit may play a role in modulating interoceptive sensitivity to alcohol.
In a study in a non-treatment seeking alcoholic population, gabapentin treatment did not change reports of subjective high or intoxication as assessed by the Subjective High Assessment Scale (SHAS) and Biphasic Alcohol Effects Scale (BAES) in a bar-lab setting (Myrick et al., 2007). The lack of an interaction between gabapentin on subjective sensitivity to alcohol is in contrast to the present findings in which gabapentin potentiated the interoceptive effects of low alcohol doses. Clearly it is difficult to directly compare the results from the bar-lab study to that of the present preclinical work; however, it is interesting to note that gabapentin label indications recommend avoiding or minimizing alcohol consumption while taking the drug due to potentiation of side effects such as sleepiness and dizziness. Such gabapentin-induced potentiation of alcohol effects is consistent with the results of the present discrimination assessments. Moreover, given the relevance of alcohol dependence-induced neuroadaptations to gabapentin efficacy (see later discussion), it is possible that an alcohol-dependent population may not show gabapentin-potentiated sensitivity to alcohol relative to a non-dependent population. Indeed, in addition to a favorable safety profile, no drug substitution or gabapentin misuse was observed in the double-blind, randomized clinical trial in alcohol-dependent subjects (Mason et al., 2014).
Interestingly, in the present work gabapentin pretreatment (60 mg/kg) increased alcohol self-administration, with parallel reductions in locomotor rate (Experiment 4; Figure 4A,B,D). While simultaneous measurement of locomotor activity within the self-administration context is advantagous, assessment of the effects of gabapentin on spontaneous locomotor behavior may be better evaluated in an open field. Indeed, prior work has shown no effects on open field locomotor behavior at similar or higher doses (Watson et al., 1997, Itzhak and Martin, 2000); but see (Munro et al., 2012). Moreover, the escalation in alcohol self-administration was not entirely expected, as previous preclinical work showed no effect of systemic gabapentin pretreatment on alcohol self-administration in non-dependent animals (Roberto et al., 2008). However, it is interesting to note that intra-amygdala (central nucleus; CeA) gabapentin pretreatment induced a non-significant tendency for increased alcohol self-administration in non-dependent rats, while reducing self-administration in alcohol-dependent rats (Roberto et al., 2008). The authors relate this data pattern to be consistent with opposing electrophysiological effects of gabapentin in the CeA of non-dependent and dependent rats (i.e., increases in GABA current vs. decreases in ethanol-induced GABA current following gabapentin application, respectively). Therefore, it is possible that the self-administration conditions of the present work were able to reveal a gabapentin-induced increase in self-administration following gabapentin pretreatment. Indeed, there are differences between that work and the present work (i.e., route of gabapentin administration; fixed ratio requirements; rat strains) that may account for the different findings. Indeed, further replication of the present findings will be important.
One of the aims of the present work was to characterize the discriminative stimulus effects of gabapentin in parallel to the self-administration assessment in an effort to inform interpretation of any gabapentin-induced changes in drinking. A consideration to address is that the gabapentin dose (60 mg/kg) that increased alcohol self-administration was not specifically tested in the discrimination assessments. First, we were concerned that a high gabapentin dose (120 mg/kg) would result in a non-specific reduction in general locomotor activity in the self-administration trained rats. Second, given that 30 and 120 mg/kg gabapentin doses had similar effects in the discrimination studies, it is likely that a dose of 60 mg/kg would also potentiate alcohol discriminative stimulus effects. Therefore, a possible behavioral mechanism underlying the increase in alcohol self-administration may be related to drug priming. For example, in self-administration models, a priming injection of the self-administered drug or drugs with similar discriminative stimulus effects as the self-administered drug can potentiate (i.e., prime) drug-seeking behavior (Anker and Carroll, 2010, DiChiara and Reinhart, 1995, Fattore et al., 2003, Gerber and Stretch, 1975, de Wit and Stewart, 1981) and taking behavior (Norman et al., 1999, McKee et al., 2009, O’Malley et al., 2002, Brown et al., 2008). Such an explanation is supported by the discrimination assessments, in which gabapentin doses above 30 mg/kg had partial alcohol-like effects and potentiated the interoceptive effects of low alcohol doses. Therefore, gabapentin pretreatment may have served as a priming cue that contributed to initiation and subsequent enhancement of ongoing operant alcohol self-administration.
Together, the present work shows that gabapentin can potentiate the interoceptive effects of alcohol and increase alcohol self-administration. Given the importance of interoceptive drug cues in drug priming and maintaining self-administration, the current findings define a specific behavioral mechanism (i.e., potentiation of interoceptive alcohol effects) by which gabapentin may have the potential to increase alcohol drinking, specifically in non-dependent populations (e.g., social drinkers). Increasing availability of treatment options for AUDs is essential and with a favorable safety profile and efficacy at maintaining abstinence and treating relapse-related symptoms in alcohol-dependent individuals (Mason et al., 2014) gabapentin may be a promising candidate. Given its widespread and current indicated usage in treating seizure disorders and neuropathic pain, the present work suggests that it may also be an important consideration to examine the emergence of maladaptive drinking patterns in social drinkers currently treated with gabapentin.
Highlights.
Increased sensitivity to experimenter- and self-administered alcohol following gabapentin pretreatment
Gabapentin pretreatment has partial alcohol-like effects
Increased self-administration following gabapentin pretreatment
Acknowledgments
This work was supported, in part, by funds from the National Institutes of Health AA019682 (JB) and the Bowles Center for Alcohol Studies.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.5 References
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168:709–17. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology. 2012;220:809–22. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2376–86. doi: 10.1038/npp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology. 2013;72:139–47. doi: 10.1016/j.neuropharm.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. European journal of pharmacology. 2006;551:71–5. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcoholism, clinical and experimental research. 2008;32:1429–38. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JT, Randall A. Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse. 2005;55:262–9. doi: 10.1002/syn.20115. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learning & memory. 2008;15:857–65. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2328–38. doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacological reviews. 2005;57:411–25. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Fadda F, Gessa GL. Blockade of ethanol discrimination by isradipine. European journal of pharmacology. 1994;265:167–70. doi: 10.1016/0014-2999(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. The European journal of neuroscience. 2004;20:1566–76. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- De Beun R, Lohmann A, Schneider R, De Vry J. Comparison of the stimulus properties of ethanol and the Ca2+ channel antagonist nimodipine in rats. European journal of pharmacology. 1996;306:5–13. doi: 10.1016/0014-2999(96)00198-7. [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Dichiara TJ, Reinhart PH. Distinct effects of Ca2+ and voltage on the activation and deactivation of cloned Ca(2+)-activated K+ channels. The Journal of physiology. 1995;489(Pt 2):403–18. doi: 10.1113/jphysiol.1995.sp021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels. Biochimica et biophysica acta. 2013;1828:1541–9. doi: 10.1016/j.bbamem.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Meder WP, Whetzel SZ. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse. 2002;45:171–90. doi: 10.1002/syn.10094. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. The European journal of neuroscience. 2003;17:1723–6. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E, Vetulani J. Diverse effects of GABA-mimetic drugs on cocaine-evoked self-administration and discriminative stimulus effects in rats. Psychopharmacology. 2007;192:17–26. doi: 10.1007/s00213-006-0694-7. [DOI] [PubMed] [Google Scholar]
- Fink K, Meder W, Dooley DJ, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. British journal of pharmacology. 2000;130:900–6. doi: 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. The Journal of clinical psychiatry. 2007;68:1691–700. doi: 10.4088/jcp.v68n1108. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of biological chemistry. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacology, biochemistry, and behavior. 1975;3:1055–61. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug Discrimination: Practical Considerations. In: GLENNON RA, YOUNG R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. Somerset, NJ: John Wiley & Sons, Inc; 2011. [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacology, biochemistry, and behavior. 1999;64:261–7. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Green-Jordan K, Grant KA. Modulation of the ethanol-like discriminative stimulus effects of diazepam and phencyclidine by L-type voltage-gated calcium-channel ligands in rats. Psychopharmacology. 2000;149:84–92. doi: 10.1007/s002139900344. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Effects of L-type voltage-sensitive calcium channel modulators on the discriminative stimulus effects of ethanol in rats. Alcoholism, clinical and experimental research. 1999;23:806–14. [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacology. 2001;154:13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology. 2000;151:226–33. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, Fisher KR, Besheer J. Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol. 2015 doi: 10.1016/j.alcohol.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. The American journal of psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Bienkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA internal medicine. 2014;174:70–7. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald LM, Sheppard WF, Staveley SM, Sohal B, Tattersall FD, Hutson PH. Discriminative stimulus effects of tiagabine and related GABAergic drugs in rats. Psychopharmacology (Berl) 2008;197:591–600. doi: 10.1007/s00213-008-1077-z. [DOI] [PubMed] [Google Scholar]
- Mckee SA, Harrison EL, O’malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological psychiatry. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder WP, Dooley DJ. Modulation of K(+)-induced synaptosomal calcium influx by gabapentin. Brain research. 2000;875:157–9. doi: 10.1016/s0006-8993(00)02610-x. [DOI] [PubMed] [Google Scholar]
- Mirijello A, D’angelo C, Ferrulli A, Vassallo G, Antonelli M, Caputo F, Leggio L, Gasbarrini A, Addolorato G. Identification and Management of Alcohol Withdrawal Syndrome. Drugs. 2015 doi: 10.1007/s40265-015-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CA, Geisel O, Banas R, Heinz A. Current pharmacological treatment approaches for alcohol dependence. Expert opinion on pharmacotherapy. 2014;15:471–81. doi: 10.1517/14656566.2014.876008. [DOI] [PubMed] [Google Scholar]
- Munro G, Storm A, Hansen MK, Dyhr H, Marcher L, Erichsen HK, Sheykhzade M. The combined predictive capacity of rat models of algogen-induced and neuropathic hypersensitivity to clinically used analgesics varies with nociceptive endpoint and consideration of locomotor function. Pharmacology, biochemistry, and behavior. 2012;101:465–78. doi: 10.1016/j.pbb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31:221–7. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Hall JF, Tsibulsky VL. Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain research. 1999;831:165–74. doi: 10.1016/s0006-8993(99)01423-7. [DOI] [PubMed] [Google Scholar]
- O’malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–50. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, Dooley DJ, Pomerleau F, Huettl P, Gerhardt GA. Amperometric measurement of glutamate release modulation by gabapentin and pregabalin in rat neocortical slices: role of voltage-sensitive Ca2+ alpha2delta-1 subunit. The Journal of pharmacology and experimental therapeutics. 2011;338:240–5. doi: 10.1124/jpet.110.178384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology. 2015;232:2443–54. doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–71. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD. Discrete versus cumulative dosing in dose-response discrimination studies. European journal of pharmacology. 1997;326:113–8. doi: 10.1016/s0014-2999(97)85404-0. [DOI] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology. 2006;6:108–13. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nature protocols. 2006;1:1194–206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13:170–6. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, De Guglielmo G, Kallupi M, Cannella N, Gerra G, Massi M, Ciccocioppo R. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacology. 2012;220:87–96. doi: 10.1007/s00213-011-2457-3. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy research. 1998;29:233–49. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Uchitel OD, Di Guilmi MN, Urbano FJ, Gonzalez-Inchauspe C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels. 2010;4:490–6. doi: 10.4161/chan.4.6.12864. [DOI] [PubMed] [Google Scholar]
- Van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. European journal of pharmacology. 2002;449:221–28. doi: 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience and biobehavioral reviews. 2012;36:1857–69. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Watson WP, Robinson E, Little HJ. The novel anticonvulsant, gabapentin, protects against both convulsant and anxiogenic aspects of the ethanol withdrawal syndrome. Neuropharmacology. 1997;36:1369–75. doi: 10.1016/s0028-3908(97)00118-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]