Abstract
Detailed studies in animal models to assess the importance of aging animals in cardiovascular research are rather scarce. The increase in mouse models used to study cardiovascular disease makes the establishment of physiologic aging parameters in myocardial function in both male and female mice critical. Forty-four FVB/N mice were studied at multiple time points between the ages of 3 and 16 mo using high-frequency echocardiography. Our study found that there is an age-dependent decrease in several systolic and diastolic function parameters in male mice, but not in female mice. This study establishes the physiologic age- and gender-related changes in myocardial function that occur in mice and can be measured with echocardiography. We report baseline values for traditional echocardiography and advanced echocardiographic techniques to measure discrete changes in cardiac function in the commonly employed FVB/N strain.
Keywords: Aging, Myocardial function, Echocardiography, Strain imaging, Gender
INTRODUCTION
As humans age, there is a documented decrease in diastolic (Kuzentsova et al. 2009) and, to a lesser degree, systolic function (Scalia et al. 2010). It is suggested that these changes are associated with age-related left ventricular (LV) remodeling, including changes in mass, volume and geometry, as well as increased LV stiffness and increased interstitial collagen deposition (Bradshaw et al. 2010). It has also been found that increasing age is associated with a decrease in exercise capacity and resistance to ischemic injury (Lesnefsky et al. 1996; Willems et al. 2005). Though the mechanisms are not yet well established, advanced age and male gender are known to be major risk factors for cardiovascular diseases (Aguirre et al. 1994; Lakatta and Levy 2003; Rich 2006) and are two of the most important prognostic indicators in ischemic heart disease (Lopes et al. 2009).
With the development of genetically modified mice and the generation of acute and chronic infarction and heart failure models, the mouse has become a valuable tool in understanding the pathophysiology of cardiac diseases and in evaluating therapeutic targets (Christensen et al. 1997; Collins et al. 2003; Yutzey and Robbins 2007). Though many of the current murine studies describe the age of the mice as a major factor and most rely on “adult” mice, they do not typically consider gender (for a review, Chen and Rockamn 2008). We propose that because age and gender are important factors in human cardiovascular disease, they should be considered in murine studies. Most, but not all (Betsyaku et al. 2002; Haghighi et al. 2001; Stypmann et al. 2006), studies that have examined gender- or age-related changes have found that these factors play a significant role in cardiovascular pathophysiology in both wild-type (Hinton et al. 2008; Przyklenk et al. 2008) and genetically modified (Bradshaw et al. 2010; Dai et al. 2009; Olsson et al. 2001; Pereira et al. 2010; Reddy et al. 2007; Yang et al. 2008) mice.
Echocardiography has been used to assist basic research in cardiovascular disease for several decades (Scherrer-Crosbie and Kurtz 2010), and the few studies that have evaluated age-related changes with traditional echocardiography have, for the most part, confirmed these age and gender effects (Betsyaku et al. 2002; Dai and Rabinovitch 2009; Reddy et al. 2007). Traditionally, M-mode-derived values of heart function and size have been used, including LV volume, LV mass, ejection fraction (EF) and fractional shortening (FS), but with recent advances in imaging technology, including high-frequency probes and vector velocity algorithms to measure strain, advanced mouse echocardiography has emerged as an accurate non-invasive tool for the evaluation of myocardial function and morphology in mouse models beyond the standard measurements (Collins et al. 2001; Li et al. 2007; Luo and Konofagou 2008; Luo et al. 2006; Spurney et al. 2004; Zhang et al. 2007). We have incorporated the advances of high-resolution (approximately 30 mm) ultrasound- and vector velocity imaging (VVI)-based strain and displacement analysis in the non-invasive characterization of the cardiac phenotype for physiologic aging- and gender-related changes in wild-type (WT) FVB/N mice. This mouse strain was selected as it is commonly used in engineering transgenic mice and in several models of heart failure (Beetz et al. 2009; Qin et al. 2010; Rajan et al. 2010) and ischemia reperfusion (Van den Borne et al. 2009; Wittköpper et al. 2010).
There is no comprehensive study of the aging murine heart at multiple time points that would outline this process, and there is a need for a gender comparison between male and female mice as they age. Herein, we provide a thorough data set with mature adult mice (≤16 mo), at several time points and with a heart rate at near-physiologic levels (475–540 bpm); thus, our study may be used as reference for aging studies on murine models.
METHODS
Animal procedures were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati’s College of Medicine.
Materials
Forty-four WT (FVB/N) mice were divided into four different age groups, including young mice as a baseline (12 males and 10 females 3 mo old) and a mature adult subgroup (12 females and 10 males), which were followed at 12, 14 and 16 mo of age, allowing for progressive monitoring of their age-related changes with a total of 88 independent echocardiographic measurements. Two subgroups were used as the mice were part of control groups within other studies.
Echocardiography
Mice were anesthetized by direct inhalation of isoflurane (1.5%–2%), chest hair was removed and each mouse was placed on a heated platform (38 °C) with paws attached to electrocardiography (ECG) leads. A rectal probe was inserted to monitor core temperature, which was maintained at 36–37 °C with the use of a heating lamp and the heated platform.
A continuous tracing was obtained throughout the echocardiography study. Echocardiographic imaging was obtained using a Vevo 2100 imaging system (Visual-Sonics, Toronto, ON, Canada) with an MS400 (30-MHz centerline frequency) probe.
Both para-sternal long-axis (PSLAX) and short-axis (SAX) views in B-mode were obtained. The B-mode-guided M-mode view at the papillary muscle level was obtained for the evaluation of end-diastolic and end-systolic left ventricular wall and chamber dimensions in both the PSLAX and SAX views.
Pulse wave Doppler (PD) of mitral valve inflow was obtained from the PSLAX view, followed by tissue Doppler (TD) from the same view at the posterior wall adjacent to the mitral annulus. As in humans, the E wave (mitral valve early diastolic flow [MV E]) and A wave (atrial contraction flow [MV A]) were measured, as was the TD-obtained E′ wave (mitral valve early diastolic flow via TD [MV E′]) and A′ wave (atrial contraction flow via TD [MV A′]) as measures of diastolic function.
Using the PSLAX M-mode, the following parameters were measured: end-diastolic dimensions of the interventricular septum in diastole (IVS;d), posterior wall diameter in diastole (LVPW;d), intra-ventricular diameter in diastole (IVD;d), end-systolic dimensions of the intra-ventricular septum in systole (IVS;s), posterior wall diameter in systole (LVPW;s), intra-ventricular diameter in systole (IVD;s). Ejection fraction (EF), fractional shortening (FS), left ventricular end diastolic volume (LV Vol;d) and left ventricular end systolic volume (LV Vol;s) were calculated as previously described (Collins et al. 2003; Haghighi et al. 2001).
To compute VVI-derived parameters, SAX images were acquired at a frame rate greater than 200 frames per second at a depth between 4 and 11 mm. At this frame rate, at least 20 frames were captured per heart-beat, ensuring that no aliasing occurred. B-mode images from the SAX were transferred as 2-D gray-scale images to a separate computer station for image processing. Radial displacement and circumferential strain were calculated from the SAX view for each individual wall segment using VevoStrain software (Vevo 2100, Version 1.1.1 B1455, VisualSonics). The software creates vector velocity tracings from the acquired images. The strain and strain rate are calculated based on speckle tracking (Bauer 2011). This approach has been used for murine cardiac phenotyping and has compared favorably, though with more variability, with the more expensive myocardial strain imaging based on magnetic resonance imaging (Azam et al. 2012). To perform the measurement, the observer, blinded to the age and gender of the patients, loaded the B-mode cine loop into VevoStrain software. ECG measurements taken synchronously with the B-mode cine loop were used to select three to four cardiac cycles out of the cine loop. An M-mode trace across the heart of the selected cardiac cycles was visually inspected to qualitatively confirm that ultrasound imaging of heart contractility matched the ECG data. A frame during diastole was selected, and the endocardial border was traced by the observer. The epicardial border was automatically selected by the software and subsequently confirmed by the observer. The VVI software was initiated to compute the displacement, velocity, strain and strain rate. Appropriate speckle tracking was verified by manual inspection of the software-determined movement of the traced borders over three cardiac cycles. B-mode cine loops were selected to minimize image artifacts and ensure good visualization of the borders. The 2-D echo measurements were repeated by a second blinded observer, and inter-observer variability was calculated.
Statistical analysis
All data was collected and processed on SigmaPlot (Version 11, Systat, San Jose, CA, USA). A comparison between female and male groups was done with Student’s t-test; for age-related changes, a two-way analysis of variance for age and gender was performed on individual variables. Inter-observer variability for all measurements was assessed using the Z-test. The statistical software used was R Version 2.15.1. For all studies, p < 0.05 was considered statistically significant. Data are expressed as means ± standard errors of the mean where noted.
RESULTS
Technically adequate images were obtained for all animals at all time points for B-mode, M-mode and VVI imaging. Average time for obtaining each data set was between 7 and 10 min. Average heart rate by age and gender is presented in Tables 1 and 2, respectively. Average time for subsequent processing of the images, including VVI, was approximately 10 to 12 min. The total time dedicated to the capture and analysis of the study for each mouse at each time point was between 17 and 22 min. The additional time required was due to anechoic regions (i.e., regions without speckle) within the borders. In these cases, the decreased presence of speckle resulted in an increased processing time to achieve adequate speckle tracking and, thus, VVI. In all cases, proper tracking was visually confirmed by the observer within the VevoStrain software.
Table 1.
Echocardiography parameters combined for male and female FVB/N mice at different ages
| 3 mo | 12 mo | 14 mo | 16 mo | |
|---|---|---|---|---|
| Left ventricular volume (μL) | ||||
| Diastole, LV Vol;d | 59.91 ± 1.96 | 68.22 ± 2.99* | 68.54 ± 2.47* | 68.14 ± 3.10* |
| Systole, LV Vol;s | 23.02 ± 1.22 | 24.88 ± 1.17 | 27.46 ± 1.88 | 25.80 ± 2.07 |
| Left ventricular mass (μg) | 129.87 ± 7.56 | 149.34 ± 13.16 | 145.12 ± 12.37 | 147.92 ± 8.76 |
| Ejection fraction, EF (%) | 62.53 ± 1.08 | 63.97 ± 1.76 | 61.05 ± 1.71 | 61.82 ± 2.35 |
| Fractional shortening, FS (%) | 33.38 ± 0.78 | 33.97 ± 1.52 | 32.78 ± 1.20 | 33.22 ± 1.62 |
| Mitral valve atrial contraction flow, MV A (mm/s) | 479.79 ± 47.93 | 548.97 ± 75.32 | 622.02 ± 110.27 | 565.38 ± 100.37 |
| Mitral valve atrial contraction flow via tissue Doppler, MV A′ | 15.64 ± 1.44 | −19.01 ± 1.06 | −17.47 ± 1.99 | −19.41 ± 1.19 |
| Mitral valve early diastolic flow, MV E (mm/s) | 1050.17 ± 69.99 | 1359.95 ± 118.72 | 1267.68 ± 102.11 | 1280.21 ± 127.52 |
| Mitral valve early diastolic flow via tissue Doppler, MV E′ | −23.46 ± 1.58 | −30.02 ± 2.16 | −26.85 ± 1.69 | −27.12 ± 1.73 |
| MV E/MV E′ | −50.76 ± 5.30 | −47.94 ± 4.79 | −50.30 ± 4.44 | −47.11 ± 3.89 |
| Heart rate | 440.41 ± 12.43 | 452.36 ± 7.97 | 423.76 ± 9.38 | 454.2 ± 10.24 |
| Average circumferential strain (μm) | −21.25 ± 0.9 | −21.46 ± 1.14 | −18.06 ± 1.39 | −19.18 ± 3.28 |
| Average radial displacement (μm) | 0.39 ± 0.02 | 0.43 ± 0.02 | 0.39 ± 0.02 | 0.40 ± 0.06 |
p < 0.05 versus 3 mo.
Table 2.
Echocardiography parameters in female and male FVB/N mice at different ages
| 3 mo |
12 mo |
14 mo |
16 mo |
|||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Left ventricular volume | ||||||||
| Diastole, LV Vol;d (μL) | 59.87 ± 3.02 | 59.94 ± 2.64 | 67.14 ± 2.69 | 69.53 ± 2.28 | 58.30 ± 1.69 | 83.89 ± 2.61*,† | 58.58 ± 3.16 | 83.42 ± 3.82*,† |
| Systole, LV Vol;s (mL) | 23.35 ± 1.79 | 22.78 ± 1.68 | 23.29 ± 1.77 | 26.78 ± 1.37 | 20.44 ± 1.27 | 37.99 ± 2.63*,† | 17.96 ± 1.44 | 39.98 ± 3.13*,† |
| Left ventricular mass (μg) | 111.75 ± 5.40 | 152.59 ± 11.38 | 146.99 ± 28.59 | 151.38 ± 55.95 | 128.15 ± 14.64 | 165.56 ± 18.11 | 123.26 ± 7.04 | 179.63 ± 7.46* |
| Ejection fraction, EF (%) | 61.97 ± 1.62 | 62.97 ± 1.46 | 65.93 ± 1.76 | 61.62 ± 1.44 | 65.04 ± 1.92 | 55.07 ± 2.55† | 69.96 ± 1.57* | 52.85 ± 1.79*,† |
| Fractional shortening, FS (%) | 32.97 ± 1.18 | 33.70 ± 1.04 | 35.76 ± 1.52 | 31.82 ± 1.31 | 35.41 ± 1.40 | 28.82 ± 1.77 | 38.51 ± 1.59 | 26.93 ± 1.14† |
| Mitral valve atrial contraction flow, MV A (mm/s) |
381.1 ± 58.09 | 551.55 ± 64.82 | 403.325 ± 49.24 | 629.89 ± 106.66 | 410.92 ± 42.80 | 917.58 ± 197.39† | 657.16 ± 140. | 404.77 ± 98.15 |
| Mitral valve atrial contraction flow via tissue Doppler, MV A′ |
−15.28 ± 1.40 | −15.83 ± 2.33 - | −17.51 ± 1.38 | 20.50 ± 1.50 | −15.95 ± 1.44 | N/A | −21.40 ± 1.88 | −17.13 ± .88 |
| Mitral valve early diastolic flow, MV E (mm/s) |
1088.93 ± 127.71 | 1014.93 ± 71.40 | 1464.84 ± 206.22 | 1255.07 ± 120.91 | 1192.74 ± 145.17 | 1364.02 ± 143.88 | 1363.62 ± 184.25 | 1141.20 ± 148.54 |
| Mitral valve early diastolic flow via tissue Doppler, MV E′ |
−24.23 ± 1.30 | −22.83 ± 2.73 | −28.32 ± 3.83 | −31.71 ± 2.10 | −26.96 ± 2.12 | −26.70 ± 2. | −30.74 ± 2.29 | −22.46 ± 1.31 |
| MV E/MV E′ | −49.85 ± 7.58 | −51.51 ± 7.69 | −55.00 ± 7.41 | −40.00 ± 4.88 | −46.21 ± 4.90 | −56.42 ± 7.29 | −45.48 ± 6.10 | −48.98 ± 5.01 |
| Heart rate | 418.00 ± 22.52 | 458.42 ± 11.42 | 445.08 ± 13.33 | 457.5 ± 7.78 | 412.7 ± 10.91 | 436.00 ± 15.28 | 461.75 ± 13.80 | 442.88 ± 15.24 |
| Average circumferential strain (μm) | −21.25 ± 1.18 | −21.07 ± 1.48 | −23.05 ± 2.01 | −20.67 ± 1.39 | −20.24 ± 2.53 | −16.76 ± 1.58 - | −3.51 ± 4.24 | −17.72 ± 3.09 |
| Average radial displacement (μm) | 0.41 ± 0.02 | 0.38 ± 0.02 | 0.42 ± 0.03 | 0.44 ± 0.03 | 0.41 ± 0.03 | 0.38 ± 0.03 | 0.45 ± 0.06 | 0.41 ± 0.07 |
p <0.05 versus 3 mo of same gender.
p <0.05 versus female at same age.
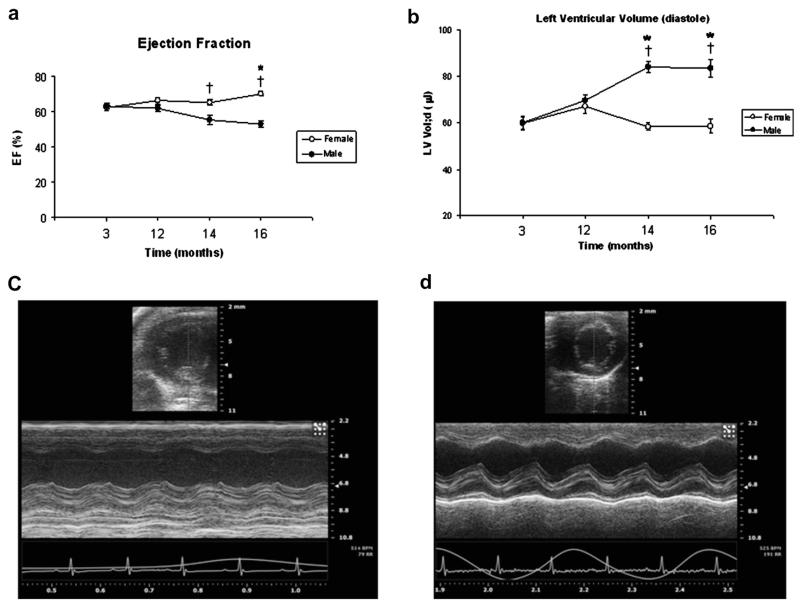
When gender was not taken into consideration, systolic function, as measured by EF, FS and strain, was maintained until the age of 16 mo. However, LV Vol;d increased with age starting at 12 mo (59.91 ± 1.96 at 3 mo vs. 68.22 ± 2.99, 68.54 ± 2.47 and 68.14 ± 3.10 at 12, 14 and 16 mo, respectively, with p < 0.05) (Table 1). Subgroup analysis of systolic function by gender and age revealed that male and female mice exhibit differences in cardiac function as they age. The male mice underwent a rapid and earlier decline in EF with a significant increase in LV Vol;d and LV Vol;s at 14 and 16 mo of age (p < 0.05) and a higher LV mass at 16 mo (Table 2), whereas female mice maintained their systolic function and chamber size up to 16 mo (Table 2, Fig. 1).
Fig. 1.
(a, b) Age-related changes in (a) ejection fraction (EF) and (b) left ventricle end-diastolic volume (LV Vol;d) in male versus female wild-type FVB/N mice. (c, d) Representative B-mode and M-mode images from echocardiography for male (c) and female (d) wild-type FVB/N mice at 16 mo of age. *p < 0.05 compared with mice of the same gender at 3 mo of age. †p < 0.05 compared with female mice of the same age.
We found that diastolic function, as defined by an E/A ratio less than 1, decreased as early as 12 mo in males (8 of 9 mice), but not in females (0 of 8). This decrease was found at all older time points in males and was significantly different in comparison to young male mice (3 months: 2.09 ± 0.33, 12 months: 0.66 ± 0.20, 14 months: 0.56 ± 0.33, 16 months: 0.55 ± 0.07, with p < 0.05 for all time points in comparison to 3 mo). Diastolic function in females did not differ significantly at any time point (3 months: 2.79 ± 0.47, 12 months: 2.76 ± 0.37, 14 months: 3.28 ± 0.80, 16 months: 2.35 ± 0.38). Other commonly used parameters of diastolic function, such as E′ and E/E′, did not significantly differ across all aging time points in both genders (Table 1).
Minor changes in systolic function were also found as measured via VVI. The circumferential strain values exhibited a non-statistically significant downward trend in males, whereas females remained unchanged up to 16 mo (Fig. 2). Radial displacement was unchanged in both groups (Table 2).
Fig. 2.
(a) Age-related changes in circumferential strain of male versus female wild-type FVB/N mice. (b–e) Representative circumferential strain and radial displacement in short-axis images from echocardiography for male (b and d, respectively) and female (c and e, respectively) wild-type FVB/N mice at 16 mo of age.
Inter-observer variability
There was excellent inter-observer agreement as indicated by a strong correlation coefficient with p values for correlation <0.001 for all parameters. However, on paired t-tests for radial displacement and circumferential strain, some differences were noted on average measurements, even though a strong correlation was present. Furthermore, inter-observer variability analysis revealed that there was no difference between observers in measuring EF, FS and systolic and diastolic volumes (Table 3).
Table 3.
Inter-observer variability indicating excellent correlation
| Variable | Correlation coefficient |
|---|---|
| Systolic volume | 0.76* |
| Diastolic volume | 0.70* |
| Ejection fraction | 0.72* |
| Fractional shortening | 0.73* |
| Diastolic diameter | 0.67* |
| Systolic diameter | 0.72* |
| Displacement | 0.67* |
| Strain | 0.67* |
Correlation values for inter-observer measurements.
p < 0.01.
DISCUSSION
This study indicates that there are age- and gender-related changes in both systolic and diastolic function as measured via traditional and advanced echocardiographic parameters. The use of a high-frequency probe (~30 MHz) and VVI improved the data quality and permitted measurements that would otherwise not be feasible in assessing the age- and gender-related changes. High-frequency imaging improves spatial resolution, which in turn allows for a more precise determination of traditional ECG parameters. Additionally, the improved spatial resolution reduces the speckle-spot size, which improves speckle tracking. This speckle tracking allowed for the displacement and strain measurements in VVI to be made. As Bauer et al. (2011) reported earlier, these approaches can be more sensitive than conventional cardiac performance and further permit regional, rather than global, assessments of function. Others have noted that strain measurements can be used as an early biomarker of changes in cardiac performance (Jurcut et al. 2008, Shao et al. 2013). Speckle-tracking methods of computing strain are angle independent, unlike Doppler-derived strain measurements. The advantages of speckle tracking in assessing cardiac performance have been reviewed by Ram et al. (2011).
With respect to diastolic function, our work is in agreement with that of several other groups who have found a decrease in diastolic function via varying techniques including isolated myocytes (Li et al. 2005), isovolumic left heart preparations (Olsson et al. 2001) and traditional echocardiography (Przyklenk et al. 2008; Scherrer-Crosbie and Kurtz 2010; Taffet et al. 1997). Furthermore, Olsson et al. (2001) reported a gender and age effect in a transgenic mouse model of familial hypertrophic cardiomyopathy, with males exhibiting earlier and more dramatic decreases in diastolic function and LV cavity dilation, whereas Baumann et al. (2008) reported gender- and strain-dependent differences in cardiac function in two different mouse lines using high-resolution echocardiography.
Dai and co-workers (Dai and Rabinovitch 2009a; Dai et al. 2009b) are one of the groups that have recently published age-related changes in cardiac function using echocardiography. In their studies, they have reported decreasing diastolic function beginning at 12 mo of age that reaches statistical significance only at 16 mo without tracking gender difference (Olsson et al. 2001; Scherrer-Crosbie and Kurtz 2010). We have confirmed this change, but also found it to be gender dependent; documenting a dramatic decrease in diastolic function in male mice at 12 mo and only a minimal change in female mice up to 16 mo.
Interestingly, we found that only the PD-derived E and A waves change with aging in a fashion similar to that observed in humans, with the E/A ratio steadily declining until 60 y of age (Kuzentsova et al. 2009). In contrast, the TD parameters of diastolic function that also change with age in humans, such as E′ and E/E′, did not change significantly in our mouse population. We believe that this is due to significant technical difficulties in obtaining adequate apical images as a result of the mouse chest anatomy and is not based on inherent differences in aging, making it quite difficult to extrapolate specific ages in mice to humans.
Systolic function, as measured via EF and FS, also exhibited a significant age-related effect which has been previously described via pressure-volume loops (Yang et al. 1999). We clearly indicate that there is a gender difference in aging FVB/N mice via advanced echocardiography, with the males undergoing an earlier and more significant decrease in systolic function at 14 mo and the female mice maintaining their systolic function until 16 mo of age. Advanced echocardiography provided further information regarding myocardial contractility changes with aging. Even though there was a decrease in EF in male mice at 14 mo, intrinsic myocardial contractility as measured via circumferential strain did not drop significantly until 16 mo. This intriguing finding can be partially explained by the increase in left ventricular volume during systole which was observed at earlier time points. This dilation resulted in a larger diameter during diastole and, therefore, a decrease in the estimated EF. Thus, even though the EF decreased initially, the intrinsic myocardial contractility, as measured via VVI, remained unchanged. This finding correlates well with previous studies that found that strain measured from VVI is a reproducible measurement of contractility and that it decreases early in the course of drug-induced cardiomyopathy and consequently can serve as marker of a disease progression (Anversa et al. 2005; Bachner et al. 2010; Cho et al. 2009; Nesbitt et al. 2009).
We also found that the male FVB/N mice underwent left ventricular remodeling with an increase in LV Vol;d and LV Vol;s. These changes were found to occur at approximately the same time points as the diastolic dysfunction, suggesting that both structural and functional changes occur through potentially similar mechanisms, which have been previously suggested (alterations in calcium-handling proteins, phospholamban and sodium calcium exchange) (Kass et al. 2004). We also noted that the septal wall maintained its thickness with aging. This can be related to the right ventricle support of the septal wall, which may lead to atrophy of the free wall (posterior wall) (Gao et al. 2002; Lopez-Candales et al. 2010; Reddy et al. 2007). These findings are consistent with previous work that evaluated ischemic tolerance in C57/Bl6 mice and found that there was overall impaired recovery of ventricular contractility after ischemia evident by 12 mo of age in males, but not in females (Juhaszova et al. 2005; Willems et al. 2005). This may be attributed to improved systolic function at this age and less diastolic dysfunction in female mice with decreased end-diastolic pressure and volume compared with age-matched males, as indicated in our study.
There are several previous studies that looked for similar changes in myocardial function and did not find any significant differences associated with aging that merit further discussion (Betsyaku et al. 2002; Bradshaw et al. 2010; Heimdal et al. 1998; Hinton et al. 2008; Stypmann et al. 2006). The studies by Stypmann et al. (2006) and Bradshaw et al. (2010) limited their mouse population to only two age groups (8 and 52 wk and 3 and 18 mo, respectively), used lower-frequency probes (12 and 15 MHz, respectively) and used ketamine/xylazine for anesthesia, which slowed the heart rate to about 320 bpm. Similarly, Reddy et al. (2007) used a smaller mouse population, had only two time points and did not clarify whether males or females were used. Other researchers, such as Hinton et al. (2008) and Dai and co-workers (Dai and Rabinovitch 2009; Dai et al. 2009), looked at function in mice at multiple time points, used isoflurane for anesthesia and obtained results similar to ours, but did not analyze (or at least did not report) gender differences as we did, and neither used VVI in evaluating contractility. Hence, in comparison to the previous work, we believe our study to be a more complete and in-depth analysis of cardiac aging via echocardiography as we encompassed older mice, multiple time points, higher-frequency probes, advanced VVI technology and, most importantly, maintained the heart rate at near-physiologic levels of 475–540 bpm (Desai et al. 1997). Interestingly, although we did note a trend toward decreased LV function as measured via VVI that appeared to mirror the increase in LV mass (particularly in the male subgroup), these changes were not statistically significant. These findings echo those observed by Kusunose et al. (2012) in various species including mice. Nonetheless, the lack of statistical significance in VVI-derived parameters is in contrast to the differences observed by Shao et al. (2013), though they sought to evaluate the technique in an ischemic heart mouse model while we studied healthy mice.
The aforementioned observed changes in myocardial function have important implications in the study of cardiomyopathies, hypertrophy, ischemia and remodeling in young and aged mice. Importantly, not all researchers have made the distinction between male and female mice when reporting the deteriorating heart function caused by ischemia or other heart disease (Bauer et al. 2011; Bujak et al. 2008; Luo and Konofagou 2008; Luo et al. 2006; Sebag et al. 2005; Thibault et al. 2007). Many studies used only male mice (Li et al. 1995, 2005), whereas others expressly studied the gender-related differences during ischemia/reperfusion (for a review, see Murphy and Steenbergen 2007), and some investigators reported significant or no differences in infarct size in males versus females (Du Toit et al. 2007; Przyklenk et al. 1995; Thibault et al. 2009). Therefore, even though there remains some controversy in the field (Scherrer-Crosbie et al. 2007), the vast majority of studies seem to agree that in aged mice, there is lower function and increased adverse outcome in males (Murphy and Steenbergen 2007). Our findings can be used as researchers continue to investigate the differences between normal physiologic aging, pathologic cardiomyopathy and heart failure as studied at different time points. Furthermore, this study can also be useful as other researchers attempt to elucidate whether age is a risk factor for ischemic injury in humans and animals or the cumulative risk exposures through time (Murphy and Steenbergen 2007).
Limitations
This study reveals an important limitation regarding murine echocardiography and the exact probe positioning necessary for TD. The difficulty in obtaining adequate apical images clearly limited our capacity to study diastolic function with some of the parameters used in humans. Though we were able to distinguish changes in diastolic function using traditional PD imaging, we cannot recommend the use of TD of the posterior wall from PSLAX as an adequate measurement of diastolic function in murine models. We further note that the conclusions of this study were limited by its retrospective nature: the same animals were not followed longitudinally, resulting in the need to use two subgroups to cover the investigated age range. Additionally, the retrospective nature of the study prohibited any histologic analysis that could have been correlated with the high-frequency imaging.
CONCLUSIONS
Our study emphasizes the role of high-resolution and advanced echocardiography technology as a non-invasive method for evaluating myocardial function in mice models in different age groups. This study indicates that the cardiac function of young and old WT FVB/N mice differs in male mice. Therefore, one of the difficulties in translating basic research in mouse models of heart disease to clinical application may be the common usage of young mice (Bujak et al. 2008), whereas the patients typically being treated for heart disease are older. Equating the age of our mice, described as “mature adults,” to specific age ranges in humans is inherently challenging. Thus, the reported changes in murine cardiac function presented here may be used in cardiac research, especially for heart failure and post-infarct remodeling mouse models, which usually involve only young mice.
Acknowledgments
The authors gratefully acknowledge the E. G. Kranias research team for providing some of the mice used in the present study.—This work was supported in part by NIH Grant HL091478 (W.K.J.).
Footnotes
Conflicts of Interest: The authors have indicated that they have no conflicts of interest regarding the content of this article.
REFERENCES
- Aguirre FV, Mcmahon RP, Mueller H, Kleiman NS, Kern MJ, Desvigne-Nickens P, Hamilton WP, Chaitman BR. Impact of age on clinical outcome and postlytic management strategies in patients treated with intravenous thrombolytic therapy: Results from the TIMI II study. Circulation. 1994;90:78–86. doi: 10.1161/01.cir.90.1.78. [DOI] [PubMed] [Google Scholar]
- Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, Bolli R. Myocardial aging—A stem cell problem. Basic Res Cardiol. 2005;100:482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- Azam S, Desjardins CL, Schluchter M, Liner A, Stelzer JE, Yu X, Hoit BD. Comparison of velocity vector imaging echocardiography with magnetic resonance imaging in mouse models of cardiomyopathy. Circulation Cardiovasc Imaging. 2012;5:776–781. doi: 10.1161/CIRCIMAGING.111.972406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachner N, Tsadoky Y, Adam D. Increase in endocardial rotation during doxorubicin treatment. Ann NY Acad Sci. 2010;1188:128–132. doi: 10.1111/j.1749-6632.2009.05092.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Cheng S, Jain M, Ngoy S, Theodoroulos C, Trujillo A, Lin F-C, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011;108:908–916. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann PQ, Sobel BE, Zaman T, Schneider DJ. Gender-dependent differences in echocardiographic characteristics of murine hearts. Echocardiography. 2008;25:739–748. doi: 10.1111/j.1540-8175.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Beetz N, Hein L, Meszaros J, Gilsbach R, Barreto F, Meissner M, Hoppe UC, Schwartz A, Herzig S. Transgenic simulation of human heart failure-like L-type Ca21-channels: Implications for fibrosis and heart rate in mice. Cardiovasc Res. 2009;84:396–406. doi: 10.1093/cvr/cvp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsyaku T, Kovacs A, Saffitz JE, Yamada KA. Cardiac structure and function in young and senescent mice heterozygous for a connexin43 null mutation. J Mol Cell Cardiol. 2002;34:175–184. doi: 10.1006/jmcc.2001.1499. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Lan AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: Role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–H622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rockamn HA. Receptor signaling pathways in heart failure: Transgenic mouse models. In: Mebazaa A, Gheorghiade, Zannad FM, Parrillo JE, editors. Acute heart failure. Springer; Berlin/New York: 2008. pp. 89–111. [Google Scholar]
- Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DT. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–624. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- Christensen G, Wang Y, Chien KR. Physiological assessment of complex cardiac phenotypes in genetically engineered mice. Am J Physiol Heart Circ Physiol. 1997;272:H2513–H2524. doi: 10.1152/ajpheart.1997.272.6.H2513. [DOI] [PubMed] [Google Scholar]
- Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altred mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- Collins KA, Korcarz CE, Shorff SG, Bednarz JE, Fentzke RC, Lin H, Leiden JM, Lang RM. Accuracy of echocardiographic estimates of left ventricular mass in mice. Am J Physiol Heart Circ Physiol. 2001;280:H1954–H1962. doi: 10.1152/ajpheart.2001.280.5.H1954. [DOI] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Edmond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK. Bernstein Cardiovascular indexes in the mouse at rest and with exercise: New tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- Du Toit EF, Genade S, Carlini S, Moolman JA, Brunner F, Lochner A. Efficacy of ischaemic preconditioning in the Enos overexpressed working mouse heart model. Eur J Pharmacol. 2007;556:115–120. doi: 10.1016/j.ejphar.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gao XM, Kiriazis H, Moore XL, Feng XH, Sheppard K, Dart A, Du XJ. Lower risk of postinfarct rupture in mouse heart overexpressing beta 2-adrenergic receptors: Importance of collagen content. J Cardiovasc Pharmacol. 2002;40:632–640. doi: 10.1097/00005344-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Haghighi K, Schmidt AG, Hoit BD, Brittsan AG, Yatani A, Lester JW, Zhai J, Kimura Y, Dorn GW, II, MacLennan DH, Kranias EG. Super-inhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem. 2001;276:24145–24152. doi: 10.1074/jbc.M102403200. [DOI] [PubMed] [Google Scholar]
- Heimdal A, Stoylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11:1013–1019. doi: 10.1016/s0894-7317(98)70151-8. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, Yutzey KE. Mouse heart valve structure and function: Echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008;294:H2480–H2488. doi: 10.1152/ajpheart.91431.2007. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: Preventing the heart-break of old age. Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Jurcut R, Wildiers H, Ganame J, D’hooge J, Paridaens R, Voigt JU. Detection and monitoring of cardiotoxicity—What does modern cardiology offer? Support Care Cancer. 2008;16:437–445. doi: 10.1007/s00520-007-0397-6. [DOI] [PubMed] [Google Scholar]
- Kass DA, Bronwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure. Circ Res. 2004;94:1533. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- Kusunose K, Penn MS, Zhang Y, Cheng Y, Thomas JD, Marwick TH, Popovic ZB. How similar are the mice to men? Between-species comparison of left ventricular mechanics using strain imaging. PLoS One. 2012;7(6):e40061. doi: 10.1371/journal.pone.0040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzentsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, Gonzalez A, Herregoals MC, Fagard RH, Diez J, Staessen JA. Prevelance of left ventricular diastolic dysfunction in a general population. Circ Heart Failure. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II. The aging heart in health: Links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Lundergan CF, Hodgson JM, Nair R, Reiner JS, Greenhouse SW, Califf RM, Ross AM. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: The GUSTO-I angiographic experience. J Am Coll Cardiol. 1996;28:331–337. doi: 10.1016/0735-1097(96)00148-9. [DOI] [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang XP, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glucation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Garson CD, Xu Y, Beyers RJ, Epstein F, French BA, Hossack JA. Quantification and MRI validation of regional contractile dysfunction in mice post myocardial infarction using high resolution ultrasound. Ultrasound in Med Biol. 2007;33:894–904. doi: 10.1016/j.ultrasmedbio.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kloner RA. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? Thromb Thrombolysis. 1995;2:21–25. doi: 10.1007/BF01062713. [DOI] [PubMed] [Google Scholar]
- Lopes RD, Alexander KP, Manoukian SV, Bertrand ME, Feit F, White HD, Pllack CV, Jr, Houhstra J, Gersh BJ, Stone CW, Ohman EM. Advanced age, antithrombotic strategy and bleeding in non-ST segement elevation acute coronary syndrome: Results from the ACITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;53:1021–1030. doi: 10.1016/j.jacc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Lopez-Candales A, Edelman K, Candales MD. Right ventricular apical contractility in acute pulmonary embolism: The McConnell sign revisited. Echocardiography. 2010;27:614–620. doi: 10.1111/j.1540-8175.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Fujikura K, Konofagou EE. Detection of murine infarcts using myocardial elastography at both high temporal and spatial resolution; Proceedings of the 28th IEEE EMBS Annual International Conference; New York City. August 30–September 3, 2006; pp. 1552–1555. ThC08.5. [DOI] [PubMed] [Google Scholar]
- Luo J, Konofagou EE. High-frame rate, full-view myocardial elastography with automated contour tracking in murine left ventricles in vivo. IEEE Trans Ultrasonics. Ferroelectr Freq Control. 2008;55:240–248. doi: 10.1109/TUFFC.2008.633. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: Methods and clinical applications. Int J Cardiovasc Imaging. 2009;25:9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Palmer BM, Leinwand LA, Moore RL. Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2001;280:1136–1144. doi: 10.1152/ajpheart.2001.280.3.H1136. [DOI] [PubMed] [Google Scholar]
- Pereira TM, Nogueria BV, Lima LCF, Porto ML, Arruda JA, Vasquez EC, Meyrelles SS. Cardiac and vascular changes in elderly atherosclerotic mice: The influence of gender. Lipids Health Dis. 2010;9:87. doi: 10.1186/1476-511X-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K, Maynard M, Darling CE, Whittacker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1399–1403. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Ovize M, Bauer B, Kloner RA. Gender does not influence acute myocardial infarction in adult dogs. Am Heart J. 1995;129:1108–1113. doi: 10.1016/0002-8703(95)90390-9. [DOI] [PubMed] [Google Scholar]
- Qin F, Lennon-Edwards S, Lancel S, Biolo A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ, Colucci WS. Cardiac-Specific Overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in Gaq-overexpressing transgenic mice. Circ Heart Failure. 2010;3:306–313. doi: 10.1161/CIRCHEARTFAILURE.109.864785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz S, Bulcao CF, D’Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation. 2010;121:410–418. doi: 10.1161/CIRCULATIONAHA.109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R, Mickelsen DM, Theodoropoulus C, Blaxall BC. New approaches in small animal echocardiography imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:765–780. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Amador-Noguez D, Darlington GJ, Scholz BA, Michael LH, Hartley CJ, Entman ML, Taffet GE. Cardiac function in young and old little mice. J Gerontol A. 2007;62:1319–1325. doi: 10.1093/gerona/62.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MW. Epidemiology, clinical features, and prognosis of acute myocardial infarction in the elderly. Am J Geriatr Cardiol. 2006;15:7–11. doi: 10.1111/j.1076-7460.2006.05273.x. [DOI] [PubMed] [Google Scholar]
- Scalia GM, Khoo SK, O’Neill S. Age-related changes in heart function by serial echocardiography in women age 40–80 year. J Women’s Health. 2010;19:1741–1745. doi: 10.1089/jwh.2009.1752. [DOI] [PubMed] [Google Scholar]
- Scherrer-Crosbie M, Kurtz B. Ventricular remodeling and function: Insights using murine echocardiography. J Mol Cell Cardiol. 2010;48:512–517. doi: 10.1016/j.yjmcc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer-Crosbie M, Rodrigues ACT, Hataishi R, Picard MH. Infarct size assessment in mice. Echocardiography. 2007;24:90–96. doi: 10.1111/j.1540-8175.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Sebag IA, Handschumacher MD, Ichinose F, Morgan JG, Hataishi R, Rodrigues ACT, Guerrero L, Steudel W, Raher MJ, Halpern EF, Dermeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Quantitative assessment of regional myocardial function in mice by tissue Doppler imaging. Circulation. 2005;111:2611–2616. doi: 10.1161/CIRCULATIONAHA.104.474411. [DOI] [PubMed] [Google Scholar]
- Shao Y, Redfors B, Tang MS, Assarsson U, Omerovic E. Novel simple approach for detection of regional perturbations of cardiac function in mouse models of cardiovascular disease. Echocardiography. 2013;1:1–7. doi: 10.1111/echo.12138. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Leatherbury L, Lo CW. High–frequency ultrasound database profiling growth, development, and cardiovascular function in C57BL/6J mouse fetuses. JAm Soc Echocardiogr. 2004;17:893–900. doi: 10.1016/j.echo.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Stypmann J, Engelen MA, Epping C, Van Rijen HVM, Milberg P, Bruch C, Breithardt G, Tiemann K, Eckardt L. Age and gender related reference values for transthoracic Doppler-echocardiography in anesthetized CD1 mouse. Int J Cardiovasc Imaging. 2006;22:353–356. doi: 10.1007/s10554-005-9052-9. [DOI] [PubMed] [Google Scholar]
- Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol. 1997;52:B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- Thibault H, Gomez L, Donal E, Augeul L, Scherrer-Crosbie M, Ovize M, Derumeaux G. Regional myocardial functionafter myocardial infarction in mice: A follow-up study by strain rate imaging. J Am Soc Endocardiogr. 2009;22:198–205. doi: 10.1016/j.echo.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Thibault H, Gomez L, Donal E, Pontier G, Scherrer-Crosbie M, Ovize M, Derumeaux G. Acute myocardial infarction in mice: Assessment of transmurality by strain rate imaging. Am J Physiol Heart Circ Physiol. 2007;293:H496–H502. doi: 10.1152/ajpheart.00087.2007. [DOI] [PubMed] [Google Scholar]
- Van den Borne SWM, van de Schans VAM, Strzelecka AE, Vervoort-Peters HTM, Lijnen PM, Cleutjens JPM, Smits JFM, Daemen MJAP, Janssen BJA, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Wittköpper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsöld B, Kirchhof P, Maier SL, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: Analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol. 1999;277:H1906–H1913. doi: 10.1152/ajpheart.1999.277.5.H1906. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Han W, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su Y, Du X, Gao X. Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physiol Heart Circ Physiol. 2008;294:H1815–H1822. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Robbins J. Principles of genetic murine models for cardiac disease. Circulation. 2007;115:792–799. doi: 10.1161/CIRCULATIONAHA.106.682534. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Takagawa J, Sievers RE, Khan MF, Viswanathan MN, Springer ML, Foster E, Yeghiazarians Y. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1187–H1192. doi: 10.1152/ajpheart.00895.2006. [DOI] [PubMed] [Google Scholar]