Abstract
In recent years, experience with transcatheter aortic valve implantation has led to improved outcomes in elderly patients with severe aortic stenosis (AS) who may not have previously been considered for intervention. These patients are often frail with significant comorbid conditions.
As the prevalence of AS increases, there is a need for improved assessment parameters to determine the patients most likely to benefit from this novel procedure. This review discusses the diagnostic criteria for severe AS and the trials available to aid in the decision to refer for aortic valve procedures in the elderly.
Keywords: Aortic Stenosis, Transcatheter Aortic Valve Replacement, Surgical Aortic Valve Replacement, Elderly, Frailty, Referral, Quality of Life, Risk Stratification
Introduction
Aortic stenosis (AS) has become a major cause of morbidity and mortality among a growing population of older adults.(1) In response to this clinical challenge, transcatheter aortic valvular implantation (TAVI) has rapidly evolved. Since the initial approval in Europe in 2007, the number of TAVI procedures has exponentially increased with over 100,000 valves placed worldwide.(1–4) The vast majority of these valves were implanted in older adults (the average age of TAVI in the US is ~84 years).(5)
It has become critically important for physicians caring for senior adults with AS to understand the risks and benefits of TAVI placement. The decision to refer for TAVI requires careful consideration of numerous factors including severity of disease, individual patient anatomy, comorbidity, overall prognosis, quality of life, and patient preference. The purpose of this review is to discuss AS in elderly patients and to evaluate the factors that can aid in the decision to refer for TAVI.
Epidemiology, Pathophysiology, and Natural History
AS is a disease of the elderly. The largest epidemiologic study from the United States used echocardiographic data from 11,911 patients in three preventive trials from the National Heart, Lung, and Blood Institute. The study demonstrated a prevalence of moderate to severe AS of 2.8% in patients >75 years of age, compared to only 0.2% in patients age 18–45.(6) The European Heart Survey evaluated 5,001 patients in 25 countries. In this cohort, 56% of patients with AS were greater than 70 years of age and 36% had at least one other comorbidity.(7, 8)
The classic description of AS by Morrow in 1968 defined a disease affecting primarily people in their fifth or sixth decade of life with a history of congenital bicuspid aortic valve or rheumatic valvular disease.(9, 10) Over the past half-century, the demographics of AS have changed due to an increase in life-expectancy and a dramatic decrease in the prevalence of rheumatic heart disease. Today, the primary form of AS is fibrocalcific disease. The second leading cause is bicuspid valve disease, typically manifesting in patients <70 years of age.(3, 7, 11, 12)
AS is an obstruction that develops insidiously with progressive calcification, inflammation, and lipid deposition on the valve.(13–19) Risk factors for the development of fibrocalcific AS are similar to those that increase the risk of atherosclerotic coronary disease; i.e. age, smoking, hypertension, and elevated cholesterol.(12) It has also been hypothesized that abnormal shear stress across the valve escalates the rate of calcium and lipid deposition. In bicuspid aortic valves, the increased shear forces lead to the development of significant obstruction approximately ten years earlier than patients with trileaflet AS.(3)
As the degree of obstruction increases in AS, left ventricular (LV) pressure increases, resulting in hypertrophy, fibrosis, and diastolic dysfunction. This can lead to decreased coronary reserve, increased myocardial ischemia, and eventually cause progressive LV dysfunction. In their 1968 paper, Ross and Braunwald famously hypothesized that with progressive pressure overload, the LV loses compensatory reserve causing symptoms to develop with a very poor overall prognosis.(20) The classically described symptoms of AS are angina, syncope, and congestive heart failure. When these symptoms develop, a characteristic, rapid progression of the disease process is seen.(20, 21) Without intervention, the two year mortality of AS is estimated to be as high as 50–80%.(22, 23) The most recent randomized data of high-risk patients with severe AS deemed to be inoperable candidates revealed a mortality of 50% at 1 year, demonstrating that the original hypothesis of Ross and Braunwald remains relevant today.
Diagnosis Using Echo Data
The decision to refer for TAVI starts with proper diagnosis of AS in a patient with correlating symptoms. Echocardiographic diagnosis is made based on several criteria. The valve should appear calcified and thickened with restricted leaflet opening on standard transthoracic echocardiography. The peak and mean gradient can then be calculated using the simplified Bernoulli equation with the maximal velocity obtained by continuous wave (CW) Doppler. The highest velocity is obtained from multiple imaging positions to reduce the risk of underestimating the peak gradient across the valve. The gradient is then used to calculate the aortic valve area (AVA) using the continuity equation (which requires the measurement of the LV outflow tract (LVOT) velocity time integral (VTI) with pulse wave (PW) Doppler, the aortic valve VTI with CW, and the LVOT area). Based on these measurements, severe AS is defined as an AVA <1 cm2 (critical <0.8 cm2), a mean gradient > 40 mm Hg, or a peak velocity > 4 m/s.(24, 25).†
Low Flow and Low gradient AS
Frequently, in patients of advanced age, the criteria for severe AS (see table 1) is complicated by a low AVA (<1 cm2) with a lower than expected aortic gradient (mean <40 mm Hg). This condition is designated low gradient AS and is commonly seen in patients with low LV ejection fraction (EF). Patients may also have low stroke volume due to severe diastolic dysfunction and are classified as low flow AS.
Table 1.
Echocardiograpic Diagnostic Criteria for AS
| Diagnostic Criteria | Echo measurements | Limitations |
|---|---|---|
| AVA <1 cm2 | LVOT area*, AV VTI with CW, LVOT gradient with PW |
LVOT diameter if incorrect can cause large error in AVA. |
| AVA index < 0.6 cm2/m2 | Divide the AVA by BSA | Weight used is not ideal body weight |
| Peak jet velocity >4 m/s | CW through aortic valve | CW Doppler must be aligned correctly |
| Mean gradient > 40 mmHg | AV CW using Bernoulli equation | Same as above |
|
Dimension less index (Velocity ratio) < 0.25 |
Velocity ratio = V·LVOT/V·AS | Cannot take into account individual variations in LVOT size |
| Aortic valve planimetry | Direct measurement of area on 2D/3D echo |
Limited and rarely used in 2D echo due to aortic calcification leading to reverberation of signal. |
(AV = aortic valve, BSA = Body surface area, VTI = velocity time integral, LVOT= left ventricular outflow tract, Bernoulli equation is ΔP = 4v2)
AVA calculated from LVOT diameter.
In patients with a small AVA and a gradient less than 40 mm Hg, the clinician must determine if the aortic valve is truly stenotic; or if instead, the limited valve opening is due to a weak LV that is unable to generate the force needed to open the valve (termed pseudo-stenosis). Understanding the approach to these patients is important, as this problem may be seen in up to 5–10% of patients with AS.(10, 26, 27)
Dobutamine stress echo (DSE) is usually used to assess the presence of pseudo-stenosis in low-gradient AS. On echocardiographic evaluation, patients with pseudo-stenosis, when challenged with dobutamine, have an increase in AVA with little to no change in aortic valve gradient (AVA will generally improve to > 1.2 cm2).(28) Patients undergoing DSE who experience an increased transvalvular gradient with no improvement in AVA are more likely to require aortic valve replacement. In patients in this “true” stenosis group, DSE can be additionally valuable by assessing contractile reserve. Contractile reserve is defined as an increase in stroke volume by 20% and has been associated with a lower operative mortality.(27, 29)
Patients with low-gradient severe AS carry as much as 20% greater operative morbidity and mortality with surgical aortic valve replacement (SAVR) than those patients with high gradient severe AS. However, five-year mortality outcomes still favor treatment.(30, 31) Le Ven et al. analyzed patients with low-flow, low gradient severe AS compared to patients with high gradient AS undergoing TAVI. Patients with low-flow severe AS were seen to have a markedly higher 30-day mortality (11.4% vs. 5.9% respectively p=0.01). On univariate analysis, low EF in patients with low gradient AS was correlated with higher cumulative mortality [HR 1.61 (95% CI 1.19 to 2.49, p=0.002)].(32) Low gradient AS has also been shown to correlate with greater frequency of other comorbidity; i.e. pulmonary hypertension, PAD, severe mitral regurgitation (MR), and coronary artery disease (CAD).(33, 34) The results of the True or Pseudo Severe Aortic Stenosis (TOPAS) registry will aid in further understanding outcomes in low flow AS patients.
Medical Management of Severe AS
No effective medical therapy has been shown to affect mortality in AS. Medical treatment is primarily used to optimize CV status and alleviate symptoms in conjunction with aortic valve replacement or for palliation of symptoms. While awaiting surgery, lifestyle modification, such as limiting physical exertion, is generally recommended. In those patients who are not SAVR or TAVI candidates, medical therapy to improve preload and afterload can be attempted. The main goals of medical therapy include volume status optimization and hypertension management. (35)
While hypertension has been associated with the development of symptoms at an earlier stage, blood pressure reduction entails intrinsic risks.(36, 37) Diuretics can decrease afterload by reducing overall blood volume; however, they can also diminish necessary preload needed to maintain cardiac output in the setting of severe AS. Beta blockade can reduce contractility and is generally contraindicated. Vasodilators such as hydralazine and nifedipine, while reducing additional afterload, will not greatly affect the fixed obstruction at the level of the valve and can lower systemic blood pressure and coronary perfusion pressure.
In critically ill patients awaiting surgery or TAVI, balloon valvuloplasty (BAV) may be attempted. BAV can result in short term symptomatic improvement. However, in the ensuing months after BAV, the stenosis tends to re-occur; and survival is not improved when compared to medical therapy (3-year survival of only 17% in the BAV group in one study).(38) Additionally, the risk of vascular complications, bleeding, and stroke are significant post-BAV.(39)
Outcomes without invasive management in AS have been very poor. A classic study of patients with severe AS who were recommended for surgery but refused showed a three year mortality of 79% in the medically treated group.(21) Modern data from the Placement of Aortic Transcatheter Valve Trial (PARTNER) studies in patients who were not surgical candidates showed similarly high two-year mortality rates (mortality of 68% in the medical treatment arm).(22)
SAVR
The 2008 ACC guidelines for valvular heart disease give three class I recommendations for SAVR (see table 2).(40) In the elderly, SAVR is generally reserved for those with severe symptomatic AS and who are reasonable operative candidates. Surgeons frequently use validated risk scores such as the Society of Thoracic Surgeons score (STS) or the EuroSCORE as an objective way to assess risk.(41, 42) No specific guidelines address which patients are of prohibitive risk; however, in clinical trials, an STS score of 8–15% predicited mortality has been used as the risk cut-off for SAVR. A larger database review shows that the majority of patients receiving SAVR have an STS of < 5%.(10)
Table 2.
Criteria for Referral for AVR, TAVI, and BAV
| SAVR |
|
| TAVI |
|
| BAV |
|
(LV = left ventricular, EF = ejection fraction)
STS score based on referral criteria for partner trials – initially STS ≥ 10, it was later decreased to ≥8.
i.e. those comorbid conditions associated with increased mortality with SAVR (liver disease, significant COPD, pulmonary hypertension, etc.)
The STS database shows the mortality for isolated SAVR has improved by about 1% over the past decade and is currently estimated as 2.6%. When concomitant procedures are required, the operative risk is increased. For example, the mortality risk of SAVR plus bypass surgery in 2011 was 4.7%, and SAVR plus mitral replacement was 7.1%.(43)
Transcatheter Aortic Valve Implantation
Despite the strikingly high mortality with symptomatic AS, a large percentage (30–40%) of patients evaluated for SAVR were deemed inoperable or refused the surgical procedure.(40, 44, 45) Age was the most frequently sited co-morbidity prohibiting surgical intervention.(6, 8, 46) TAVI was developed as an alternative therapeutic option for this group of patients. The first, major randomized trials performed to prove the efficacy and safety of TAVI were done in a series of publications by the PARTNER group.
The initial trial of TAVI in 2010 by Leon et al. was performed in patients who were considered too high-risk to undergo surgery.(47) Three hundred fifty-eight patients with symptomatic, severe AS defined as AVA < 0.8 cm2, mean aortic gradient > 40 mm Hg, or a peak velocity of > 4 m/s were evaluated and deemed to be inoperable by two surgeons using an STS score > 10% or a coexisting condition that would be associated with a >50% predicted probability of death within 30 days of surgery.
In this trial, a one-year mortality of 30.7% was seen in the TAVI arm -- compared to 50.7% in the medical arm (p<0.001). The results at two years demonstrated a mortality of 34% in the TAVI group and 68% in the standard therapy group (p<0.001) with corresponding cardiac death 31% and 62% respectively (p<0.001).(22)
TAVI vs. SAVR
The 2011 PARTNER trial evaluated patients considered acceptable, although high-risk surgical candidates, and randomized them in a 1:1 fashion to TAVI vs. SAVR.(48) Six hundred ninety-nine high-risk patients with severe symptomatic AS were randomized. At thirty days, the rate of death from any cause was 2.4% in the transcatheter group and 6.5% in the surgical group (p=0.07). Mortality was 24.2% and 26.8% at 1 year respectively (p=0.44), meeting criteria for non-inferiority (p=0.001).
Although there were comparable mortality rates in the trial, significant differences in the frequency of major complications after TAVI were seen. The proportion of major stroke at 30 days was slightly higher in the TAVI group: 3.8% vs. 2.1% (p= 0.07). Vascular complications were significantly higher in TAVI at 30 days (11% vs. 3.2%, p<0.001). Surgical patients experienced more post-procedural bleeding (9.3% vs. 19.5%, p<0.001) and new onset atrial fibrillation (8.6% vs. 16.0%, p=0.006). The need for post-procedural pacemaker placement was similar between the 2 groups (3.8 vs. 3.6 %, p=0.89).
The two-year outcome in this high-risk cohort was recently published showing a mortality of 34% in TAVI and 35% in SAVR (p=0.78).(49) At 30 days, the risk of stroke was not significantly higher with TAVI than with SAVR (4.6 vs. 2.4%, p=0.12). Post-operative AVA was similar between the two groups. Paravalvular aortic regurgitation (AR) was more frequent after TAVI; and importantly, even mild paravalvular AR was strongly associated with increased late mortality (p<0.001).
Anticoagulation and Bleeding in SAVR and TAVI in the Elderly
The need for adjunctive antiplatelet and antithrombotic treatment after TAVI and SAVR must also be considered, particularly in elderly patients at increased bleeding risk during and after the procedure. Peri-procedural bleeding has been shown to be significantly higher with SAVR than TAVI (19.5% vs. 9.3%, p<0.001).(48) Major bleeding events have been associated with increased morbidity and mortality with both procedures (adjusted HR 2.49, 95% CI 1.85–3.37; p<0.001) and have been linked with increased rates of acute kidney insufficiency requiring dialysis in SAVR.(50)
Anticoagulation choice after TAVI has largely been empirically determined as clinical evidence is lacking. The current recommendations by the ACCF/AATS/SCAI/STS consensus document state that patients who are post-TAVI should be placed on aspirin and clopidogrel therapy. However, no recommendation of the duration of clopidogrel therapy was given nor was the loading dose specified.
The choice of anti-thrombotic therapy in patients receiving SAVR varies according to center, patient comorbidities (i.e. concomitant atrial fibrillation), and valve type. The STS database of prosthetic SAVR showed that 49% of patients are discharged on aspirin alone, 12% on warfarin alone, and 23% with warfarin plus aspirin. A 2012 document comparing 25,656 patients post-SAVR showed an individual risk of stroke or bleeding event of 1% on aspirin monotherapy at 3 months.(51) The cohort placed on aspirin plus coumadin had a lower risk of embolic stroke but had a significantly higher risk of bleeding when compared to patients on aspirin monotherapy (relative risk of bleeding: 2.80, 95% CI: 2.18 to 3.60, p<0.0001).
Predicting Optimal Patients for TAVI
The prototypical tenet of geriatric care of “start low and go slow” and “less is more” contrasts the approach implied by the data in the PARTNER trials, which demonstrate improved outcomes with an aggressive invasive approach. The inherent risks associated with invasive procedures in this population make it vitally important that providers identify the subsets of older adults who will benefit from TAVI or SAVR. Selecting patients to achieve a high benefit-to-risk ratio is a substantial clinical challenge. The remainder of the document will evaluate the data available to guide the clinician through this process. Table 2 offers a summary of referral criteria for each of the different treatment modalities for severe AS.
Perioperative Risk Assessment
Given the complexity of the decision to refer for SAVR or TAVI, objective risk calculators have been developed to estimate surgical risk. The STS score was specifically designed to analyze the risk of aortic valve surgery and remains the most commonly used score in the evaluation TAVI and SAVR patients in North America.(41) It contains 24 covariates derived from testing 67,292 patients undergoing isolated SAVR between 2002 and 2006. Emergency surgery, history of endocarditis, and history of previous cardiac surgery are the most heavily weighted variables in the score.
It is important to recognize the STS score does not incorporate several key risk factors; i.e., aortic anatomy, cirrhosis, and pulmonary hypertension. It fails to consider several features that are crucial to the elderly population: frailty, cognitive functioning, nutritional status, and quality of life metrics.(52)
As part of the recent SURgical replacement and Transcatheter Aortic Valve Implantation trial (SURTAVI), a novel risk-stratification process was used to include the risk factors that are missing from the STS score. To construct an alternative score, Van Mieghem et al. chose age plus ten risk factors: concomitant CAD, frailty, LV dysfunction with EF < 35%, baseline neurologic dysfunction, pulmonary disease, peripheral arterial disease (PAD), renal disease, history of sternotomy, diabetes, and pulmonary hypertension.(53, 54) The SURTAVI assessment model has not been validated in a large prospective fashion, but may prove to be a reasonable addition to the STS score in aiding in the decision of TAVI vs. SAVR.
The STS risk score and alternative available risk scores (i.e. EuroSCORE) have been criticized for poor risk prediction in the highest risk patients (the group of patients in which accurate risk assessment is most vital).(33) The recognized need for a TAVI specific risk score has resulted in the development of a risk model based on registry data. The model is currently undergoing evaluation and is yet to be published.
Predictors of Morbidity and Mortality in the PARTNER Trials
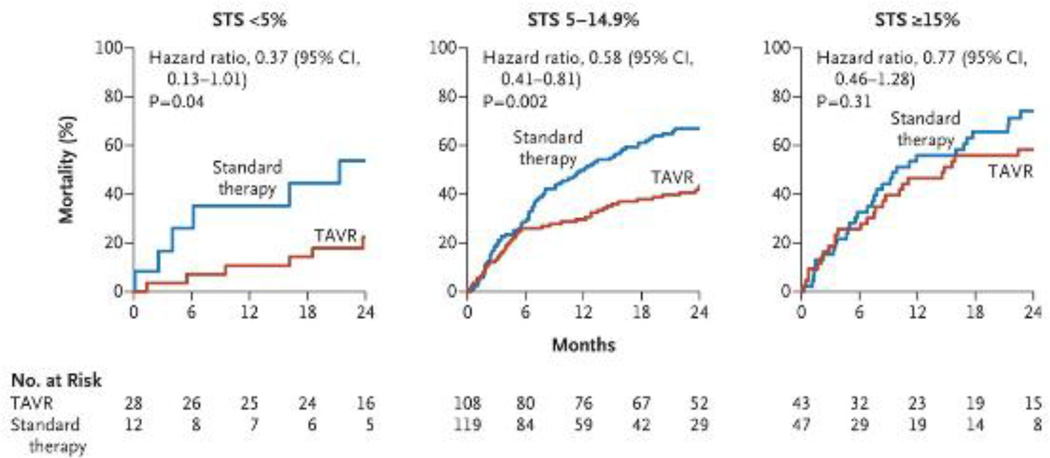
In the 2-year analysis of all participants in the inoperable (very high-risk) PARTNER cohort, Makkar et al. performed several key inquires of the comorbid conditions associated with morbidity and mortality after TAVI. First, stratification of 2-year mortality based on tertile of STS risk (<5%, 5–14.9% and ≥15%) was performed. Those patients in with an STS score ≤ 14.9% had a significantly lower mortality with TAVI than with those with STS ≥ 15% (HR 0.58; CI =0.41–0.81).(22) (See figure 1.) The study also found several other comorbidities that were statistically linked to death, including body mass index (HR per unit increase 0.95; 95% CI, 0.91 to 0.98; p=0.005); history of stroke (HR 2.99; 95% CI, 1.19 to 7.51; p=0.01); and chronic obstructive pulmonary disease (COPD).
Figure 1. Two Year Mortality Stratified According to the Society of Thoracic Surgeons (STS) Risk Score.
Stratification according to STS categories revealed a significant association with 2-year mortality (<5%, 5 to 14.9%, and ≥ 15 - with higher scores indicating greater surgical risk). From Makkar RR, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis.New Engl J Med. 2012 May 3;366(18):1696-704 Reprinted with permission from Massachusetts Medical Society.(22)
Kodali et al. performed a two-year analysis of the high-risk cohort receiving TAVI vs. SAVR.(49) The trial showed similar multivariate predictors of increased mortality as was seen in the inoperable cohort (see table 3). Body mass index, stroke, liver disease, STS score, and mitral regurgitation were associated with increased mortality. Pre-procedural creatinine level, prior vascular surgery, and a mean aortic gradient increase of > 10 mm Hg after TAVI were independently associated with increased mortality in the TAVI group but not in the SAVR group. History of coronary artery bypass grafting, STS score, liver disease and degree of mitral regurgitation were associated with worse outcomes with SAVR.
Table 3.
Comorbidities and Risk Factors Contributing to TAVI outcomes of Morbidity or Mortality
| Risk Factor | HR/OR, 95% CI, P value | Trial Evaluating |
|---|---|---|
| STS score | Dewey et al.(79) | |
| EuroSCORE | Nashef et al.(42) | |
| SURTAVI score | Van Mieghem et al.(53) | |
| BMI* | HR 0.96; CI,0.94 to 0.98; p<0.001 | Kodali et al.(49) |
| Stroke history | HR 2.99; CI, 1.19 to 7.51; p=0.01 | Makkar et al.(22) |
| COPD requiring oxygen | HR 1.69; CI, 1.05 to 2.73; p=0.03 | Makkar et al. |
| Liver disease | HR 2.24; CI, 1.30 to 4.00; p= 0.006 | Kodali et al. |
| Age** | OR 1.09, CI 1.03 to 16 p=0.005 | Auffret et al.(80) |
| Frailty | HR 3.5; CI, 1.4 to 8.5; p<0.007 | Green et al.(68) |
| Cognitive Impairment | OR 2.85 (1.32–6.17) 0.1 | Stortecky et al.(67) |
| Malnutrition*** | OR 1.30 (1.03–1.66) 0.03 | Stortecky et al. |
| Trans-apical approach | OR 3.12; CI, 1.43 to 6.82; p=0.004 | Van der Boon et al.(81) |
| PH ≥ 60 mm Hg | OR 7.56; CI, 2.58 to 22.17; p<0.001 | Auffret et al. |
| Low-Flow | HR 1.48; CI, 1.13 to 1.97; p=0.005 | Le Ven et al.(32) |
| Peri-procedural Cr | HR 1.06; CI, 1.00 to 1.13, p=0.04 | Kodali et al. |
| Prior Vascular surgery | HR 1.85; CI, 1.01 to 3.39, p=0.05 | Kodali et al. |
| Moderate to severe MR | HR 1.36 CI, 1.02 to 1.82, p=0.04 | Kodali et al. |
HR = Hazard Ratio, OR= Odds Ratio, CI= 95% Confidence Interval, BMI = Body Mass Index, COPD = Chronic Obstructive Pulmonary Disease, PH = Pulmonary Hypertension, Cr= Creatinine, MR = Mitral Regurgitation
Lower BMI seen to be protective; the hazard ratio represents an increase of 1
for each increase of 1 year
based on mini nutritional assessment (82)
Quality of Life and Disability
TAVI has been associated with improved quality of life in high-risk patients in multiple studies. For the elderly, this is frequently paramount as the quality of life often supersedes the quantity of life.(49, 55–62). Several metrics have been used to assess quality of life before and after TAVI. These include Medical Outcomes Study Short-Form 36 Health Survey (SF-36), New York Heart Association (NYHA) class, frequency of re-hospitalization, and 6-minute walk test.
In the inoperable (very high-risk) cohort of the PARTNER trial, objective quality of life metrics favored TAVI over medical therapy. A NYHA class I or II symptom rating was seen in 83% of the TAVI treatment arm compared to 43% in the standard therapy arm. The rate of re-hospitalization for cardiac reasons was lower after TAVI, as was the median number of days alive and out of the hospital (699 days vs. 355 days, p<0.001).
Krane et al. used the SF-36 survey to assess 99 patients after TAVI. SF-36 scores increased across several domains with TAVI: physical functioning score 34.7 vs. 48.5, p< 0.001, general health score 7.1 vs. 54.1, p<0.01, vitality score 37 vs. 46.1, p<0.01, and physical heath summarized score 31.2 vs. 38.6, p<0.001.(61)
Six minute walk testing is an intuitive way to assess functional capacity after TAVI. The original PARTNER trials evaluated 6-minute walk in both cohorts. Compared to medical therapy, TAVI had a significant improvement in 6 minute walk distance (p=0.02). When compared to surgery, the TAVI group had better walking distance at one month (p=0.002), but there was no difference observed at one year (p=0.67). Another study of TAVI from Europe showed that patients were able to walk significantly farther after TAVI than before the procedure: 266 meters vs. 204 meters (p<0.001).(57)
Cognitive Function and Stroke
The risk of impairing cognitive function after TAVI due to stroke or prolonged delirium is a key consideration in the elderly. In the PARTNER inoperable cohort, the rate of stroke at 2 years was markedly higher in the TAVI group than in the medically managed group (13.8% vs. 5.5%, p=0.01). Strokes within the first 30 days were mainly ischemic (12 ischemic and 1 hemorrhagic); however, from 30 days to 2 years, the rate of hemorrhagic strokes increased (5 ischemic strokes and 4 hemorrhagic stokes in the TAVI arm vs. 1 hemorrhagic and 4 ischemic strokes in the medical therapy arm).(63) The increased rate of hemorrhagic stroke is likely attributable to the standard dual antiplatelet therapy used with TAVI.
Alternatively, in the high-risk cohort of the PARTNER trial, the stroke rate was seen to be much lower: 2.1% in the SAVR group and 3.8% in the TAVI group at 30 days. Overall risk of stroke and TIA at one year was also higher in the transcatheter group (2.4% vs. 5.5%, p=0.04).
It must be noted that with the improvement in interventional techniques and operator skill, the TAVI associated stroke risk has decreased. In recent registry data from November 2011- May 2013, the Edwards Sapien transcatheter valve (Edwards Lifescience, Irvine, CA) showed a TAVI stroke risk of only 2%.(5)
There has been only limited study of cognitive function before and after TAVI. A study by Ghanem et al. assessing the frequency of cerebral emboli with diffusion weighted magnetic resonance imaging (MRI) showed that up to 64% of patients studied had evidence of emboli. Only 5.4% (5 of 111 patients) developed diagnosable early cognitive decline and even less, 2.7% (3 of 111) experienced late cognitive decline. Only patient age (p=0.012) was associated with increased risk of diminished cognitive status after TAVI. History of stroke, use of cerebral embolic protection devices, and baseline cognitive status were not significantly correlated with increased risk of early cognitive impairment.(64)
Frailty
Frailty is a state of vulnerability in which a patient has decreased physiologic reserve resulting in a poor outcome when a stressor is applied. Fried et al. completed seminal work that defined frailty based on unintentional weight loss, walking speed, and low physical activity. Her later work added other markers to our understanding of the condition; i.e. wasting, weakness, low albumin levels, and inability to perform the activities of daily living (ADLs).(65, 66)
Frailty is an extremely common problem in patients undergoing TAVI evaluation. Stortecky et al. described 100 patients in Sweden undergoing TAVI. Approximately 50% of patients were considered frail based on a derived frailty index score.(33, 67) In another study of 102 patients undergoing assessment for TAVI, 79% of those evaluated were considered frail based on the sole parameter of gait speed.(68)
Several groups have attempted evaluate the relationship between frailty and mortality with TAVI. In Stortecky et al., a frailty index score was derived based on mini mental status examination, a “Get Up and Go” test, disability scores, and a nutritional assessment. The score was not associated with greater mortality post-TAVI, but was significantly linked to subsequent CV and cerebrovascular events (OR: 4.17; 95% CI 1.37–12.72; p=0.01).
Green et al. defined a multi-modality frailty score based on ADL disability, serum albumin, gait speed, and grip strength. The study showed that a higher score in the model correlated with a shorter predicted survival after TAVI (HR 3.51; 95% CI, 1.43–8.62; p=0.006).(68)
Frailty in TAVI is an active area of ongoing research. The multicenter Frailty-AVR study will assess outcomes in 400 SAVR and 400 TAVI patients over age 70, using 7 different frailty assessment tools. This study will define which components of a frailty evaluation are most predictive of major morbidity and mortality, as well as determining the degree of frailty that would warrant TAVI inclusion over SAVR.(69)
Active Research: Novel Technologies
Industry has been aggressively developing new valve technology to improve outcomes in TAVI. Several ongoing clinical trials have been developed to address the shortcomings of the first generation valves; i.e. large catheter size leading to vascular complications, high incidence of paravalvular AR, and frequent post-procedural complete heart block requiring pacemaker placement.(70)
The designs of the second-generation valves have mainly focused on decreasing the profile of the device to allow for delivery through an 18 French (Fr) or smaller sheath. The Edwards Sapien 3, Sapien XT, and Medtronic (Minneapolis, MN) CoreValve Evolut all tout significantly smaller Fr delivery systems. The Sapien-3 has increased radial force compared to the Sapien-XT to allow for decreased paravalvular AR. It has slightly increased height compared to the first generation heart valve for improved positioning but has been associated with a greater incidence of complete heart block when compared to Sapient XT.(71)
TAVI Care Coordination and Cardiac Rehabilitation
Transitioning care after TAVI is a crucial issue. The expert consensus document on TAVI suggests a careful transition back to the primary cardiologist after the first month post-procedure. A clear plan should be agreed upon for management of co-morbid conditions that may have been uncovered during the pre-TAVI evaluation as well as treatment of complications that arose during the procedure. Further research is required to determine the proper intervals of routine imagining after valve insertion as well as the type and duration of anti-thrombotic needed to reduce the risk of stroke.
There has been very limited research into the effects of cardiac rehabilitation (CR) on post-TAVI patients. The largest study to date by Voller et al. showed that CR is efficacious in improving 6-minute walk times and exercise capacity in both TAVI and SAVR.(72) Another small trial comparing early CR effects on SAVR and TAVI patients demonstrated similar improvements in 6-minute walk time and exercise capacity on cardiopulmonary exercise testing. There was no difference in improvement between post-TAVI vs. post-SAVR patients.(73) Further study is needed to optimize the effects of CR in this cohort, to determine the geriatric interventions most likely to confer benefit, and to elicit the cost effectiveness of CR programs in the post-procedural setting.
Conclusions
In the elderly, AS is an increasingly common problem encountered in clinical practice. The development of symptoms heralds extremely high short-term mortality and compels the patients’ physician to consider intervention options in an otherwise frail population. Prior to the advent of TAVI, the physician was forced to recommend SAVR in high-risk patients and as many as 30% were denied surgery due to frailty and comorbidity.(74)
With the advent of TAVI, dramatic improvements in outcome have occurred; i.e., decreased mortality, reduced hospitalizations, improved quality of life, and diminished disability rates. Simultaneously, overall surgical morbidity and mortality has significantly decreased. However, despite the marked benefits observed in the randomized PARTNER trials, the risk of major complications including stroke, vascular events, and mortality are considerable. While adverse events related to TAVI and SAVR are steadily improving, the uncertainty when weighing the risks and benefits of these aggressive clinical strategies in high-risk patients remains significant. The available data provokes the question: how can we best define the patients most likely to benefit from the procedure with the lowest likelihood of a complication, prolonged hospitalization, or insidious subclinical cognitive decline?
The studies mentioned above describe the factors that aid in overall risk assessment for TAVI (see table 4). Additionally, anatomical and echo characteristics can also alter the individual patient risk, i.e. PAD and low-flow AS. More recently, several groups have added frailty scores to traditionally used risk calculators. Although further validation is required, these risk factors will likely have an increasingly important role in correctly individualizing valve therapy in the near future.(53, 67)
Table 4.
Studies Assessing Frailty and Outcome in TAVI vs. Cardiac Surgery.
| Study | Parameter(s) | Result | HR/OR; 95% CI, P value* |
|---|---|---|---|
| Green et al.(68) | Gaits speed, grip strength, serum albumin, ADL status in frailty score with TAVI |
↑ frailty score correlates with ↑ 1 year mortality |
HR 3.5; CI: 1.4 to 8.5, p< 0.007 |
| Afialo et al.(83) | Frailty and disability score in cardiac surgery |
5 meter gait speed and Nagi disability score ≥ 3, ↑ mortality |
Gait: OR 2.63; CI, 1.17 to 5.90 Nagi: OR 2.98; CI, 1.35–6.56 |
| De Areza et al.(84) |
EuroSCORE + 6 minute walk test for SAVR |
6 minute walk distance associated with composite CV outcome |
HR 0.28; CI, 0.09 to 0.85, p=0.025 |
| Lee et al.(85) | Frailty based on impairment of ADLs, ambulation, or history of dementia in cardiac surgery |
Frailty - ↑ hospital mortality and ↑ hospital stay |
OR 1.8; CI, 1.1 to 3.0 for mortality |
| Schoenenberger et al.(86) |
Frailty index to assess functional decline after TAVI |
Frailty index strongly predicted functional decline |
OR 1.56; CI, 1.20 to 2.04; p= 0.001 |
| Stortecky et al.(67) |
Multidimensional Geriatric Assessment (MGA) tool to predict adverse events in TAVI |
Frailty characteristics- cognitive impairment, malnutrition, mobility impairment, limitations in ADLs - were predictive of increased mortality. |
OR 3.29; CI, 1.06 to 10.15; for MGA |
ADL = activity of daily living, CV= cardiovascular
p value given if available.
The knowledge of increased risk from enhanced prognostic tools is definitely important. However, even more imperative is the physician’s clinical response once these risk factors are identified. For example, when frailty is identified in the pre-operative phase, clinicians should preemptively attempt to reduce disability, weakness, and improve nutritional status prior to TAVI. Strength training, nutritional supplementation and testosterone therapy have been the major therapies studied to-date. (75–77) However, significant physical exertion in severe AS can lead to worsening symptoms and potentially cause pulmonary edema. Testosterone therapy has recently been associated with increased CV events.(78) This leaves nutritional support as a major means of improving geriatric functioning prior to TAVI and should be routinely incorporated into TAVI program services.
The value of the doctor-patient relationship is the last major consideration. A candid discussion with the patient and the patient’s family during the evaluation of severe symptomatic AS is essential. Interventionalists and surgeons treating patients with AS must have a thorough knowledge of geriatric principles as part of a clinical standard. Understanding these elderly patients’ goals and wishes are crucial when weighing the risks and benefits of this major procedure. This can be particularly challenging when patients and their families have varying degrees of health literacy.
As the technology and experience with TAVI grows, complications and mortality will continue to decline. Given the dramatic benefits seen in the PARTNER randomized trials, recommending against TAVI in elderly patients has become progressively more difficult. There is an enduring need for larger scale studies to elucidate patients who are most likely to suffer serious complications and to establish guideline based cut-offs to guide TAVI referral in the elderly.
ABBREVIATIONS
- ADL
Activities of Daily Living
- AR
Aortic Regurgitation
- AS
Aortic Stenosis
- AVA
Aortic Valve Area
- BAV
Balloon Valvuloplasty
- CAD
Coronary Artery Disease
- COPD
Chronic Obstructive Pulmonary Disease
- CR
Cardiac Rehabilitation
- CW
Continuous Wave
- DSE
Dobutamine Stress Echocardiography
- EF
Ejection Fraction
- LV
Left Ventricular
- LVOT
Left Ventricular Outflow Tract
- MR
Mitral Regurgitation
- MRI
Magnetic Resonance Imaging
- NYHA
New York Heart Association
- PAD
Peripheral Arterial Disease
- PARTNER
Placement of Aortic Transcatheter Valve
- PW
Pulsed Wave
- SAVR
Surgical Aortic Valve Replacement
- STS
Society Thoracic Surgeons
- TAVI
Transcatheter Aortic Valve Implantation
- VTI
Velocity Time Integral;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Of note there remains debate over the optimal cut-off for AVA defining severe AS. Many experienced clinicians advocate for an echocardiographic cut-off of 0.8 cm2 and a cardiac catheterization lab cut-off of 1.0 cm2 for severe AS based on differences in the quantitative hemodynamics used to make the AVA calculation. (24)
REFERENCES
- 1.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013 Sep 10;62(11):1002–1012. doi: 10.1016/j.jacc.2013.05.015. PubMed PMID: 23727214. Epub 2013/06/04. eng. [DOI] [PubMed] [Google Scholar]
- 2.Mylotte DOR, Windecker S, et al. Transcatheter aortic valve replacement in Europe: adoption trends and factors influencing device utilization. J Am Coll Cardiol. 2013;62(3):210–219. doi: 10.1016/j.jacc.2013.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005 Feb 22;111(7):920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. PubMed PMID: 15710758. Epub 2005/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 4.P S. Transcatheter Aortic Valve Replacement: A Transformative Therapy. Prog Cardiovasc Disease. 2014 doi: 10.1016/j.pcad.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA : the journal of the American Medical Association. 2013 Nov 20;310(19):2069–2077. doi: 10.1001/jama.2013.282043. PubMed PMID: 24240934. Epub 2013/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 6.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006 Sep 16;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. PubMed PMID: 16980116. Epub 2006/09/19. eng. [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European heart journal. 2003 Jul;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. PubMed PMID: 12831818. Epub 2003/07/02. eng. [DOI] [PubMed] [Google Scholar]
- 8.Davies WRTM. European Experience and Perspectves on Transcatheter Aortic Valve Replacment Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Morrow AG, Roberts WC, Ross J, Jr, Fisher RD, Behrendt DM, Mason DT, et al. Obstruction to left ventricular outflow. Current concepts of management and operative treatment. Annals of internal medicine. 1968 Dec;69(6):1255–1286. doi: 10.7326/0003-4819-69-6-1255. PubMed PMID: 5749681. Epub 1968/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 10.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. The Journal of thoracic and cardiovascular surgery. 2012 Sep;144(3):e29–e84. doi: 10.1016/j.jtcvs.2012.03.001. PubMed PMID: 22898522. Epub 2012/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014 Jan 21;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. PubMed PMID: 24352519. Epub 2013/12/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaden JJNV, Enriquez-Sarano M. The Global Burden of Aortic Stenosis. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003 Oct;170(2):205–211. doi: 10.1016/s0021-9150(03)00284-3. PubMed PMID: 14612199. Epub 2003/11/13. eng. [DOI] [PubMed] [Google Scholar]
- 14.Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arteriosclerosis, thrombosis, and vascular biology. 1997 Mar;17(3):547–552. doi: 10.1161/01.atv.17.3.547. PubMed PMID: 9102175. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 15.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001 Mar 20;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. PubMed PMID: 11257079. Epub 2001/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, et al. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995 Oct 15;92(8):2163–2168. doi: 10.1161/01.cir.92.8.2163. PubMed PMID: 7554197. Epub 1995/10/15. eng. [DOI] [PubMed] [Google Scholar]
- 17.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994 Apr;23(5):1162–1170. doi: 10.1016/0735-1097(94)90606-8. PubMed PMID: 8144784. Epub 1994/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arteriosclerosis, thrombosis, and vascular biology. 1999 May;19(5):1218–1222. doi: 10.1161/01.atv.19.5.1218. PubMed PMID: 10323772. Epub 1999/05/14. eng. [DOI] [PubMed] [Google Scholar]
- 19.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994 Aug;90(2):844–853. doi: 10.1161/01.cir.90.2.844. PubMed PMID: 7519131. Epub 1994/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968 Jul;38(1 Suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. PubMed PMID: 4894151. Epub 1968/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, et al. The effect of aortic valve replacement on survival. Circulation. 1982 Nov;66(5):1105–1110. doi: 10.1161/01.cir.66.5.1105. PubMed PMID: 7127696. Epub 1982/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. The New England journal of medicine. 2012 May 3;366(18):1696–1704. doi: 10.1056/NEJMoa1202277. PubMed PMID: 22443478. Epub 2012/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 23.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. The Annals of thoracic surgery. 2006 Dec;82(6):2111–2115. doi: 10.1016/j.athoracsur.2006.07.048. PubMed PMID: 17126120. Epub 2006/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 24.Oh JK, Taliercio CP, Holmes DR, Jr, Reeder GS, Bailey KR, Seward JB, et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol. 1988 Jun;11(6):1227–1234. doi: 10.1016/0735-1097(88)90286-0. PubMed PMID: 3366997. Epub 1988/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009 Jan;22(1):1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101-2. PubMed PMID: 19130998. Epub 2009/01/10. eng. [DOI] [PubMed] [Google Scholar]
- 26.Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction:result of aortic valve replacement in 52 patients. Circulation. 2000 Apr 25;101(16):1940–1946. doi: 10.1161/01.cir.101.16.1940. PubMed PMID: 10779460. Epub 2000/04/26. eng. [DOI] [PubMed] [Google Scholar]
- 27.Kulik A, Burwash IG, Kapila V, Mesana TG, Ruel M. Long-term outcomes after valve replacement for low-gradient aortic stenosis: impact of prosthesis-patient mismatch. Circulation. 2006 Jul 4;114(1 Suppl):I553–I558. doi: 10.1161/CIRCULATIONAHA.105.001180. PubMed PMID: 16820636. Epub 2006/07/06. eng. [DOI] [PubMed] [Google Scholar]
- 28.deFilippi CR, Willett DL, Brickner ME, Appleton CP, Yancy CW, Eichhorn EJ, et al. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. The American journal of cardiology. 1995 Jan 15;75(2):191–194. doi: 10.1016/s0002-9149(00)80078-8. PubMed PMID: 7810504. Epub 1995/01/15. eng. [DOI] [PubMed] [Google Scholar]
- 29.Monin JL, Quere JP, Monchi M, Petit H, Baleynaud S, Chauvel C, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003 Jul 22;108(3):319–324. doi: 10.1161/01.CIR.0000079171.43055.46. PubMed PMID: 12835219. Epub 2003/07/02. eng. [DOI] [PubMed] [Google Scholar]
- 30.Tribouilloy C, Levy F, Rusinaru D, Gueret P, Petit-Eisenmann H, Baleynaud S, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009 May 19;53(20):1865–1873. doi: 10.1016/j.jacc.2009.02.026. PubMed PMID: 19442886. Epub 2009/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 31.Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006 Apr 11;113(14):1738–1744. doi: 10.1161/CIRCULATIONAHA.105.568824. PubMed PMID: 16585393. Epub 2006/04/06. eng. [DOI] [PubMed] [Google Scholar]
- 32.Le Ven F, Freeman M, Webb J, Clavel MA, Wheeler M, Dumont E, et al. Impact of low flow on the outcome of high-risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013 Aug 27;62(9):782–788. doi: 10.1016/j.jacc.2013.05.044. PubMed PMID: 23770162. Epub 2013/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 33.Sintek MZA. Patient Evaluation and Selection for Transcatheter Aortic Valve Replacvment: The Heart Team Approach. Prog in Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Lauten A, Zahn R, Horack M, Sievert H, Linke A, Ferrari M, et al. Transcatheter aortic valve implantation in patients with low-flow, low-gradient aortic stenosis. JACC Cardiovascular interventions. 2012 May;5(5):552–559. doi: 10.1016/j.jcin.2012.04.001. PubMed PMID: 22625194. Epub 2012/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 35.Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, et al. Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension. 2003 Jun;41(6):1268–1272. doi: 10.1161/01.HYP.0000070029.30058.59. PubMed PMID: 12707297. Epub 2003/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 36.Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010 Aug;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. PubMed PMID: 20530299. Epub 2010/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 37.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA : the journal of the American Medical Association. 2013 Dec 18; doi: 10.1001/jama.2013.284427. PubMed PMID: 24352797. Epub 2013/12/20. Eng. [DOI] [PubMed] [Google Scholar]
- 38.Otto CM, Mickel MC, Kennedy JW, Alderman EL, Bashore TM, Block PC, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation. 1994 Feb;89(2):642–650. doi: 10.1161/01.cir.89.2.642. PubMed PMID: 8313553. Epub 1994/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 39.Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation. 1991 Dec;84(6):2383–2397. doi: 10.1161/01.cir.84.6.2383. PubMed PMID: 1959194. Epub 1991/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 40.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 Sep 23;52(13):e1–e142. doi: 10.1016/j.jacc.2008.05.007. PubMed PMID: 18848134. Epub 2008/10/14. eng. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. The Annals of thoracic surgery. 2009 Jul;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. PubMed PMID: 19559823. Epub 2009/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 42.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1999 Jul;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. PubMed PMID: 10456395. Epub 1999/08/24. eng. [DOI] [PubMed] [Google Scholar]
- 43.Adult Cardiac Surgery Database Executive Summary 10 years. [cited 2013 March 2];STS report Period Ending 3/31/2010. Available from: http://www.sts.org/sites/default/files/documents/20112ndHarvestExecutiveSummary.pdf.
- 44.Bouma BJ, van Den Brink RB, van Der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart (British Cardiac Society) 1999 Aug;82(2):143–148. doi: 10.1136/hrt.82.2.143. PubMed PMID: 10409526. Pubmed Central PMCID: PMC1729124. Epub 1999/07/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar RLA, Colombo A, et al. Self Expanding Prostheses for Trabscatheter Aortic Valve Replacement. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Pollak PMMM, Holmes DR., Jr Quality, Economics and National Guidelines for Transcatheter Aortic Valve Replacement. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010 Oct 21;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. PubMed PMID: 20961243. Epub 2010/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 48.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine. 2011 Jun 9;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. PubMed PMID: 21639811. Epub 2011/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 49.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. The New England journal of medicine. 2012 May 3;366(18):1686–1695. doi: 10.1056/NEJMoa1200384. PubMed PMID: 22443479. Epub 2012/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 50.Genereux P, Cohen DJ, Williams MR, Mack M, Kodali SK, Svensson L, et al. Bleeding Complications after Surgical Aortic Valve Replacement (SAVR) Compared with Transcatheter Aortic Valve Replacement (TAVR): Insights from the PARTNER I Trial. J Am Coll Cardiol. 2013 Nov 13; doi: 10.1016/j.jacc.2013.10.058. PubMed PMID: 24291283. Epub 2013/12/03. Eng. [DOI] [PubMed] [Google Scholar]
- 51.Brennan JM, Edwards FH, Zhao Y, O’Brien S, Booth ME, Dokholyan RS, et al. Early anticoagulation of bioprosthetic aortic valves in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. J Am Coll Cardiol. 2012 Sep 11;60(11):971–977. doi: 10.1016/j.jacc.2012.05.029. PubMed PMID: 22921973. Epub 2012/08/28. eng. [DOI] [PubMed] [Google Scholar]
- 52.Wong CY, Green P, Williams M. Decision-making in transcatheter aortic valve replacement: the impact of frailty in older adults with aortic stenosis. Expert review of cardiovascular therapy. 2013 Jun;11(6):761–772. doi: 10.1586/erc.13.45. PubMed PMID: 23750685. Epub 2013/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 53.van Mieghem NM, Head SJ, van der Boon RM, Piazza N, de Jaegere PP, Carrel T, et al. The SURTAVI model: proposal for a pragmatic risk stratification for patients with severe aortic stenosis. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012 Jun 20;8(2):258–266. doi: 10.4244/eijv8i2a40. PubMed PMID: 22912963. Epub 2012/08/23. eng. [DOI] [PubMed] [Google Scholar]
- 54.Banjona PSR, Greason K, Hartzell S. Outcome of Surgical Aortic Valve Replacment: The Benchmark for Percutaneous Therapies. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Georgiadou P, Kontodima P, Sbarouni E, Karavolias GK, Smirli A, Xanthos T, et al. Long-term quality of life improvement after transcatheter aortic valve implantation. American heart journal. 2011 Aug;162(2):232–237. doi: 10.1016/j.ahj.2011.06.004. PubMed PMID: 21835282. Epub 2011/08/13. eng. [DOI] [PubMed] [Google Scholar]
- 56.Gotzmann M, Bojara W, Lindstaedt M, Ewers A, Bosche L, Germing A, et al. One-year results of transcatheter aortic valve implantation in severe symptomatic aortic valve stenosis. The American journal of cardiology. 2011 Jun 1;107(11):1687–1692. doi: 10.1016/j.amjcard.2011.01.058. PubMed PMID: 21439537. Epub 2011/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 57.Gotzmann M, Hehen T, Germing A, Lindstaedt M, Yazar A, Laczkovics A, et al. Short-term effects of transcatheter aortic valve implantation on neurohormonal activation, quality of life and 6-minute walk test in severe and symptomatic aortic stenosis. Heart (British Cardiac Society) 2010 Jul;96(14):1102–1106. doi: 10.1136/hrt.2009.180661. PubMed PMID: 19884109. Epub 2009/11/04. eng. [DOI] [PubMed] [Google Scholar]
- 58.Bekeredjian R, Krumsdorf U, Chorianopoulos E, Kallenbach K, Karck M, Katus HA, et al. Usefulness of percutaneous aortic valve implantation to improve quality of life in patients >80 years of age. The American journal of cardiology. 2010 Dec 15;106(12):1777–1781. doi: 10.1016/j.amjcard.2010.08.017. PubMed PMID: 21055715. Epub 2010/11/09. eng. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011 Nov 1;124(18):1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. PubMed PMID: 21969017. Epub 2011/10/05. eng. [DOI] [PubMed] [Google Scholar]
- 60.Ussia GP, Mule M, Barbanti M, Cammalleri V, Scarabelli M, Imme S, et al. Quality of life assessment after percutaneous aortic valve implantation. European heart journal. 2009 Jul;30(14):1790–1796. doi: 10.1093/eurheartj/ehp171. PubMed PMID: 19443421. Epub 2009/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 61.Krane M, Deutsch MA, Bleiziffer S, Schneider L, Ruge H, Mazzitelli D, et al. Quality of life among patients undergoing transcatheter aortic valve implantation. American heart journal. 2010 Sep;160(3):451–457. doi: 10.1016/j.ahj.2010.05.038. PubMed PMID: 20826252. Epub 2010/09/10. eng. [DOI] [PubMed] [Google Scholar]
- 62.Du H, Newton PJ, Salamonson Y, Carrieri-Kohlman VL, Davidson PM. A review of the six-minute walk test: its implication as a self-administered assessment tool. European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2009 Mar;8(1):2–8. doi: 10.1016/j.ejcnurse.2008.07.001. PubMed PMID: 18694656. Epub 2008/08/13. eng. [DOI] [PubMed] [Google Scholar]
- 63.Ribiero HBUM, Allende R, et al. Balloon-Expandable Prostheses for Transcatheter Aortic Valve Replacement. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Ghanem A, Kocurek J, Sinning JM, Wagner M, Becker BV, Vogel M, et al. Cognitive trajectory after transcatheter aortic valve implantation. Circulation Cardiovascular interventions. 2013 Dec 1;6(6):615–624. doi: 10.1161/CIRCINTERVENTIONS.112.000429. PubMed PMID: 24129642. Epub 2013/10/17. eng. [DOI] [PubMed] [Google Scholar]
- 65.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. PubMed PMID: 11253156. Epub 2001/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 66.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. PubMed PMID: 15031310. Epub 2004/03/20. eng. [DOI] [PubMed] [Google Scholar]
- 67.Stortecky S, Schoenenberger AW, Moser A, Kalesan B, Juni P, Carrel T, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovascular interventions. 2012 May;5(5):489–496. doi: 10.1016/j.jcin.2012.02.012. PubMed PMID: 22625186. Epub 2012/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 68.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovascular interventions. 2012 Sep;5(9):974–981. doi: 10.1016/j.jcin.2012.06.011. PubMed PMID: 22995885. Pubmed Central PMCID: PMC3717525. Epub 2012/09/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leon MDGH, Fontana GP. Challenges and Future Opportunities for Transcatheter Aortic Valve Therapy. Prog Cardiovasc Dis. 2014 doi: 10.1016/j.pcad.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Fanning JP, Platts DG, Walters DL, Fraser JF. Transcatheter aortic valve implantation (TAVI): valve design and evolution. International journal of cardiology. 2013 Oct 3;168(3):1822–1831. doi: 10.1016/j.ijcard.2013.07.117. PubMed PMID: 23972363. Epub 2013/08/27. eng. [DOI] [PubMed] [Google Scholar]
- 71.Binder RK, Rodes-Cabau J, Wood DA, Webb JG. Edwards SAPIEN 3 valve. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012 Sep;8(Suppl Q):Q83–Q87. doi: 10.4244/EIJV8SQA15. PubMed PMID: 22995118. Epub 2013/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 72.Voller H, Salzwedel A, Nitardy A, Buhlert H, Treszl A, Wegscheider K. Effect of cardiac rehabilitation on functional and emotional status in patients after transcatheter aortic-valve implantation. European journal of preventive cardiology. 2014 Feb 27; doi: 10.1177/2047487314526072. PubMed PMID: 24577878. Epub 2014/03/01. Eng. [DOI] [PubMed] [Google Scholar]
- 73.Russo N, Compostella L, Tarantini G, Setzu T, Napodano M, Bottio T, et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. European journal of preventive cardiology. 2013 Jun 11; doi: 10.1177/2047487313494029. PubMed PMID: 23757283. Epub 2013/06/13. Eng. [DOI] [PubMed] [Google Scholar]
- 74.Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? European heart journal. 2005 Dec;26(24):2714–2720. doi: 10.1093/eurheartj/ehi471. PubMed PMID: 16141261. Epub 2005/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 75.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. J Am Coll Cardiol. 2014 Mar 4;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070. PubMed PMID: 24291279. Epub 2013/12/03. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. The New England journal of medicine. 1994 Jun 23;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. PubMed PMID: 8190152. Epub 1994/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 77.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: A meta-analysis. Journal of the American Geriatrics Society. 2006 Nov;54(11):1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. PubMed PMID: 17087692. Pubmed Central PMCID: PMC1752197. Epub 2006/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA : the journal of the American Medical Association. 2013 Nov 6;310(17):1829–1836. doi: 10.1001/jama.2013.280386. PubMed PMID: 24193080. Epub 2013/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 79.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 2008 Jan;135(1):180–187. doi: 10.1016/j.jtcvs.2007.09.011. PubMed PMID: 18179938. Epub 2008/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 80.Auffret V, Boulmier D, Oger E, Bedossa M, Donal E, Laurent M, et al. Predictors of 6-month poor clinical outcomes after transcatheter aortic valve implantation. Archives of cardiovascular diseases. 2014 Jan;107(1):10–20. doi: 10.1016/j.acvd.2013.10.005. PubMed PMID: 24361056. Epub 2013/12/24. eng. [DOI] [PubMed] [Google Scholar]
- 81.van der Boon RM, Marcheix B, Tchetche D, Chieffo A, Van Mieghem NM, Dumonteil N, et al. Transapical versus transfemoral aortic valve implantation: a multicenter collaborative study. The Annals of thoracic surgery. 2014 Jan;97(1):22–28. doi: 10.1016/j.athoracsur.2013.09.088. PubMed PMID: 24263012. Epub 2013/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 82.Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clinics in geriatric medicine. 2002 Nov;18(4):737–757. doi: 10.1016/s0749-0690(02)00059-9. PubMed PMID: 12608501. Epub 2003/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 83.Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circulation Cardiovascular quality and outcomes. 2012 Mar 1;5(2):222–228. doi: 10.1161/CIRCOUTCOMES.111.963157. PubMed PMID: 22396586. Epub 2012/03/08. eng. [DOI] [PubMed] [Google Scholar]
- 84.de Arenaza DP, Pepper J, Lees B, Rubinstein F, Nugara F, Roughton M, et al. Preoperative 6-minute walk test adds prognostic information to Euroscore in patients undergoing aortic valve replacement. Heart (British Cardiac Society) 2010 Jan;96(2):113–117. doi: 10.1136/hrt.2008.161174. PubMed PMID: 19561363. Epub 2009/06/30. eng. [DOI] [PubMed] [Google Scholar]
- 85.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010 Mar 2;121(8):973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. PubMed PMID: 20159833. Epub 2010/02/18. eng. [DOI] [PubMed] [Google Scholar]
- 86.Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) European heart journal. 2013 Mar;34(9):684–692. doi: 10.1093/eurheartj/ehs304. PubMed PMID: 23008508. Epub 2012/09/26. eng. [DOI] [PubMed] [Google Scholar]