Abstract
Extracellular matrix proteins such as fibronectin (FNT) play crucial roles in cell proliferation, adhesion, and migration. For better understanding of these associated cellular activities, various microscopic manipulation tools have been used to study their intracellular signaling pathways. Recently, it has appeared that acoustic tweezers may possess similar capabilities in the study. Therefore, we here demonstrate that our newly developed acoustic tweezers with a high-frequency lithium niobate ultrasonic transducer have potentials to study intracellular calcium signaling by FNT-binding to human breast cancer cells (SKBR-3). It is found that intracellular calcium elevations in SKBR-3 cells, initially occurring on the microbead-contacted spot and then eventually spreading over the entire cell, are elicited by attaching an acoustically trapped FNT-coated microbead. Interestingly, they are suppressed by either extracellular calcium elimination or phospholipase C (PLC) inhibition. Hence, this suggests that our acoustic tweezers may serve as an alternative tool in the study of intracellular signaling by FNT-binding activities.
Keywords: Acoustic tweezers, High-frequency ultrasound transducer, Fibronectin, SKBR-3 breast cancer cells, Intracellular calcium elevation
1. Introduction
Precise manipulation techniques using optical-, magnetic-, and acoustic tweezers (or trapping) have been developed to spatially control microscopic cells or polymer beads for quantitative analysis of many cellular properties either directly or indirectly. In particular, a micron-sized polystyrene bead (hereinafter called a microbead) is often coated with fibronectin (FNT), one of extracellular matrix (ECM) proteins which play important roles in cell adhesion, migration, growth and differentiation [1,2]. This remarkably complex protein has become the subject of exciting discoveries regarding new integrin sites and molecules [3]. For example, the FNT-integrin binding promoted calcium influx in various cells including T-cells, endothelial cells, and trophoblast cells [4–6]. However, it has been so far known little about how the FNT triggers such diverse intracellular signaling transduction responsible for transmitting information inside the cells.
By using optical tweezers, surface-modified microbeads with FNT were attached to various cells in order to examine their mechanical properties [7] as well as the linkage of FNT-integrin-cytoskeleton in fibroblast cells [8]. A FNT-coated microbead serving like a force probe was also tethered to a human umbilical vein endothelial cell, and displaced from the trap center (or the focus) for stretching actin fibers of the cell [8]. Despite the precision and versatility of optical tweezers as a contactless manipulation tool, however, they have a few practical shortcomings such as limited use in optically transparent media and relatively weak trapping force in the pico-newton (pN) range [9].
Magnetic trapping methods were employed to apply a shear stress on a mouse embryonic fibroblast by handling a 4.5 μm FNT-coated superparamagnetic microbead linked to the adhesion contact on the apical cell surface [10]. Magnetic force was generated from a solenoid with a sharp-tipped steel needle, laterally pulling the cell-bound magnetic microbead with a force range of nano-newtons (nN). In order to exert a trapping force on a cell directly, magnetic microbeads were inserted inside a cell [11,12], which may cause unexpected rupture that adversely affects cell viability or function.
Several acoustic techniques have been developed as an alternative tool for cell manipulation. In particular, they can readily be applied to light opaque media and operated with relatively moderate energy use in comparison to optical and magnetic trapping approaches. Surface standing acoustic wave (SSAW), for instance, was utilized for achieving various particle patterns of fluorescent polystyrene microbeads, erythrocytes, and Escherichia coli in microfluidic channels [13]. Agglomerates of 10 μm polystyrene microbeads were also transported by moving the Bessel-function pressure fields emitted from a 2.35 MHz 16-element circular array transducer [14]. The phase delay of an excitation sinusoidal signal given to each element was adjusted to change the location of a trapped microbead in an enclosed area by the transducer itself.
In contrast to those SSAW trapping techniques, we have recently devised a two-dimensional transverse (or lateral) trapping method to manipulate micron-sized cells or particles with single element or array focused ultrasonic transducers. It was experimentally realized that individual lipid droplets and leukemia cells were trapped with a single element focused transducer at 30 MHz and 200 MHz, respectively [15,16]. A 26 MHz linear phased array was also exploited for directing a polystyrene microbead to a targeted position via electronic scanning of the array elements [17]. More recently, a 193 MHz lithium niobate (LiNbO3) focused transducer was applied to studying the elastic property of breast cancer cells (MCF-7). In the study, a 5 μm FNT-coated polystyrene microbead, which was tagged to a MCF-7 cell, was pulled toward the focus to mechanically deform cell membrane. A dependence of the membrane’s stretched length on the trapping strength was evaluated as a function of excitation voltage amplitude to the transducer [18].
For further suggesting the versatility of our acoustic tweezers other than in cellular mechanistic studies pursued so far, this paper demonstrates that our newly developed acoustic tweezers with a high-frequency lithium niobate ultrasonic transducer have also potentials to study intracellular calcium signaling in human breast cancer cells. In particular, in order to show the capability of the acoustic tweezers in cell signaling study, we examine whether attachment of an acoustically trapped FNT-coated microbead to SKBR-3 cells elicits the intracellular calcium elevation in the cells. The LiNbO3 transducers are here used to trap a single FNT-coated polystyrene microbead that is bound to a SKBR-3 cell membrane. The calcium variation inside the cell is monitored by using fluorescence imaging of Fluo-4 AM (acetoxymethyl ester), a calcium fluorescent indicator. The effect of FNT-cell binding on the intracellular calcium level is also compared with the case of a non-FNT-coated microbead. We furthermore investigate calcium propagation over the cell and the dependence of calcium elevation on extra-calcium and phospholipase C (PLC) levels during the FNT-microbead attachment. The results convincingly demonstrate the potential of acoustic tweezers as a cell manipulation tool in studying intracellular signaling mechanisms caused by FNT binding to the cell surface, and therefore may shed light on the effect of FNT on adhesion, invasion, and migration of breast cancer cells.
2. Material and methods
2.1. Working principle of acoustic tweezers (or trapping)
Let two incident rays in a Gaussian intensity field strike a polystyrene microbead in water as shown in Fig. 1. Both longitudinal and shear waves propagate inside the microbead, while only a longitudinal mode is present in water. As passing through the microbead, each incoming ray propagates along different paths from which it initially takes. Changes in the direction lead to the momentum transfer, applying the acoustic radiation force on the microbead (Fa or Fb). Due to the asymmetric intensity distribution, a net (trapping) force F (=Fa + Fb) may direct the microbead toward the central beam axis. The trapping force can be viewed as a sum of two components, scattering and gradient forces. The scattering force, Fs, arising from reflection, repels the microbead away from the beam axis. In contrast, the gradient force, Fg is originated from refraction and attracts the microbead toward the center axis. Typically, a restoring force required for acoustic tweezers can be acted on the microbead when the gradient force is greater than the scattering force [19].
Fig. 1.
Illustration of the acoustic trapping mechanism.
2.2. Microbead coating with FNT
Human breast cancer cell lines (SKBR-3, ATCC, Manassas, VA, USA) are utilized for this study, as highly expressing integrin receptors that serve as a crossbridge for cell-ECM protein interaction on the membrane. Their ECM binding is a key factor to determine cellular growth, apoptosis, invasion, and metastasis. Specific types of integrins associated with certain cancer cells have been selected for a targeted therapy during cancer mechanotransduction process. Due to high affinity interaction between integrin and FNT, 5 μm and 15 μm polystyrene microbeads (Polybead®, Polysciences Inc., Warrington, PA, USA) are coated with FNT (F1141, Sigma–Aldrich, St. Louis, MO, USA) for monitoring calcium changes in the cell.
2.3. Transducer characteristics
A press-focused high-frequency LiNbO3 transducer with a center frequency of 193 MHz and −6 dB bandwidth of 114% is employed to trap a 5 μmFNT-coated microbead. The aperture diameter and focal length of the transducer are 0.65 mm and 0.75 mm, respectively (f-number = 1.1). A pulse-echo test of the transducer with a 2.5 μm tungsten wire target is carried out in both axial and lateral directions with an ultrasonic pulser-receiver (5910PR, Panametrics, Waltham, MA, USA). The measured lateral beam width and axial depth of focus are found to be 9.0 μm and 65.9 μm[20]. In contrast, a press-focused high-frequency LiNbO3 transducer with a center frequency of 47 MHz and −6 dB bandwidth of 74% is employed to trap a 15 μm FNT-coated microbead since the 193 MHz transducer has not offered reliable acoustic trapping of the 15 μm FNT-coated microbead. The aperture diameter and focal length of the transducer are 3.0 mm and 5.0 mm, respectively (f-number = 1.7). Lateral acoustic pressure is measured by a hydrophone (HPM04/01, Precision Acoustics, UK) with a driving voltage of 9.5 Vpp. The measured −6-dB lateral beam width is found to be 85 μm at a focal depth (Fig. 2b)
Fig. 2.
(a) System configuration for microbead trapping experiment. (b) Pulse-echo characteristics of a 47 MHz LiNbO3 transducer. (Upper: a photographic image of transducer, lower-left: pulse-echo signals, and lower-right: frequency characteristics of the pulse-echo signals.) The red line indicates −6 dB bandwidth of 74% (c) Illustration of the experimental procedure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.4. Experimental procedure
The acoustic trapping system, as shown in Fig. 2a, is utilized to immobilize a FNT-coated microbead as well as carry out fluorescence imaging of a SKBR-3 breast cancer cell. Fluorescent calcium indicators, Fluo-4 AM (Invitrogen, Carlsbad, CA, USA), are loaded into SKBR-3 cells pre-cultured in complete Dulbecco’s modified eagle medium (Invitrogen, Carlsbad, CA, USA) at 37 °C for 36 h, prior to monitoring intracellular calcium changes in the cell. 1 μM indicator solution diluted with Hank balanced salted solution (HBSS) (Invitrogen, Carlsbad, CA, USA) is added in a 35 mm petri-dish containing the cells, as being incubated at room temperature for 30 min. After the Fluo-4 AM solution is aspirated, the cells are thoroughly washed with phosphate buffered saline (PBS) solution (pH = 7.4) (Invitrogen, Carlsbad, CA, USA) and 2 mL HBSS solution, containing ~5 × 104 FNT-coated microbeads, is added into the cell dish. The cell dish is then placed on top of a microstage in an inverted epi-fluorescence microscope (IX71, Olympus, Center Valley, PA, USA). In order to hold up an FNT-coated microbead at a certain distance from a targeted cell, a 200 MHz sinusoidal burst waveform with the peak-to-peak voltage amplitude of 6.3 Vpp is supplied to the transducer (center frequency: 193 MHz) for the trapping of a 5 μm microbead whereas a 47 MHz sinusoidal burst waveform with the peak-to-peak voltage amplitude of 9.5 Vpp is supplied to the transducer (center frequency: 47 MHz) for the trapping of a 15 μm microbead. The duty factor (DF) and pulse repetition frequency (PRF) are 1% and 1 kHz, respectively. In our previous study, we utilized the 200 MHz sinusoidal bursts for excitation of the same transducer to trap a 5 μm microbead. Therefore, we here also utilized the same 200 MHz sinusoidal burst waveform for acoustic trapping in order to achieve the similar acoustic trapping condition. In fact, the higher frequency of ultrasound produces the narrower beam, better enabling acoustic trapping of a single 5 μm microbead [20]. The trapped microbead is transversely directed toward the cell for setting up a bead-cell binding. Fluorescence cell images (Ex: 488 nm and Em: 532 nm) for monitoring intracellular calcium changes are acquired every 0.5 s via a CCD camera (ORCA-Flash2.8, Hamamatsu Photonics, Hamamatsu, SZK, Japan) implemented with the microscope. During the image acquisition, the microbead position is retained at a contact site on the cell membrane for approximately 25 s (Fig. 2c).
2.5. Reagents and inhibitor treatment
To determine the dependence of FNT-binding induced calcium elevation on calcium influx across the plasma membrane, calcium-free HBSS (Invitrogen, Carlsbad, CA, USA) is utilized to eliminate extracellular calcium. We also examine the effect of PLC activity on the calcium elevation by applying U73122 (U73122, Sigma–Aldrich, St. Louis, MO, USA), a PLC inhibitor, over the cells at 5 μM for 10 min to block the release of inositol trisphosphate (IP3).
2.6. Cell viability test
Cell viability is tested with a cell-permeant dye, Calcein AM (Invitrogen, Carlsbad, CA, USA) to check whether the microbead-cell pair undergoing the trapping force damages cell function [21]. The cells are incubated in 1 μM Calcein solution for 30 min. Fluorescence images are then acquired at designated moments to compare changes in cell viability due to the trapping force application. For statistical analysis, mean and standard deviation of fluorescence level at each moment are obtained with the sample size of n = 12. A two-tailed paired t-test is also undertaken with the level of significance set at p-value < 0.05.
3. Results
3.1. Intracellular Ca2+ elevations Induced by cell attachment of a trapped FNT-coated microbead
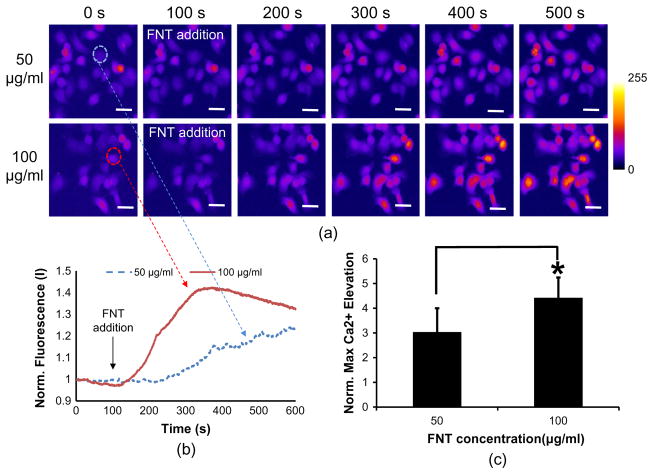
The trapped FNT-coated microbead attachment to a SKBR-3 cell yields significant calcium elevations (Fig. 3a, upper), whereas the non-FNT-coated microbead hardly elicits any notable calcium change within the cell (Fig. 3a, lower) (Supplementary video 1 and 2). Fig. 3b illustrates the normalized temporal calcium variations corresponding to both cases. The cell clearly exhibits transient calcium elevations when the trapped FNT-coated microbead is attached to the cell surface. The calcium level is then gradually reduced to ~1 until t = 100 s. The normalized maximum value is 1.67 ± 0.56 for the FNT-coated microbead binding, while 1.03 ± 0.08 (n = 10) for the non-FNT case. When tethered to the cell, consequently, these FNT-coated microbeads are more likely to increase intracellular calcium activities than otherwise pure microbeads (p-value = ~0.009 < 0.05). In addition, the calcium elevation level in SKBR-3 cells by 5 μm FNT-coated microbead binding is compared with that in SKBR-3 cells by 15 μm FNT-coated microbead binding. Interestingly, the calcium elevations in the cells by the 15 μm FNT-coated microbead binding are significantly higher than those in the cells by the 5 μm FNT-coated microbead binding (Fig. 4). The normalized maximum value is 6.56 ± 2.33 for the 15 μm FNT-coated microbead (p-value = ~0.0001 < 0.05). Finally, calcium elevations in a SKBR-3 cell by free FNT binding are examined in order to confirm whether the calcium elevations in SKBR-3 cells are caused by FNT coated on the microbead. When free FNTs at 50 and 100 μg/ml (final concentration) are added in the cells, respectively, the cells exhibit calcium elevations as response to the FNT addition at both concentrations (Fig. 5a). In particular, it is found that FNTs at 100 μg/ml elicit higher calcium elevations in the cells than FNTs at 50 μg/ml (Fig. 5b and c). Therefore, these results suggest that the calcium elevations in SKBR-3 cells by the acoustically trapped FNT-coated microbead binding are indeed elicited by FNT binding and also the calcium elevations are dependent on FNT binding concentrations.
Fig. 3.
Calcium elevation in SKBR-3 cells due to FNT-coated microbeads: (a) comparison of calcium elevation between FNT-coated- (upper) and non-FNT-coated microbead attachment (lower). Each arrow indicates a trapped microbead location (b) Temporal profiles of calcium elevation arising from FNT-coated- (curved line) and non-FNT-coated microbead binding (flat line). (c) Quantitative analysis of calcium elevation due to FNT-coated- (right) and non-FNT-coated microbeads (left). (n = 9, p-value = ~0.009 < 0.05).
Fig. 4.

Calcium elevations in SKBR-3 cells induced by the 5 μm (n = 10) and 15 μm (n = 12) FNT-coated microbead binding (p-value = ~0.00001 < 0.05).
Fig. 5.
Calcium elevation in SKBR-3 cells by free FNT binding (a) Calcium fluorescence images of SKBR-3 cells at the indicated time-points (50 μg/ml: upper and 100 μg/ml: lower). (b) Temporal calcium changes in SKBR-3 cells due to free FNT binding at 50 (blue-dotted line) and 100 μg/ml (red-solid line) (representative ones). The black-solid arrow indicates the time point when free FNTs are added into the cell. (c) Quantitative analysis of calcium elevations due to free FNT binding at 50 (left) and 100 μg/ml (right) (n = 13, p-value = ~0.045 < 0.05). The scar bar indicates ~20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Ca2+ Propagation Induced by FNT-coated microbead attachment using acoustic tweezers
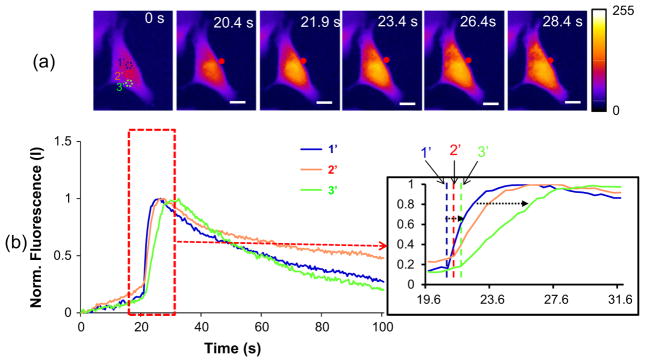
When the FNT-coated microbead is attached to the cell (Fig. 6a, t = 20.8 s), the initial calcium elevation is localized in the region 1′ that is adjacent to the attached FNT-coated microbead (t = 22.4 s), and then found in the regions 2′ and 3′, sequentially (t = 24.0 s and t = 30.8 s). The temporal calcium fluorescence changes are shown in Fig. 6b. The initial time of calcium rise is t = 20.8 s in the region 1′, whereas t = 21.2 s and t = 22.0 s in the regions 2′ and 3′, respectively (Fig. 6b). The maximum calcium elevation also starts at t = 26.0 s in the region 1′, while at t = 27.6 s and t = 30.0 s in the regions 2′and 3′, respectively. The results indicate that the calcium elevation is initially elicited in a contact spot where the FNT-coated microbead is attached, and then spread over the neighboring regions in the SKBR-3 cell later on. Hence, FNT-coated microbead attachment is likely to provoke more calcium influx that further leads to a global intracellular calcium elevation throughout the cell.
Fig. 6.
Calcium propagation over a SKBR-3 cell when the FNT microbead is attached to the cell: (a) Sequential fluorescence images obtained at the indicated time points after the microbead attachment (b) Temporal fluorescence changes at the selected areas [1′: contact spot, 2′: middle area, and 3′: far area) of the cell. The scar bar indicates ~10 μm.
3.3. Dependence of calcium elevation on extra-calcium level and PLC activity
In the previous section, it is found that the initial calcium elevation is locally elicited by the cell attachment of a trapped FNT-coated microbead and then spread over the entire cell, which implies that a localized calcium elevation may arise from calcium influx caused by FNT binding activity. To verify this finding, the effect of extracellular calcium level on intracellular calcium rise is examined by trapping a FNT-coated microbead. Fig. 7 shows that extracellular calcium elimination is found to substantially inhibit intracellular calcium elevation in SKBR-3 cells due to FNT-microbead attachment to the cells in comparison to the control cells (no treatment) (p-value = ~0.003 < 0.05, n = 10).
Fig. 7.

Quantitative analysis of calcium elevation in SKBR-3 cells under calcium free HBSS (No calcium) and treated with PLC inhibitors (U73122) (n = 10).
In addition, the influence of PLC activity on FNT-binding induced calcium elevation in SKBR-3 cells is investigated using acoustic trapping of a FNT-coated microbead since PLC has been known to be primarily involved in FNT-induced elevation of cytoplasmic calcium in various cells including blastocyst [22,23]. According to the t-test (p-value = ~0.03 < 0.05), it can readily be seen that the PLC inhibitor (U73122) suppresses the calcium elevation while a FNT-coated microbead is attached to the cell (Fig. 7). The normalized maximum fluorescence is 1.67 ± 0.28 for the control cells, whereas 1.30 ± 0.38 for the PLC-treated cells (n = 10). These results therefore demonstrate that calcium elevations in the SKBR-3 cells by the trapped FNT-coated microbead attachment may involve the PLC activity as shown in other types of cells.
3.4. Trapping force effect on cell viability
Fig. 8a shows the viability change of SKBR-3 cells before- and after ultrasonic interrogation. No noticeable change in Calcien fluorescence intensity that measures the cell viability occurs after FNT-coated microbead attachment to the cell at the trap (>25 s) (p-value: 0.56 > 0.05). The difference of mean intensities between t = 0 s and t = 120 s is 0.8%, indicating that mechanical disturbance by a trapped microbead does not negatively alter the cell viability under the given trapping condition. In addition, the viabilities of SKBR-3 cells are examined in 24 h after the 5 μm and 15 μm FNT-coated microbead attachment, respectively. The SKBR-3 cells are still viable in 24 h after the 5 μm (Fig. 8b, left) and 15 μm (Fig. 8b, right) FNT-coated microbead are bound to the cells.
Fig. 8.
Quantitative analysis of SKBR-3 cell viability influenced by an acoustically trapped microbead: (a) Plot of mean Calcien florescence intensity as a function of time after FNT-microbead binding (n = 12). The arrow indicates the moment when a trapped FNT-coated microbead is attached to a cell. (b) Cell viability in 24 h after the attachment of a 5 μm (left) and a 15 μm (right) FNT-microbead to cells. The arrows indicate the cells undergoing the FNT-coated microbead binding activity.
4. Discussion
Various types of acoustic tweezers with a very high-frequency ultrasonic transducer have been used to manipulate cells and micron size of particles [15,18]. In this paper, the acoustic tweezers, which generate highly focused microbeams at 200 MHz and 47 MHz, are utilized to trap a 5 μm and a 15 μm functionalized microbead to study intracellular calcium signaling activated by FNT binding activities. Note that higher frequency yields a sharper beam allowing better precision to be achieved and smaller particles trapped. Hence, the ultrasonic transducer having much higher center frequency of 200 MHz is utilized to bind 5 μm polystyrene microbeads to the cell membrane. In Fig. 3, it is shown that attachment of the acoustically trapped FNT-coated microbead to the SKBR-3 cells clearly induces significant intracellular calcium elevations compared to attachment of the trapped non-FNT microbead to the cells. Our previous studies have shown that 200 MHz ultrasound microbeams themselves were able to promote intracellular calcium elevations in highly-invasive breast cancer cells (MDA-MB-231), but not in weakly-invasive breast cancer cells such as SKBR-3 cells [24]. In addition, such a high-frequency ultrasonic stimulation resulted in considerable cytoplasmic calcium elevations in human umbilical vein endothelial cells [25]. In those studies, the calcium response of the cells to high-frequency ultrasonic stimulation was dependent on the applied acoustic pressure. Therefore, it was shown that both umbilical vein endothelial cells and highly-invasive cancer responded less actively at the lower acoustic excitation (input voltage = ~8 Vpp and ~9.5 Vpp) [24]. Similarly, the calcium response of SKBR-3 cells is here little affected by non-FNT-coated microbead attachment under acoustic excitation at 6.3 Vpp (Fig. 3), suggesting that a pure microbead contact to the cell does not significantly contribute to the calcium elevation in the cell. This suggests that such calcium increase in SKBR-3 cells is primarily triggered by FNT binding activity, demonstrating that acoustic tweezers are very useful in studying the intracellular calcium signaling in cancer cells by FNT binding activities.
Fig. 4 illustrates that the calcium elevation in SKBR-3 cells due to FNT-coated microbead binding is likely to be dependent on the size of the trapped FNT-coated microbead. The 15 μm FNT-coated microbead provides a larger FNT contact area than the 5 μm FNT-coated microbead, thus allowing more FNT-binding activities. Therefore, this result shows that the larger FNT-coated microbead elicits higher calcium elevation in SKBR-3 cells. Similarly, in the free FNTs binding experiment, FNTs at 100 μg/ml elicit higher calcium elevation in SKBR-3 cells than FNTs at 50 μg/ml, suggesting the intracellular calcium elevation induced by FNT binding activities may depend on the FNT-binding concentration (Fig. 5). Therefore, these results suggest that the FNT binding activities in the associated study using acoustic tweezers can be controlled by also adjusting of the size of the microbead besides the concentration of FNTs coated on the microbead.
Previous works have shown that spreading of endothelial cells on ECM proteins including FNT triggers intracellular calcium elevations [5,26]. The calcium elevation was induced by an influx of calcium through the plasma membrane via voltage-independent channels. Also, the ECM proteins have shown to be very crucial factors in breast cancer cell invasions, growths, and survivals [27]. However, in spite of the important roles of ECM proteins such as FNT in the cells, the calcium responses of breast cancer cells, particularly SKBR-3 cells, to FNT-binding activities have not been so far explored. In this work, our developed acoustic tweezers allow us to examine the detailed calcium propagation over the breast cancer cells (SKBR-3) induced by synchronized FNT binding activities, revealing that the attachment of a FNT-coated microbead to the cells induces the local calcium increase in the spot where a FNT-coated microbead contacts and further triggers global intracellular calcium elevations throughout the cell (Fig. 6). In contrast, the FNT addition itself elicits global calcium elevation of the cell, not localized increase (Fig. 5). Also, it is observed that the calcium elevations over the cells are gradually increased compared to the FNT-coated microbead binding to the cells, which resulting in rapid calcium elevations (Fig. 5b). It may be caused by the non-synchronized FNT binding to the cells. Furthermore, in the associated mechanistic study using acoustic tweezers, it is found that the calcium elevations are reduced by extracellular calcium elimination and PLC activity inhibition (Fig. 7). Therefore, these results demonstrate that the intracellular calcium elevations by acoustically trapped FNT-coated microbead binding are likely to be promoted by calcium influx, which is a similar result from another study [26], suggesting that the acoustic trapping technique has the potential to elucidate molecular mechanisms in the mechanistic study of cancer cells related to ECM.
In our previous study related to MCF-7 breast cancer cells [18], the cell function was intact even when the same transducer was driven with a higher input voltage at 12.6 V than currently applied in this work (6.3 Vpp). Similarly, the cell viability was tested for human umbilical vein endothelial cells under a different condition (PRF = 1 kHz, voltage amplitude = 19.0 V, and Duty factor = 0.5%) but no significant change was obtained. As the results shown in our previous studies, the results in Fig. 8 also indicate that acoustic trapping forces at the given voltage, PRF, and duty cycle do not significantly reduce the viability of SKBR-3 cancer cells.
5. Conclusions
This paper demonstrates that our acoustic tweezers devised with a single element lithium niobate (LiNbO3) ultrasonic transducer offer us to monitor intracellular calcium elevation induced by FNT binding activities on single cell basis. It is shown that the intracellular calcium level of a breast cancer cell (SKBR-3) can be significantly elevated by maneuvering a FNT-coated microbead at acoustic trap. We found that the FNT-cell binding triggers intracellular calcium signaling whose propagation pathway can be also seen from the contact site toward the rest of the cell as well as cellular pathways of calcium elevation due to the FNT binding involve extra-calcium influx and PLC activities by using our acoustic tweezers. Therefore, the experiments carried out so far, albeit limited in scope, provide a practical example illustrating the promising nature of acoustic tweezers as a non-contact tool for studying intracellular signaling. Finally, it is worthwhile to point out that the use of acoustic tweezers can be extended to elucidate other signaling pathways relevant to various extracellular matrix proteins since the microbead can be coated with other ECM proteins.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants (R01-EB012058 and P41-EB002182) to K.K. Shung, Basic Science Program through the National Research Foundation of Korea (NRF) and DGIST HRHR grant funded by the Ministry of Science, ICT & Future Planning, and the Research Grant of Kwangwoon University in 2014 (15-HRLA-01, NRF-2014M3A9D7070668, NRF-2014R1A1A20549 34, and NRF-2012R1A1A1015778) to J.Y. Hwang and J. Lee.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ultras.2015.06.017.
References
- 1.Bychkov SM. Fibronectins (review) Vopr Med Khim. 1983;29:2–15. [PubMed] [Google Scholar]
- 2.Hynes RO. Fibronectins. Sci Am. 1986;254:42–51. doi: 10.1038/scientificamerican0686-42. [DOI] [PubMed] [Google Scholar]
- 3.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 4.Rock MT, Brooks WH, Roszman TL. Calcium-dependent signaling pathways in T cells. Potential role of calpain, protein tyrosine phosphatase 1b, and p130Cas in integrin-mediated signaling events. J Biol Chem. 1997;272:33377–33383. doi: 10.1074/jbc.272.52.33377. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Mayernik L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev Biol. 2002;245:270–279. doi: 10.1006/dbio.2002.0644. [DOI] [PubMed] [Google Scholar]
- 7.Musielak M. Red blood cell-deformability measurement: review of techniques. Clin Hemorheol Microcirc. 2009;42:47–64. doi: 10.3233/CH-2009-1187. [DOI] [PubMed] [Google Scholar]
- 8.Nishizaka T, Shi Q, Sheetz MP. Position-dependent linkages of fibronectin-integrin-cytoskeleton. Proc Natl Acad Sci USA. 2000;97:692–697. doi: 10.1073/pnas.97.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabry B, Klemm AH, Kienle S, Schaffer TE, Goldmann WH. Focal adhesion kinase stabilizes the cytoskeleton. Biophys J. 2011;101:2131–2138. doi: 10.1016/j.bpj.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanase M, Biais N, Sheetz M. Magnetic tweezers in cell biology. Methods Cell Biol. 2007;83:473–493. doi: 10.1016/S0091-679X(07)83020-2. [DOI] [PubMed] [Google Scholar]
- 12.de Souza N. Pulling on single molecules. Nat Methods. 2012;9:873–877. doi: 10.1038/nmeth.2149. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Ahmed D, Mao X, Lin SC, Lawit A, Huang TJ. Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW) Lab Chip. 2009;9:2890–2895. doi: 10.1039/b910595f. [DOI] [PubMed] [Google Scholar]
- 14.Courtney CRP, Drinkwater BW, Demore CEM, Cochran S, Grinenko A, Wilcox PD. Dexterous manipulation of microparticles using Bessel-function acoustic pressure fields. Appl Phys Lett. 2013;102:123508, 123501–123505. [Google Scholar]
- 15.Lee J, Lee C, Kim HH, Jakob A, Lemor R, Teh SY, Lee A, Shung KK. Targeted cell immobilization by ultrasound microbeam. Biotechnol Bioeng. 2011;108:1643–1650. doi: 10.1002/bit.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Lee C, Shung KK. Calibration of sound forces in acoustic traps. IEEE Trans Ultrason Ferroelectr Freq Control. 2010;57:2305–2310. doi: 10.1109/TUFFC.2010.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng F, Li Y, Hsu HS, Liu C, Tat Chiu C, Lee C, Ham Kim H, Shung KK. Acoustic trapping with a high frequency linear phased array. Appl Phys Lett. 2012;101:214104. doi: 10.1063/1.4766912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JY, Lee C, Lam KH, Kim HH, Lee J, Shung KK. Cell membrane deformation induced by a fibronectin-coated polystyrene microbead in a 200-MHz acoustic trap. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:399–406. doi: 10.1109/TUFFC.2014.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J. Numerical analysis for transverse microbead trapping using 30 MHz focused ultrasound in ray acoustics regime. Ultrasonics. 2014;54:11–19. doi: 10.1016/j.ultras.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Raum K, O’Brien WD., Jr Pulse-echo field distribution measurement technique for high-frequency ultrasound sources. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44:810–815. [Google Scholar]
- 21.Knight MM, Roberts SR, Lee DA, Bader DL. Live cell imaging using confocal microscopy induces intracellular calcium transients and cell death. Am J Physiol Cell Physiol. 2003;284:C1083–1089. doi: 10.1152/ajpcell.00276.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Mayernik L, Armant DR. Trophoblast adhesion of the peri-implantation mouse blastocyst is regulated by integrin signaling that targets phospholipase C. Dev Biol. 2007;302:143–153. [Google Scholar]
- 23.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JY, Lee NS, Lee C, Lam KH, Kim HH, Woo J, Lin MY, Kisler K, Choi H, Zhou Q, Chow RH, Shung KK. Investigating contactless high frequency ultrasound microbeam stimulation for determination of invasion potential of breast cancer cells. Biotechnol Bioeng. 2013;110:2697–2705. doi: 10.1002/bit.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang JY, Lim HG, Yoon CW, Lam KH, Yoon S, Lee C, Chiu CT, Kang BJ, Kim HH, Shung KK. Non-contact high-frequency ultrasound microbeam stimulation for studying mechanotransduction in human umbilical vein endothelial cells. Ultrasound Med Biol. 2014;40:2172–2182. doi: 10.1016/j.ultrasmedbio.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz MA, Brown EJ, Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- 27.Nam JM, Onodera Y, Bissell MJ, Park CC. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res. 2010;70:5238–5248. doi: 10.1158/0008-5472.CAN-09-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.