Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive stimulation method that can induce transient polarity-specific neuroplastic changes in cortical excitability lasting up to 1 h post-stimulation. While excitability changes with stimulation over the primary motor cortex have been well documented, the functional effects of stimulation over premotor regions are less well understood. In the present experiment, we tested how cathodal and anodal tDCS applied over the region of the supplementary motor area (SMA) affected preparation and initiation of a voluntary movement. Participants performed a simple reaction time (RT) task requiring a targeted wrist-extension in response to a go-signal. In 20% of RT trials a startling acoustic stimulus (SAS) was presented 500 ms prior to the “go” signal in order to probe the state of motor preparation. Following the application of cathodal, anodal, or sham tDCS (separate days) over SMA for 10 min, participants performed blocks of RT trials at 10 min intervals. While sham stimulation did not affect RT or incidence of early release by the SAS, cathodal tDCS led to a significant slowing of RT that peaked 10 min after the end of stimulation and was associated with a marked decrease in the incidence of movement release by the SAS. In contrast, anodal tDCS resulted in faster RTs, but the incidence of release was unchanged. These results are consistent with the SMA playing a role in the pre-planning of movements and that modulating its activity with tDCS can lead to polarity-specific changes in motor behavior.
Keywords: tDCS, Motor preparation, Supplementary motor area, Reaction time, Startle, Neural activation
1. Introduction
The supplementary motor area (SMA) has long been known to play a role in the control of movement [1], particularly in the advance preparation and initiation of voluntary actions [2]. Experiments in non-human primates have provided evidence that the SMA contains a large proportion of movement-related neurons that are active throughout the preparatory time interval and demonstrate a gradual increase in firing rate that peaks near the onset of movement [3–5]. Similarly, scalp surface EEG and subdural electrocortigography (ECoG) studies in humans have shown that self-initiated movements are preceded by a slow rising movement-related potential over the region of the SMA that begins as much as 3 s prior to movement [6–9]. SMA neurons have also been shown to be preferentially active prior to self-paced, self-initiated movements, yet SMA activity is also seen during some forms of externally cued movements, such as instructed-delay tasks [8,10]. These findings demonstrate the generalized role of the SMA in the early preparation of voluntary actions.
The SMA may also contribute to the initiation of movement. This idea is based on evidence that many SMA neurons show activity that is time-locked to the onset of muscle activity [11,12]: electrical stimulation of the SMA evokes a complex pattern of motor output [1,13–15], and lesions of the SMA are associated with a transient akinetic state [16–18], including deficits in gait initiation and execution [19].
Despite the consensus that the SMA is involved in movement preparation and initiation, the role the SMA plays in contributing to preparatory motor set, organizing of the spatial and temporal parameters of motor output, and the release of action is unclear [20]. Non-invasive brain stimulation methods can be used to alter the excitability of underlying cortical areas, and thus provides the means to probe the effects of stimulation on motor behavior. However, the results of studies using transcranial magnetic stimulation (TMS) have been equivocal. For example, early and late single-pulse transcranial magnetic stimulation (TMS) applied over SMA did not affect either reaction time (RT) or movement time in healthy humans [21,22]; while on the other hand, repetitive TMS has been shown to either improve [23] or degrade [24] motor performance in patients with Parkinson’s disease. Another means of modulating cortical excitability is through the use of transcranial direct current stimulation (tDCS). By applying small amount of direct current (e.g. .5–1 mA) over a cortical area of interest for a short period of time using scalp surface electrodes, tDCS has been shown to modulate cortical excitability in humans (for reviews see [25,26]). For instance, anodal stimulation applied over primary motor cortex (M1) has been shown to increase TMS-induced motor evoked potential amplitudes elicited from the site of tDCS stimulation for up to 90 min post-stimulation [27]. Conversely, decreased M1 excitability has been demonstrated using cathodal stimulation [26]. If the excitability of SMA plays a role in the preparatory state of the motor system, then anodal tDCS and cathodal tDCS applied over SMA should lead to increases or decreases, respectively, in the level of motor preparation.
In order to assess the extent and the timing of early motor preparation achieved, instructed-delay RT task paradigms can be used, as they provide precise control of the time interval between a warning (“get ready”) and imperative (“go”) stimulus. When there is some unpredictability about the timing between the warning and imperative stimulus, motor preparation can be indexed with RT, where faster RTs are associated with a greater level of advance preparatory activity (e.g., [28,29]). Additionally, the state of preparation of the intended movement during the delay interval can also be probed by delivering a startling acoustic stimulus (SAS) prior to, or in place of, the imperative cue. Under simple RT conditions, the SAS evokes an early and rapid release of the planned movement if it is sufficiently prepared ([30–32], for reviews see [33,34]).
Thus the current study aimed to investigate the functional effect of modulating SMA activity using tDCS. Specifically, we hypothesized that anodal stimulation of the SMA would lead to an increased state of motor preparation as evidenced by decreased RTs and an increase in the proportion of trials in which the SAS evoked the early release of movement. In contrast, we hypothesized that cathodal stimulation would result in increased RTs and a decrease in the proportion of movements triggered by SAS.
2. Methods
2.1. Participants
Ten healthy volunteers (8 M, 2F; 30.3 ± 10.0 years) participated in the active tDCS experiments which were completed in two separate sessions, each session corresponding to a different stimulation polarity (see Section 2.3). In addition, seven healthy volunteers (3 M, 4F; 27.0 ± 7.3 years) participated in a single sham tDCS testing session. All participants gave written informed consent, and the study was conducted in accordance with the ethical approval of the Institutional Research Board at Northwestern University, and the Research Ethics Board at the University of Ottawa, and conformed to the latest revision of the Declaration of Helsinki at the time of testing.
2.2. Task and feedback
Participants sat in a chair facing a computer monitor and gripped a handle attached to the arm of a custom wrist manipulandum that allowed measurement of wrist angular displacement. Participants performed a 20 degree right wrist extension from a home position of 10 degrees of flexion to a fixed target as quickly and accurately as possible upon the presentation of a visual “go” signal (appearance of a green square on the computer screen). The go signal occurred 2–3 s (variable) following a warning signal. Final position feedback was provided in between trials by representing the position of the manipulandum with a 1 cm tall yellow cursor line within a horizontal (1 cm × 15 cm) black rectangle presented on the computer screen with respect to a blue vertical cursor line that represented the target. Real time position feedback was only provided during practice. Further details of the experimental task and equipment have been published previously (see variable foreperiod condition, [31]). Practice trials were given prior to data collection to allow subjects to become familiar with the task and to remove learning effects [35,36]. Participants only required 10–25 practice trials to become proficient at the task. Wrist position feedback was given visually with a cursor that moved horizontally on the computer screen in direct relation to the manipulandum (for details see [31]). During testing, final position feedback was given approximately 1 s after each movement ended. In this way, knowledge of results was available, helping to stabilize movement accuracy performance.
Participants performed 7 blocks of 25 RT trials. Each block took approximately 4.5 min to complete. Two blocks were performed prior to tDCS, 1 block was performed immediately post-stimulation, and the remaining 4 blocks were initiated at 10 min intervals with respect to the end of stimulation. Participants sat quietly during the rest periods between testing blocks.
2.3. Transcranial direct current stimulation (tDCS)
Stimulation was delivered via two electrodes placed over the scalp. The “active” electrode was a sponge electrode (Empi Inc., Dupel B.L.U.E-medium butterfly 2.0 cm3) measuring 8.1 cm2 that was placed 1.8 cm anterior to the measured location of Cz (based on the international 10–20 system for EEG electrode placement). The active electrode was saturated with sterile saline (.9% NaCl) and was held in place if necessary using a standard EEG cap. The “return” electrode was a self-adhesive carbon-foam electrode (Empi Inc.) measuring 51 cm2 (approx. 8.5 cm × 6 cm) that was placed centrally on the forehead directly above the eyebrows.
The placement location for the active electrode was determined using 2 methods: First, the scalp location immediately above the centroid of SMA was landmarked based on Talairach space mapped onto standardized head coordinates [37]. Second, in a subset of participants (n = 5) this location was confirmed using transcranial magnetic stimulation (TMS, Magstim Inc.) based on previous studies [22,38,39]. In short, TMS was delivered over the vertex and motor evoked potentials (MEPs) were recorded in tibialis anterior. The stimulating coil was then moved anteriorly in 5 mm increments and the location was noted where MEPs were no longer observed. In all cases the TMS-based localization ended up being ±2 mm from the anatomically-based location of 1.8 cm anterior to Cz. Therefore, the anatomically-based landmark measurement method was used in the remaining participants to ease localization and to decrease participant discomfort.
Electrical stimulation was delivered using a Dupel iontophoresis constant current delivery device (Empi Inc.). Current was set at 1 mA and was delivered for 10 min (current density at active electrode = .123 mA/cm2). This level of stimulation is relatively high in comparison to previous studies, but still within the range of what would be considered safe [25]. A relatively large return electrode was used so that the current density of the stimulation would be sufficiently low at that site to negate the possibility of strong polarizing return effects [25]. The anodal lead was attached to the active electrode in one session, and the cathode was attached in the other session. For the sham stimulation group delivery was similar to the active stimulation (see above), however the stimulation device was only powered on while ramping up to 1 mA (<15s), then immediately shut off without the participant’s awareness. Stimulation polarity/type was unknown to participants, polarity order was balanced for active stimulation, and testing sessions were conducted at least one week apart to ensure a complete washout of any residual tDCS effects.
2.4. Startling acoustic stimulus (SAS)
Within each block, a 124 dB SAS was delivered pseudorandomly on five of the 25 trials, 500 ms prior to the imperative “go” signal during the movement preparation period (the SAS was not delivered in the first 3 trials or in 2 consecutive trials). Participants were told they would occasionally hear a loud sound but that it was irrelevant to the task. The acoustic stimulus (1 KHz, 40 ms) was created by amplifying a signal created by a tone generator (e.g., Grass model S10CTCMA). The stimulus was presented via a loud-speaker (MG Electronics, MG58H) centered 50 cm behind the head of the participant with an intensity of 124 dB(A). Stimulus intensity at the seated participant distance was confirmed using a precision sound level meter (e.g., Brüel & Kjær Impulse Precision Sound Level Meter 2204).
2.5. Recording equipment
Surface electromyographic (EMG) data were collected from right extensor carpi radialis longus (ECR), right flexor carpi radialis (FCR), and left sternocleidomastiod (SCM) as previously described [31] using bipolar surface electrodes (Delsys Bagnoli DE-2.1) connected to an external amplifier system (Delsys Bagnoli). Wrist angular position data were collected using an optical encoder connected attached to the central axis of the manipulandum. On each trial, unfiltered EMG and position data were digitally sampled at 1 kHz (National Instruments PCI-6030E via BNC-2090) for 4000 ms using a customized program written with LabVIEW software (National Instruments Inc.) and stored for offline analysis. Data collection was initiated by the computer 3000 ms prior to the imperative stimulus.
2.6. Data reduction
Movement onset was defined as the first point of a change of more than .2 deg of angular displacement from the starting position following the “go” cue. Early movement onsets were defined as trials where onset of the targeted movement occurred within 175 ms of the SAS. Peak displacement was the maximal angular excursion from the home position, while time to peak displacement was the time from displacement onset to peak displacement. Movement final position was defined as the first point at which angular velocity fell below and remained below 8 deg/s for at least 150 ms, and movement time was defined as the time from displacement onset to final position.
EMG burst onsets and offsets were determined using a threshold method as previously described [31]. Premotor RT was defined as the time from the onset of the imperative stimulus (or SAS) to the initial onset of ECR. Peak EMG amplitudes were defined as the largest EMG amplitude, rectified and filtered with a 25 Hz low pass elliptic filter, recorded within an interval of 100 ms following EMG burst onset.
2.7. Statistical analyses
For all testing sessions no differences were found between the two pretest blocks for any of the measured variables (all p values >.05), thus these two blocks were collapsed resulting in a single pretest value for each participant. For the active stimulation group dependent measures were analyzed using 2 (Trial condition: control/SAS) × 2 (tDCS polarity: anodal/cathodal) × 6 (Time: pretest, post-tests 1–5) Repeated Measures Analysis of Variance (RM ANOVA). For SAS trials, only 5 trials occurred within each testing block so the effect of one trial on overall data variability was disproportionately large. As such, analysis of SAS trial early response incidence and RT was carried out by collapsing across the 5 post-test blocks. Therefore a 2 (Polarity) × 2 (Time: pretest vs. collapsed post-test blocks) RM ANOVA was used for these comparisons. Data for a sham stimulation group was collected to determine whether repeated testing led to practice effects and/or habituation, thus data were analyzed separately using a one-way (6 Time: pretest, post-tests 1–5) RM ANOVA. Early response incidence in SAS trials and SAS trial RT for this group were analyzed using Student’s t-tests to compare between pretest and collapsed post-test data.
Prior to analysis, proportion variables were corrected for normalcy using an arcsine square root transformation. Greenhouse-Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity. Partial eta squared (ηp2) is reported to provide an estimate of the proportion of the variance that can be attributed to the tested factor. Differences with a probability of less than .05 were considered to be significant. Simple effects tests, and pre-planned comparisons using paired samples t-tests, were administered where appropriate to determine the locus of any differences.
3. Results
3.1. Premotor RT
For the active stimulation group premotor RT was analyzed to determine whether the presentation of a SAS or the application of tDCS over SMA led to RT changes. Similar to previous data [40], there was a large main effect of Trial condition (control/SAS), F(1, 9) = 313.639, p<.001, ηp2 = .972, indicating that the SAS led to much shorter RTs irrespective of the other factors (Fig. 1). In addition, there was a non-significant trend towards an interaction between Polarity and Time, F(5, 45) = 2.208, p = .070, ηp2 = .197. A significant effect would suggest that each tDCS polarity had a differential effect on RT across time (testing blocks). However, since there was also a significant 3-way Trial condition × Polarity × Time interaction, F(6, 54) = 3.106, p = .011, ηp2 = .257, this suggests that any possible Polarity × Time interaction was different for the Control trials compared to the SAS trials. In light of this 3-way interaction and large main effect for Trial condition, separate ANOVAs were carried out for both Control and SAS trials.
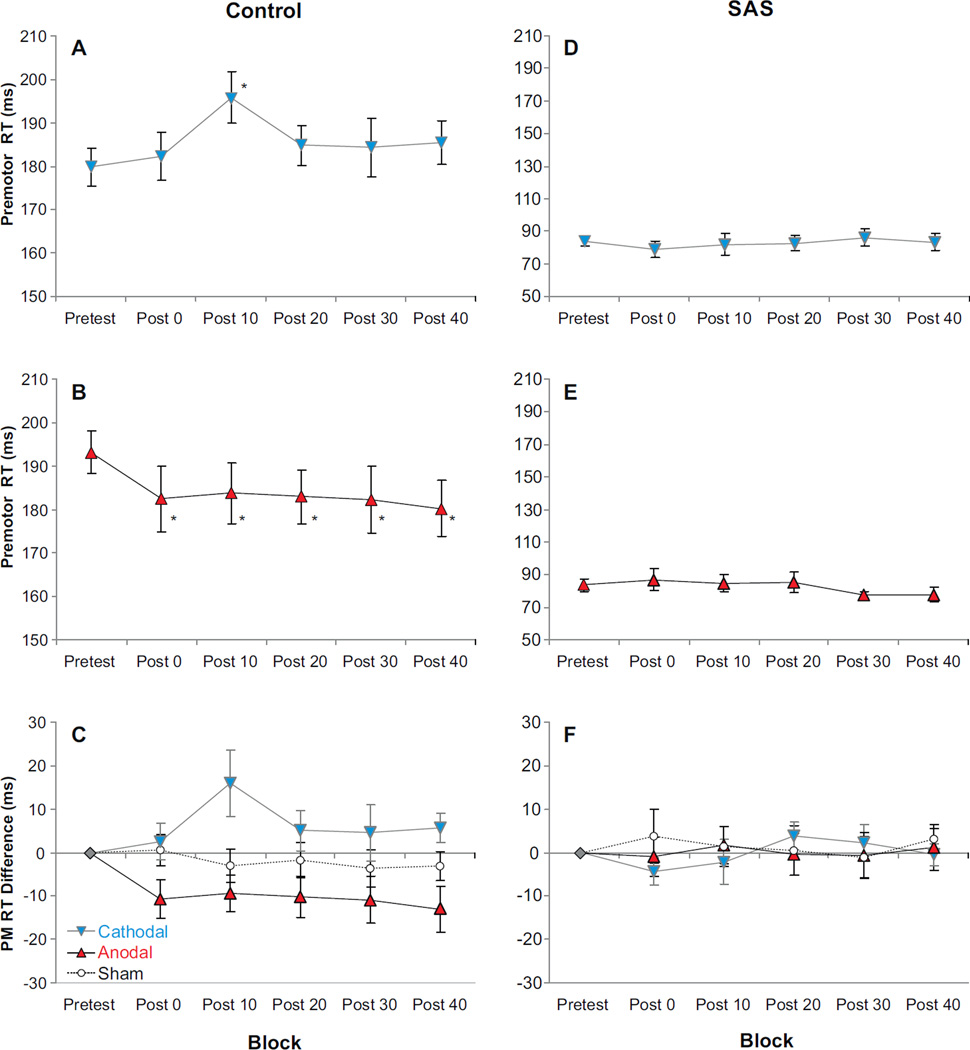
Fig. 1.
Mean (±SE) premotor reaction time (PMRT) observed for each tDCS polarity between blocks and auditory stimulus type (gray/blue = cathodal stimulation; black/red = anodal stimulation; white circles/dashed lines = sham stimulation). Panels A–C show PMRT observed in control trials, while panels D–F show PMRT observed in trials where a startling acoustic stimulus (SAS) was presented. Panels C and F show PMRT difference compared to pretest for anodal, cathodal, and sham stimulation. Panels A, B, D, and E show absolute PMRT for active tDCS sessions (significant PMRT differences of p < .05 between pretest and post-tests are marked with *). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1.1. Control trials
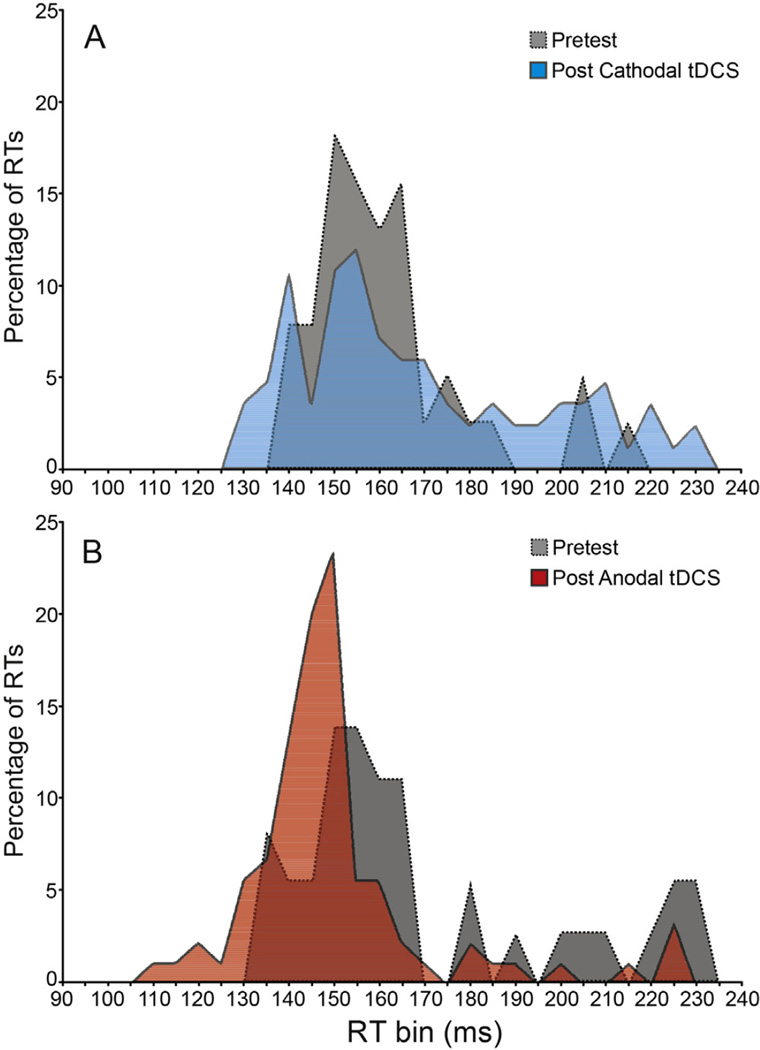
An example of the changes following tDCS in the RT distribution during control (non-SAS) trials in a representative subject is shown in Fig. 2. Cathodal tDCS resulted in a skewing of the RT distribution towards slower RTs compared to pretest measures, while anodal tDCS markedly reduced the incidence of slow RTs and shifted the distribution to toward faster RTs. A 2 (Polarity) × 6 (Time) RM ANOVA showed no main effects for Polarity, p = .815, or for Time, p = .120, however, there was a significant interaction between the factors, F(5, 45) = 3.606, p = .008, ηp2 =.286. In general, cathodal tDCS led to slower RTs following the pretest blocks while anodal tDCS led to faster RTs. Since pretest RTs were significantly different for the two testing sessions (p = .01), absolute RTs are presented in Fig. 1A and B along with normalized RT difference scores for ease of comparison (i.e., RT compared to pretest, Fig. 1C). Simple effects tests were carried out on Control trials using a 1-way ANOVA for the factor Time for each Polarity separately. For cathodal tDCS a significant effect for Time was observed, F(5,45) = 2.784, p = .028, ηp2 = 236, whereby RTs were slower following cathodal tDCS compared to pretest. However, post-hoc comparisons showed that this difference was only significant (p<.05) at 10 min post-stimulation (Fig. 1A). A significant effect for Time was also observed following anodal tDCS, F(5, 45) = 2.778, p = .029, ηp2 = .236, and planned comparisons showed that anodal tDCS led to significantly (p < .05) faster RTs compared to pretest at all times post stimulation (Fig. 1B). Sham stimulation (Fig. 1C, dashed line) showed no main effect of testing block on RT, F(5,30) = .436, p = .820, ηp2 = .068 (pretest mean= 188.1 ms).
Fig. 2.
Distributions of premotor reaction time (RT) observed in a single representative participant. Panel A shows the percentage of RTs falling within each 5 ms bin for post-cathodal tDCS blocks (light gray/blue) compared to the associated pretest (dark gray). Panel B shows the percentage of RTs falling within each 5 ms bin for post-anodal tDCS blocks (light gray/red) compared to the associated pretest (dark gray). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1.2. SAS trials
For the active stimulation group, analysis of premotor RT during SAS trials showed no main effects of stimulation Polarity, F(1,9) = .044, p = .839, ηp2 = .005, or Time, F (1, 9) = 1.380, p = .270, ηp2=.133, and no interaction between the factors, F(1, 9) = .383, p = .551, ηp2 =.041. This suggests that compared to control trials, tDCS did not have any effect on premotor RT when a SAS elicited the response (as noted above, SAS data were analyzed across collapsed post-test blocks—although uncollapsed data are presented in Fig. 1D, 1E, & 1F for comparison to control trials). Similarly, for the sham stimulation group SAS trial RT was not different in the post-test blocks compared to pretest, t(6) = .528, p = .616 (uncollapsed data shown in Fig. 1F, dashed line).
3.2. Incidence of startle and early voluntary response following SAS
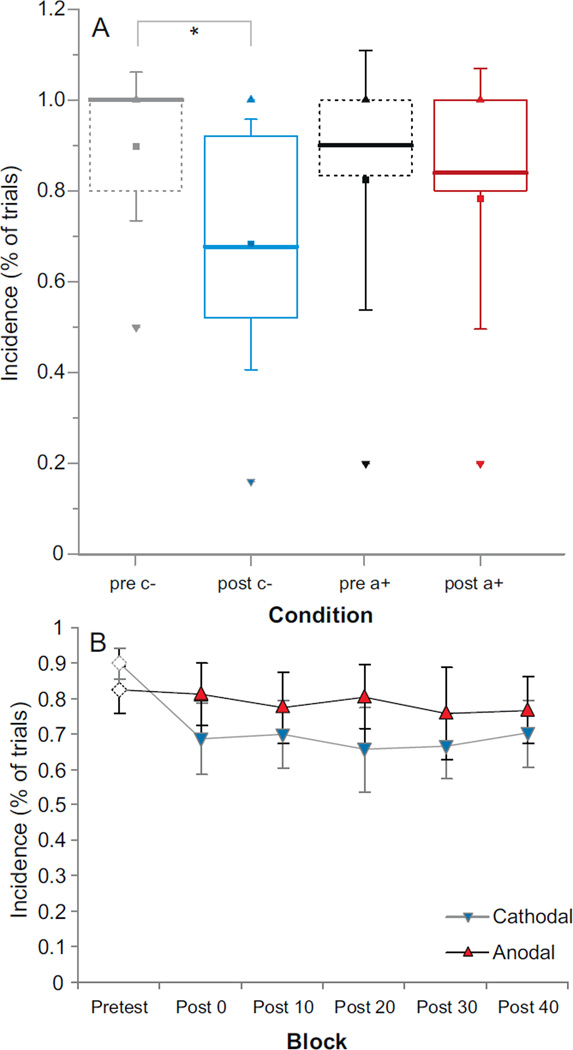
An analysis of the proportion of SAS trials that resulted in an observed startle-related burst of EMG activity in SCM showed no significant main effects or interactions (all p values >.05). That is, in all testing blocks across both groups a startle-response related burst of activity in SCM was observed in over 71% of SAS trials. Further analysis of SAS trials was undertaken to determine whether tDCS had any effect on the incidence of early voluntary responses elicited (i.e. proportion of SAS trials where targeted wrist displacement occurred within 175 ms of the SAS). Analysis showed a main effect for Time, F(1, 9) = 14.276, p = .004, ηp2 = .613, but no main effect for Polarity. However, there was a significant interaction between Polarity and Time, F(1, 9) = 8.621, p = .017, ηp2 = .489. Post-hoc analysis comparing pretest and post-test incidence indicated that there was a significantly lower incidence of early voluntary responses due to SAS following cathodal tDCS (p < .05). In contrast, there was no difference in this incidence following anodal tDCS. Analysis of the incidence of early voluntary responses elicited by the SAS for the sham tDCS group also showed no differences between pretest blocks (96.4% of trials) and post-test blocks (all >90% of trials). Active tDCS results are shown in Fig. 3A: data are presented as box-plots for pretest vs. post-test for each polarity; Fig. 3B shows data separated by testing block.
Fig. 3.
Incidence of early responses observed following presentation of a startling acoustic stimulus observed for each active tDCS polarity (gray/blue = cathodal stimulation, black/red = anodal stimulation) in pretest (dashed lines) vs. post-test blocks (solid lines). Panel A shows boxplots indicating median values (thick line) along with inter-quartile ranges between collapsed pretest blocks and collapsed post-test blocks. Mean is indicated by filled square, whiskers indicate + 1 SD from mean, and minimum ▼ as well as maximum ▲ observed values are also provided. Panel B is mean (±SE) incidence separated by post-tDCS testing block. * p < .05. pre = pretest; post = post-test; c− = cathodal; a+ = anodal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Task performance
No significant main effects or interactions were observed in any of the control trial kinematic variables for either group. Analyses were not carried out on kinematic data for the SAS trials (for rationale see Section 2.7 and [30,41]).
4. Discussion
The present experiment was conducted to determine whether the motor preparatory state in humans could be modulated by applying tDCS over the region of the SMA Here we show that following applications of tDCS, polarity-dependent changes in motor behavior were observed, with cathodal tDCS leading to slower RTs and anodal tDCS leading to faster RTs during control (non-SAS) trials. Cathodal tDCS also led to a decrease in the incidence of early response triggering by a SAS, while anodal tDCS had no effect on incidence of early responses. These results suggest that direct current stimulation over the region of the SMA led to excitability changes in neural structures or networks responsible for the preparation of movement prior to initiation. Furthermore, the findings presented here indicate that functional changes in motor behavior can be achieved through the external modulation of SMA excitability using tDCS.
Previously it has been shown that some facilitation of RT might occur following anodal tDCS applied over M1, however, these changes were attributed to facilitation of learning rather than to functional effects associated with changes in motor cortical excitability [42]. RT facilitation was also previously reported following anodal stimulation over the vertex (near the region of the SMA), however stimulation was only applied transiently during the RT interval between the go-signal and the response [43]. In the present experiment premotor RT was affected in a polarity-specific manner for up to 40 min following 10 min of tDCS applied over SMA: Anodal tDCS led to a speeding of RT in the post-tDCS blocks ranging from 9.4 to 13 ms, while cathodal tDCS led to a slowing of RT by an average of 6.8 ms (range = 2.5–16 ms) compared to pretest (although this RT effect was only significant 10 min after stimulation ended, Fig. 1A). While factors other than the tDCS may have contributed to the observed changes in RT, we feel the likelihood of this is low. For example, observed faster RTs in later testing blocks may have been a result of practice or learning effects, however, the sham stimulation group showed only a modest (.6 to 3.6 ms) and non-significant decrease in RT making this explanation unlikely. Furthermore, consistent with data from the sham stimulation group, it has been previously shown that in simple RT tasks, RT benefits arising from practice reach asymptote after only 9–12 trials [35,36]. Thus these effects were likely washed out in the familiarization trials. Second, there was no RT difference observed between pre-tDCS blocks 1 and 2, suggesting that no further RT improvements occurred in the pre-test.
Since differential effects of tDCS on RT were observed, a practice explanation would suggest that anodal tDCS had very little effect on RT, while cathodal tDCS had a very large deleterious effect on RT. Yet, recent studies have indicated that functional effects arising from cathodal tDCS tend to be less pronounced or absent in comparison to anodal tDCS (e.g., [44,45]) including when it was applied over SMA [46]. The current RT results could be considered to be in line with this observation as a significant lengthening of RT following cathodal tDCS was only seen during the time period between 10 and 20 min following stimulation. However, if added to the small RT decrease seen in the sham group, these results may become significant at other time points, although based on the current design this analysis was not possible. In contrast, anodal tDCS led to significantly faster RTs at all test times following tDCS (Fig. 1B). Although the effect size of anodal tDCS on RTs was relatively modest (partialeta squared = .236), on average, the shift in RT distribution towards faster responses compared to the “react as fast as possible” pre-stimulation control trials was a consistent feature across subjects. In particular, anodal tDCS led to a marked reduction in the incidence of slow RTs (Fig. 2B), whereas cathodal tDCS was associated with an increase in the amount of slow RTs resulting in a rightward skew of the RT distribution (Fig. 2A). Thus, despite the fact that pretest RTs were significantly different across sessions, the polarity of tDCS was predictive of the change in RT.
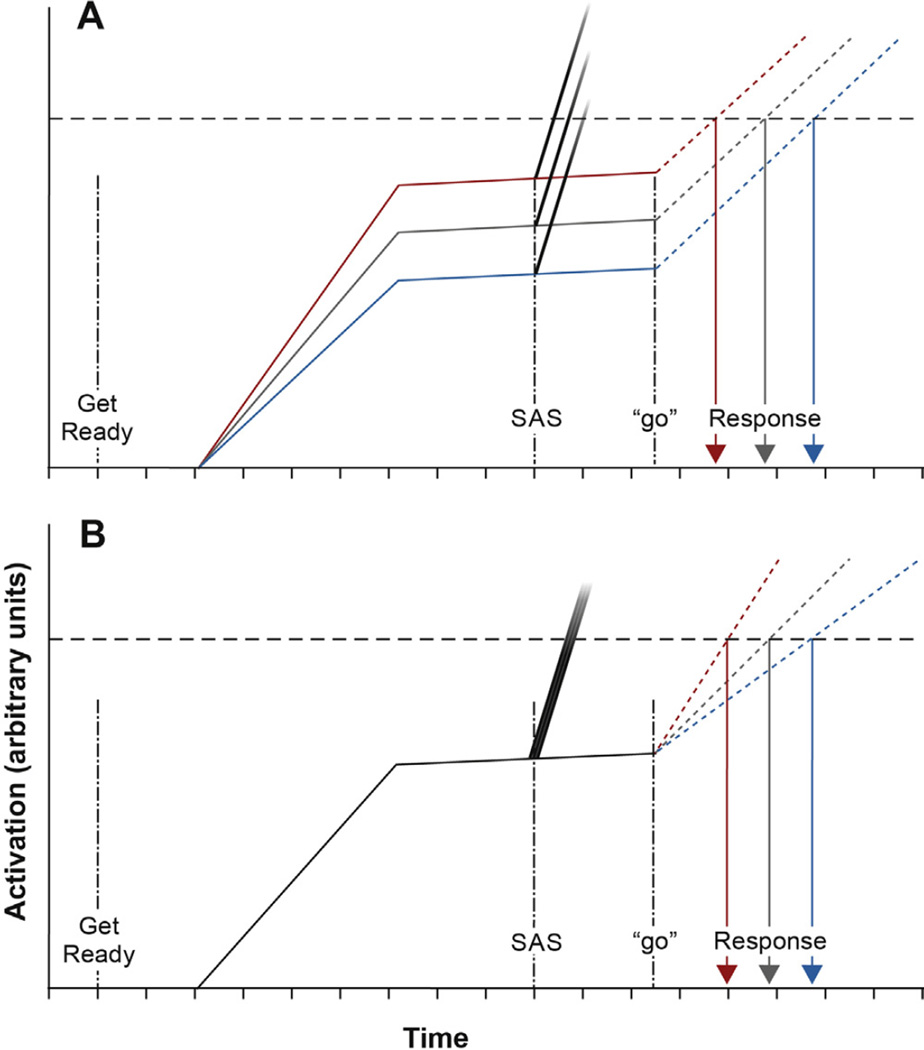
The RT results presented above thus suggest that tDCS applied over the region of the SMA affected the neural excitability of networks mediating either the level of response preparation or the rate of activation increase related to response initiation. An accumulator model of movement preparation and initiation (e.g., [40]) along with the results from the SAS trials may be used to dissociate between these possibilities. The model presented in Fig. 4 depicts a schematic representation of mean cortical activation related to motor preparatory processes [solid lines; akin to the activation level in spikes/s, described by [47], whereby activation is built up between the warning cue and estimated “go” signal. Activation is then maintained (with a slight increase as foreperiod ages) at some level below the threshold at which the release of the movement is triggered (Fig. 4, “go”). Normal initiation-related activity is depicted with dotted lines that build to surpass the threshold required for movement triggering. By modulating excitability via tDCS, the change in SMA activity could have affected the level of motor preparation (Fig. 4A) through facilitation (anodal tDCS, black/red) or suppression (cathodal tDCS, light gray/blue) of the maintained activation level in control (dark gray). This would result in a change in the amount of activation (and thus time) required to initiate the response. Conversely, a change in SMA activity may have affected initiation time by changing the rate at which initiation-related activity was built up, thus leading to changes in the amount of time required to surpass threshold (Fig. 4B). Note that either of these possibilities would result in effects on RT similar to those reported here (note that there is no reason that tDCS could not have affected both processes). However, we argue that the SAS trial early voluntary response incidence results provide some evidence that tDCS applied over the SMA predominantly affected the level of motor preparatory activation with respect to initiation threshold (as shown in Fig. 4A). In Fig. 4, the startle-related response initiation trigger is represented as a steep initiation activation occurring after the SAS but prior to the normal initiation (the probability of the startle activation reaching the required threshold to trigger the action is represented by darkness of the line at the point it crosses threshold). This model predicts no differences in the incidence of startle triggering if SMA modulation led only to changes in the rate of initiation-related processes (Fig. 4B). In contrast, the model predicts that if cathodal tDCS led to a decreased level of maintained preparatory activation, a corresponding decrease in probability of the startle eliciting the response would be observed (Fig. 4A). Moreover, it would be predicted that if the incidence of triggering was already near maximal in pretest, anodal tDCS would have little to no effect on incidence. The “early response” incidence data observed in the current experiment (Fig. 3) showed that following anodal tDCS there was no change in the incidence of early responses observed. In addition, as the incidence was similar to that seen in the sham group, this suggests that the response triggering effect of startle had likely reached a “ceiling.” In contrast, following anodal tDCS, there was a significant drop in early response triggering by startle. Together, these data are consistent with the predictions of a model suggesting that that modulation of SMA excitability had a greater effect on motor preparatory activation level with respect to some initiation threshold. Finally, regardless of stimulation polarity, if the SAS elicited an early response, premotor RT was significantly shorter than in non-SAS trials (Fig. 1D–F), and always reflected the intended, targeted movement (see [30,48]). Also, because no differences in SAS trial RT were observed between pretest and post-test for either stimulation polarity, this suggests that tDCS did not have an effect on the time taken for the startle to trigger the response, and that tDCS likely had no effect on the fast-conducting response initiation pathways that mediate the early release of movement by a SAS.
Fig. 4.
Schematic model of preparation and initiation-related neural activity with respect to a fixed threshold (horizontal dashed line) occurring with increasing time after a warning signal (“get ready”). Mean activation related to preparation is represented by solid lines, while activation related to initiation following a “go” signal is represented by dotted lines. Dark gray = activation in pretest. Black/red = activation following anodal tDCS. Light gray/blue = activation following cathodal tDCS. Fading grayscale lines represent activation in response to a startling acoustic stimulus (SAS), and the probability of the activation reaching a given amplitude is represented by the darkness of the gradient at that amplitude (darker = higher probability of seeing activation at that level). Panel A shows expected differences in response time and early response incidence if tDCS affected level of preparatory activation. Panel B shows expected differences if tDCS affected rate of initiation activation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The mechanisms by which tDCS administered over SMA might affect motor preparation are not currently clear. Application of direct current stimulation to the scalp surface in humans is presumed to lead to excitability changes in the neural tissue underlying the active electrode through direct polarizing effects on the resting membrane potential. Using TMS measures, changes in cortical excitability have been demonstrated following tDCS over primary motor cortex [25,49] and premotor cortex [50]. Anodal stimulation is considered to lead to a short term tonic depolarization, while cathodal stimulation leads to tonic hyperpolarization [25,51,52]. Longer duration modulation of membrane polarization is thought to result from changes to NMDA receptor efficacy [53]. Changes in SMA activity may have influenced preparatory-related activation via basal ganglia-thalamocortical pathways [54]. This may explain why reduced SMA activity seen in patients with Parkinson’s disease often leads to a reduced capacity to initiate movement and movement slowness [55]. Alternatively, tDCS over SMA may have affected motor preparation via alterations in the efficacy of the reciprocal cortico-cortical connections with M1 [20,56] or corti-cospinal projections to interneurons in the intermediate zone and motor neurons in the ventral horn of the spinal cord [20,57,58]. In addition, we cannot discount the possibility that the effects we observed were mediated by current spread to other regions involved in movement preparation such as dorsolateral prefrontal cortex, dorsal premotor cortex, or even primary motor cortex although this is less likely.
5. Conclusion
In summary, the present results show that following the application tDCS over the region of the SMA, polarity-specific functional changes were observed in motor behavior lasting up to 40 min. We argue that by affecting excitability of the SMA, motor activation related to the preparation of motor responses was likely enhanced or degraded by anodal or cathodal tDCS, respectively. These results show that not only can tDCS influence cortical excitability, but these changes can result in functional effects related to the production of motor responses.
HIGHLIGHTS.
Voluntary reaction time was examined following offline tDCS of SMA.
A loud, startling acoustic stimulus was used as a secondary index of preparation.
Anodal tDCS resulted in significantly faster reaction times.
Cathodal tDCS led to slowed reactions and decreased response triggering by startle.
Acknowledgments
Supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant awarded to ANC (RGPIN: 418361-2012).
Non-standard abbreviations
- ECR
extensor carpi radialis longus
- FCR
flexor carpi radialis
- RT
reaction time
References
- 1.Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- 2.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Tanji J. Comparison of neuronal activities in the monkey supplementary and precentral motor areas. Behav Brain Res. 1985;18:137–142. doi: 10.1016/0166-4328(85)90069-5. [DOI] [PubMed] [Google Scholar]
- 5.Romo R, Schultz W. Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp Brain Res. 1987;67:656–662. doi: 10.1007/BF00247297. [DOI] [PubMed] [Google Scholar]
- 6.Kornhuber HH, Deecke L. Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflügers Arch Eur J Physiol. 1965;284:1–17. [PubMed] [Google Scholar]
- 7.Deecke L, Kornhuber HH. An electrical sign of participation of the mesial ‘supplementary’ motor cortex in human voluntary finger movement. Brain Res. 1978;159:473–476. doi: 10.1016/0006-8993(78)90561-9. [DOI] [PubMed] [Google Scholar]
- 8.Cui RQ, MacKinnon CD. The effect of temporal accuracy constraints on movement-related potentials. Exp Brain Res. 2009;194:477–488. doi: 10.1007/s00221-009-1725-5. [DOI] [PubMed] [Google Scholar]
- 9.Neshige R, Luders H, Friedman L, Shibasaki H. Recording of movement-related potentials from the human cortex. Ann Neurol. 1988;24:439–445. doi: 10.1002/ana.410240313. [DOI] [PubMed] [Google Scholar]
- 10.Thaler DE, Rolls ET, Passingham RE. Neuronal activity of the supplementary motor area (SMA) during internally and externally triggered wrist movements. Neurosci Lett. 1988;93:264–269. doi: 10.1016/0304-3940(88)90093-6. [DOI] [PubMed] [Google Scholar]
- 11.Tanji J, Kurata K. Neuronal activity in the cortical supplementary motor area related with distal and proximal forelimb movements. Neurosci Lett. 1979;12:201–206. doi: 10.1016/0304-3940(79)96062-2. [DOI] [PubMed] [Google Scholar]
- 12.Tanji J, Taniguchi K, Saga T. Supplementary motor area: neuronal response to motor instructions. J Neurophysiol. 1980;43:60–68. doi: 10.1152/jn.1980.43.1.60. [DOI] [PubMed] [Google Scholar]
- 13.Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 1987;7:1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Buren JM, Fedio P. Functional representation on the medial aspect of the frontal lobes in man. J Neurosurg. 1976;44:275–289. doi: 10.3171/jns.1976.44.3.0275. [DOI] [PubMed] [Google Scholar]
- 15.Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and supplementary motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- 16.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34:301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- 17.Thaler D, Chen YC, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. I. Simple learned movements. Exp Brain Res. 1995;102:445–460. doi: 10.1007/BF00230649. [DOI] [PubMed] [Google Scholar]
- 18.Brinkman C. Supplementary motor area of the monkey’s cerebral cortex: short-and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Sala S, Francescani A, Spinnler H. Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry. 2002;72:77–85. doi: 10.1136/jnnp.72.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 21.Gregori B, Curra A, Dinapoli L, Bologna M, Accornero N, Berardelli A. The timing and intensity of transcranial magnetic stimulation, and the scalp site stimulated, as variables influencing motor sequence performance in healthy subjects. Exp Brain Res. 2005;166:43–55. doi: 10.1007/s00221-005-2337-3. [DOI] [PubMed] [Google Scholar]
- 22.Ziemann U, Tergau F, Netz J, Homberg V. Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain Res. 1997;744:32–40. doi: 10.1016/s0006-8993(96)01062-1. [DOI] [PubMed] [Google Scholar]
- 23.Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area for treatment of Parkinson’s disease. Mov Disord. 2008;23:1524–1531. doi: 10.1002/mds.22168. [DOI] [PubMed] [Google Scholar]
- 24.Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol. 2001;112:259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 28.Mowrer OH. Preparatory set (expectancy): some methods of measurement. Columbus: The American Psychological Association. 1940 [Google Scholar]
- 29.Niemi P, Näätänen R. Foreperiod and simple reaction-time. Psychol Bull. 1981;89:133–162. [Google Scholar]
- 30.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Motor Behav. 2004;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- 31.Carlsen AN, MacKinnon CD. Motor preparation is modulated by the resolution of the response timing information. Brain Res. 2010;1322:38–49. doi: 10.1016/j.brainres.2010.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol (Lond) 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- 34.Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev. 2011;35:366–376. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Pascual-Leone A, Brasil-Neto JP, Valls-Sole J, Cohen LG, Hallett M. Simple reaction time to focal transcranial magnetic stimulation. Comparison with reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115:109–122. doi: 10.1093/brain/115.1.109. [DOI] [PubMed] [Google Scholar]
- 36.Lee TD, Magill RA. The locus of contextual interference in motor-skill acquisition. J Exp Psychol Learn. 1983;9:730–746. [Google Scholar]
- 37.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart; New York: Georg Thieme; 1988. [Google Scholar]
- 38.Muri RM, Rosler KM, Hess CW. Influence of transcranial magnetic stimulation on the execution of memorised sequences of saccades in man. Exp Brain Res. 1994;101:521–524. doi: 10.1007/BF00227345. [DOI] [PubMed] [Google Scholar]
- 39.Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- 40.Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol. 2012;123:21–33. doi: 10.1016/j.clinph.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Maslovat D, Carlsen AN, Ishimoto R, Chua R, Franks IM. Response preparation changes following practice of an asymmetrical bimanual movement. Exp Brain Res. 2008;190:239–249. doi: 10.1007/s00221-008-1467-9. [DOI] [PubMed] [Google Scholar]
- 42.Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 43.Elbert T, Lutzenberger W, Rockstroh B, Birbaumer N. The influence of low-level transcortical DC-currents on response speed in humans. Int J Neurosci. 1981;14:101–114. doi: 10.3109/00207458108985821. [DOI] [PubMed] [Google Scholar]
- 44.Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Non-invasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayduk-Costa G, Drummond NM, Carlsen AN. Anodal tDCS over SMA decreases the probability of withholding an anticipated action. Behav Brain Res. 2013;257:208–214. doi: 10.1016/j.bbr.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 47.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 48.Valls-Solé J, Rothwell JC, Goulart FR, Cossu G. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol (Lond) 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nitsche MA, Paulus W. Transcranial direct current stimulation—update 2011. Restor Neurol Neurosci. 2011;29:463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 50.Furubayashi T, Terao Y, Arai N, Okabe S, Mochizuki H, Hanajima R, et al. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp Brain Res. 2008;185:279–286. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- 51.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (Lond) 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. NeuroReport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 53.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984;4:539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 56.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 57.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electro-physiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]