Abstract
Movements that are executed or imagined activate a similar subset of cortical regions, but the extent to which this activity represents functionally equivalent neural processes is unclear. During preparation for an executed movement, presentation of a startling acoustic stimulus (SAS) evokes a premature release of the planned movement with the spatial and temporal features of the tasks essentially intact. If imagined movement incorporates the same preparatory processes as executed movement, then a SAS should release the planned movement during preparation. This hypothesis was tested using an instructed-delay cueing paradigm during which subjects were required to rapidly release a handheld weight while maintaining the posture of the arm or to perform first-person imagery of the same task while holding the weight. In a subset of trials, a SAS was presented at 1500, 500, or 200 ms prior to the release cue. Task-appropriate preparation during executed and imagined movements was confirmed by electroencephalographic recording of a contingent negative variation waveform. During preparation for executed movement, a SAS often resulted in premature release of the weight with the probability of release progressively increasing from 24 % at −1500 ms to 80 % at −200 ms. In contrast, the SAS rarely (<2 % of trials) triggered a release of the weight during imagined movement. However, the SAS frequently evoked the planned postural response (suppression of bicep brachii muscle activity) irrespective of the task or timing of stimulation (even during periods of postural hold without preparation). These findings provide evidence that neural processes mediating the preparation and release of the focal motor task (release of the weight) are markedly attenuated or absent during imagined movement and that postural and focal components of the task are prepared independently.
Keywords: Imagined movement, Movement preparation, Startle, Contingent negative variation
Introduction
For decades, there has been interest in the use of imagined movement as a surrogate for real movement. The early experiments of Roland et al. (1980) showed that mental imagery of performing a sequence of finger movements was associated with activity in the supplementary motor area (SMA) but not the primary motor cortex (M1). The absence of activity in M1 during imagined movement was interpreted to reflect the SMA’s preferential role in the preparation, not execution, of movement. Subsequent electrophysiological and neuro-imaging studies have shown that there is considerably more overlap in premotor and primary motor cortex activation during executed and imagined movements than originally shown in the Roland studies (Stephan et al. 1995; Lotze et al. 1999; Gerardin et al. 2000; Cisek and Kalaska 2004; Hanakawa et al. 2008). Behavioral measures and self-report suggest that subjects perceive the timing and content of motor imagery to be similar to motor performance (Decety et al. 1989; Decety 1993; Decety and Jeannerod 1995). Similarly, electroencephalographic (EEG) studies have shown that both executed and imagined movements are preceded by a slow-rising potential with comparable timing and topography (Cunnington et al. 1996; Green et al. 1997; Jankelowitz and Colebatch 2002; Caldara et al. 2004; Carrillo-de-la-Pena et al. 2008; Kranczioch et al. 2009, 2010). Based on the congruency of location and timing of cortical activity associated with imagined and executed movements, Jeannerod (2001) proposed that mental imagery shares processes related to the planning and preparation of movement, but overt movements do not occur either because the final command to execute the movement is not sent or movement is prevented by inhibition late in the process. For this reason, motor imagery has been used as a surrogate for executed movement to enhance sports performance (Kearns and Crossman 1992; Gentili et al. 2006; Fontani et al. 2007; Olsson et al. 2008; Frank et al. 2014; Schack et al. 2014), as therapy after stroke or spinal cord injury (Dickstein et al. 2004; Jackson et al. 2004; Butler and Page 2006; Dunsky et al. 2006; Lotze and Cohen 2006; Sharma et al. 2006; Braun et al. 2008; Kho et al. 2014), as a method to derive movement-related signals to drive and control brain–machine interfaces (Mason et al. 2007), or for movements that cannot be performed within an imaging scanner (e.g., gait; Malouin et al. 2003; Bakker et al. 2007, 2008; la Fougere et al. 2010; Snijders et al. 2011). However, the efficacy of using motor imagery to enhance performance (Holmes and Calmels 2008) or train the motor cortical system in individuals with stroke, Parkinson’s disease, or multiple sclerosis (Barclay-Goddard et al. 2011; Ietswaart et al. 2011; Braun et al. 2013) has been questioned based on the limited success of interventions and concerns about the equivalence of imagined and executed movements.
Interpretation of the findings of functional neuroimaging and EEG studies that have compared imagined versus executed movements is constrained by the fact that a lack of significant difference in outcome measures (e.g., blood–oxygen-level-dependent signal) between tasks does not necessarily mean that the neural processes are equivalent. In this study, we tested whether the state of motor preparation is different between an executed movement task (EM) and kinesthetic imagery of the same movement (IM) by presenting a startling acoustic stimulus (SAS) at three time points during the movement preparation time period. A SAS triggers the premature release of preplanned movements, at latencies often less than 100 ms, when the stimulus is applied at the same time as, or prior to, the onset of an imperative stimulus to initiate movement (Valls-Solé et al. 1999; Carlsen et al. 2004a, b; MacKinnon et al. 2007, 2013; Carlsen and MacKinnon 2010; Rogers et al. 2011). Despite the short onset latencies, the temporal and spatial features of the planned movement remain unchanged (Carlsen et al. 2004b). The short-latency release of voluntary movements by a loud acoustic stimulus has been termed the StartReact phenomenon (Valls-Solé et al. 2008). Most importantly, the StartReact only occurs when the movement can be prepared in advance (Carlsen et al. 2004a), suggesting that the stimulus triggers the early release of stored motor programs. We have previously shown that presenting a SAS during the preparatory period of an instructed-delay movement task can trigger the premature release of the intended movement (Cressman et al. 2006; MacKinnon et al. 2007; Carlsen and MacKinnon 2010; Rogers et al. 2011; Alibiglou and MacKinnon 2012). The incidence of movement release has been shown to be correlated with the timing and magnitude of the movement-related cortical potential preceding movement onset (MacKinnon et al. 2013). These observations show that a SAS can be used to test time-varying changes in the state of preparation of the movement.
One previous study has used the StartReact paradigm to examine whether prepared movements can be released during motor imagery (Maslovat et al. 2013). This study showed that the SAS evoked a partial release of movement in half of the subjects tested. These results were interpreted to suggest that executed and imagined movements were prepared in a similar manner, but mental imagery was associated with “sub-threshold activation” of the output such that a startling stimulus resulted in portions of the response “leaking out.” However, it is important to note that partial responses were only seen in subjects who showed evidence of task-related muscle activity during mental imagery. In the subjects who did not show muscle activity during imagery, the SAS did not release movement. However, it is unclear whether the lack of a Start-React in these subjects reflects a higher threshold for release, absence of motor preparation, or the absence of task-related imagery. These issues highlight the difficulty of controlling for task performance during mental imagery. To better control for the presence or absence of task-related motor imagery, we recorded scalp surface EEG to ensure that the onset of executed and imagined movements was preceded by a contingent negative variation (CNV) waveform that reflects preparatory activity (Walter et al. 1964; Cunnington et al. 1996; Jankelowitz and Colebatch 2002; Caldara et al. 2004; Carrillo-de-la-Pena et al. 2008; Kranczioch et al. 2009, 2010). If kinesthetic imagery of movement relies on the same preparatory neural processes as executed movement, then the presentation of a SAS during preparation for imagined movement should evoke an early and unintentional release of the planned movement. We tested this hypothesis by applying a SAS at various time points during the preparation phase of an EM and IM task.
Methods
Subjects
Fifteen subjects participated in this experiment. Only those subjects that showed a distinct CNV during both the (extensor digitorum communis, EDC) and elbow flexors (biceps brachii, BB) during executed (black lines) and imagined (red lines) task conditions. Note the anticipatory suppression of BB immediately preceding activation of the prime mover (EDC) for the executed movement task executed and imagined movement tasks were included in the final analysis. These criteria ensured that subjects prepared the imagined and executed movements in response to the cues as instructed. Six subjects were excluded from further analysis for this reason, resulting in a total of nine right-handed subjects (seven female, two male, age = 24.6 ± 3.5 years). The participants had no musculoskeletal or neurological disorders that affected function of their upper limbs. Each subject was tested in a single session. Written consent was obtained prior to the start of the experiment, and all procedures were approved by the Institutional Review Board at Northwestern University in accordance with the Declaration of Helsinki.
Experiment tasks
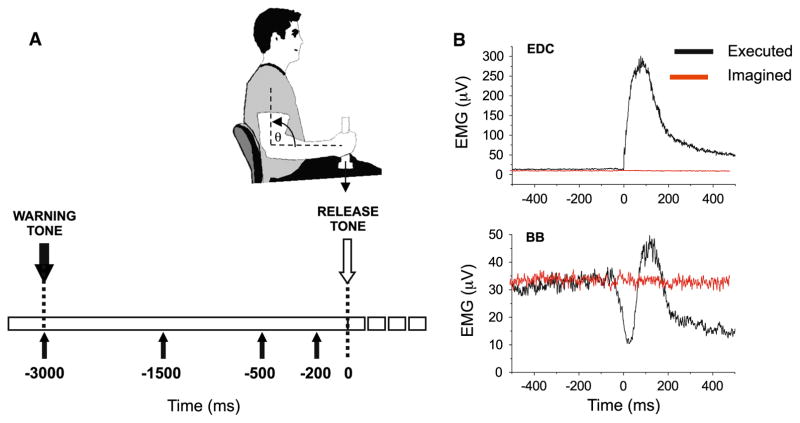
Subjects performed two tasks: an executed movement (EM) and a matched imagined movement (IM). In both tasks, subjects sat in an armless chair and held a vertical rod attached to a 1.25-lb (0.57 kg) load with their shoulder abducted to approximately 20°, forearm parallel to the ground, and elbow flexed at 90° (Fig. 1a). Subjects were required to maintain this relative arm position in space throughout the data collection epochs. For both the EM and IM tasks, an instructed-delay paradigm was used, consisting of a warning tone (1000 Hz, 80 dB) followed 3 s later by an imperative release tone (1000 Hz, 80 dB; Fig. 1a). Subjects were required to be alert and in the “ready” position several seconds prior to each data collection epoch.
Fig. 1.
a Summary of the timing of the stimuli for the instructed-delay paradigm. A warning tone (100 ms, 1000 Hz, 80 dB) was presented 3000 ms before an imperative release tone (100 ms, 1000 Hz, 80 dB). In a subset of trials, a SAS (40 ms, 1000 Hz, 123 dB) was presented at either 1500, 500, or 200 ms prior to the release cue. b Grand average-rectified EMG responses in the finger extensors
For the EM task, subjects were instructed to release the weight as soon as possible following the release tone by rapidly extending the fingers, but without changing their arm posture. This task requires a stereotypical sequence of anticipatory postural adjustments (initial suppression of elbow flexor muscles activity, followed by finger extensor activity) to ensure that the elbow angle remains at 90° (Hugon et al. 1982). This task was chosen to ensure that muscle activity associated with the instructed task was different from that evoked by a classic startle reflex (typically associated with rapid elbow flexion). Feedback of reaction time (onset of finger extension acceleration) was provided on a computer screen following the completion of each trial. Subjects completed a set of practice trials of the movement task to ensure that the task was performed correctly (rapid weight release with little or no change in elbow angle) and to establish a reaction time criteria for feedback. During the data collection sessions, reaction time feedback was provided in the following manner: If the reaction time was within less than the 50th percentile of RTs recorded during training trials, a green square was presented, and if it was longer, or prior to the imperative release tone, a red square was presented. Subjects were encouraged to achieve as many green square trials as possible. Positive verbal feedback was given by the experimenter for lowest reaction times.
For the IM task, subjects held the same weight and were instructed to prepare to release the weight in the same way as for the EM task; however, rather than releasing the weight, they were told to imagine the sensation of rapidly releasing the weight as fast as possible in response to the release tone. All other aspects of the IM task were matched to the EM task including the initial conditions (posture of the upper arm) and timing of presentation of the warning and release tones. The instructions required subjects to imagine, in the first person, the physical sensation of releasing the weight, rather than to imagine watching themselves or another person release the weight from a third-person perspective (Sirigu and Duhamel 2001). Thus, the instructions were focused on first-person imagery of the kinesthetic sensation of release and not the suppression of release. A total of four blocks of 40 trials of the EM and IM task (160 trials for each task) were performed. Subjects always started with a block of executed movements and subsequently alternated between blocks of executed and imagined movements. This order was used to ensure that subjects had sufficient practice with the EM task before performing imagined movements, since this has been shown to affect the magnitude of the CNVs (Cunnington et al. 1996) and activations observed during fMRI (Wriessnegger et al. 2014). Breaks were taken between blocks of trials and as needed to prevent fatigue.
Startling acoustic stimulus (SAS)
The SAS (40 ms, 1000 Hz) was presented using a loudspeaker positioned 50 cm behind the subject’s head at ear level. The SAS intensity was measured to be 123 dB at the ear. The SAS was presented pseudorandomly in 20 % of the trials for both the EM and IM tasks at timings of −1500, −500, and −200 ms prior to the release tone (eight SAS trials for each stimulus timing and task). An additional eight SAS control trials for each task were included during which the stimulus was presented alone at the time when the warning stimulus would have been presented (−3000 ms), that is, during a time period when subjects were alert and holding the required posture, but not yet preparing for movement. Two SAS trials were never presented in a row.
Data collection
Scalp surface EEG was recorded from a montage of 11 electrodes placed on the scalp using a cap. Signals were recorded from electrodes at the following international 10–20 locations: CZ, C3, C4, Fz, Pz, FC1, FC2; CP1, CP2, FC5, and CP5. EOG was collected using electrodes placed 1 cm above and below the right eye over the upper and lower canthi. EEG signals were referenced to linked mastoid electrodes. Bipolar electromyographic (EMG) recordings were obtained from electrodes placed over the right biceps brachii (BB), right extensor digitorum communis (EDC), and left sternocleidomastoid (SCM). The SCM muscle was recorded as a marker of the presence or absence of a stimulus-evoked startle reflex (Brown et al. 1991). As the startle reflex is, for the most part, bilaterally symmetrical (Brown et al. 1991), the left SCM was recorded since it would tend to be less likely to be activated as a part of the right limb voluntary response. Horizontal accelerations of the hand were recorded using a uniaxial accelerometer attached to the dorsum of the distal phalange of the middle finger (Model 1202F, Measurement Specialties). An accelerometer was also attached to the weight to capture the moment of release. The EEG (band pass DC-200 Hz), EOG (band pass 5–200 Hz), and EMG (band pass 30–500 Hz) signals from the BB and EDC muscles were recorded at a sample rate of 1000 Hz using a Neuroscan Synamps data acquisition system (Compumedics, Ltd.). All other signals were recorded using a Power 1401 (Cambridge Electronic Design, Inc.) data acquisition board with a sampling rate of 2000 Hz.
Data analysis
Movement release was manually marked by visual inspection of the onset of EDC activity on a trial-by-trial basis (Hodges and Bui 1996). Reaction time was then calculated as the time from the onset of the release tone (for control trials), or the SAS (for startle trials), to EDC EMG onset. Trials in which the SAS evoked a short-latency release of the weight (reaction time in EDC of less than 150 ms and verified by the inspection of the finger and load accelerometers) were identified. The incidence of release was then calculated for each SAS presentation timing (SAS-triggered releases)/(number of SAS presentations – error trials). Similarly, the incidence of trials with a SAS-evoked suppression of activity in BB was calculated, irrespective of the presence or absence of an accompanying burst in the EDC muscle. Trials with a startle reflex were identified based on the presence of an SCM EMG burst with an onset of between 50 and 150 ms (Brown et al. 1991). The incidence of trials with both an early burst in the SCM muscle in conjunction with early release of the weight or suppression of BB muscle activity was also quantified.
CNV waveforms were derived for each subject by averaging all control (non-SAS) trials relative to the onset of the imperative release tone for both the EM and IM tasks. Although EMG onset for the EM trials was after the tone, this approach ensured that the EM and IM tasks were temporally aligned. Trials were rejected if there was EDC EMG activity, eye movement, or a deviation of ±50 μV in the EEG signal between −4000 ms and the release tone. For the IM task, trials were rejected using the same criteria as the EM task with the additional criteria that trials with a burst of EMG activity in the EDC muscle that exceeded baseline levels were also rejected. This ensured that the waveform created for the IM task reflected the preparation for imagined and not executed movements (Lotze et al. 1999). Average waveforms were baseline corrected to the mean signal obtained over a 100-ms interval (−3850 to −3750 ms) between the start of the epoch (−4000 ms) and onset of the warning tone (−3000 ms). EEG amplitudes were computed for each subject by calculating the area under the rectified CNV waveform over a 100-ms interval centered around the SAS probe timings (−1500, −500, and −200 ms) and the baseline interval (centered at −3800 ms).
A task-related anticipatory postural adjustment (APA) accompanying performance of the EM task was defined by the presence of a sharply delineated reduction of the rectified BB EMG activity beginning prior to, or at the same time as, the onset of finger extensor activity. The onset and end of the BB EMG suppression were marked manually. The area under the curve during BB EMG suppression was then computed and compared to an area of the same duration calculated prior to the warning signal beginning at −3800 ms. A matched number of control (non-SAS) and SAS trials (eight each; control trials selected randomly) were used for comparison between conditions. For IM control (non-SAS) trials, BB silence onsets and offsets were not present; therefore, areas were calculated based on onset and offset times matched to EM control trials.
Statistics
Dependent variables were analyzed using repeated measures ANOVA with factors of task (EM vs. IM) and time (baseline, −1500, −500, −200 ms). Separate ANOVAs were conducted at three EEG electrode locations: Cz (vertex over the SMA), C3 (contralateral M1), and C4 (ipsilateral M1). Greenhouse–Geisser-corrected degrees of freedom were used to correct for violations of the assumption of sphericity if necessary. Incidence (ratio) variables were subjected to an arcsine square root transform prior to ANOVA testing. Tukey’s honestly significant difference (HSD) test was used for post hoc analysis of interaction effects and across repeated measures. An alpha value of p < 0.05 was set as the threshold for significance.
Results
EM task performance
The release of the weight during control (non-SAS) trials for the EM task was typically accompanied by an anticipatory reduction of EMG activity in the BB muscle in conjunction with a phasic burst of activity in the finger extensors (EDC; Fig. 1b). The average reaction time from the release tone to the start of BB EMG suppression was 107 ± 21 ms and to onset of the EDC EMG was 114 ± 19 ms. There was no significant difference between the EM and IM tasks in the mean-rectified BB EMG during the preparatory hold period (from −4000 to −500 ms; p = 0.479).
Release of the movement by startle
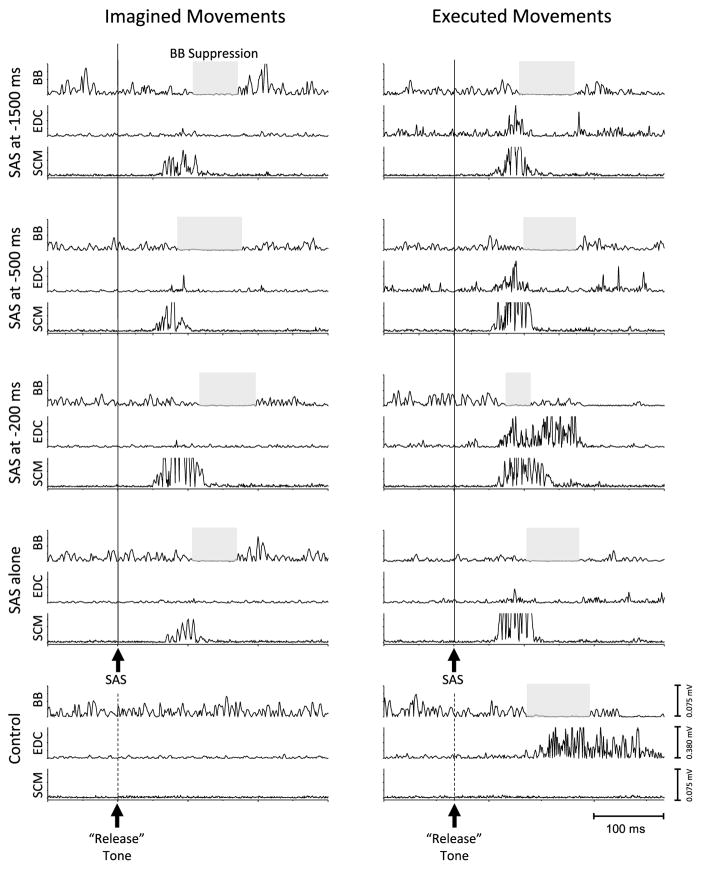
Figure 2 shows examples of EMG activity in the elbow flexors (BB), finger extensors (EDC), and sternocleidomastoid (SCM) muscles during the different trial conditions from a single subject. The presentation of a SAS during the preparation phase of the EM task frequently evoked an early release of the weight characterized by the activation of the SCM, anticipatory reduction of EMG activity in the BB muscle, followed by the activation of the finger extensors. The relative timing between BB suppression and EDC activation was similar to that seen during control (non-SAS) trials (also see Fig. 1b). In contrast, the presentation of a SAS during the IM task did not evoke activity in the EDC muscle and was not followed by a release of the weight. However, the SAS often evoked a short-latency suppression of the BB activity for the IM task.
Fig. 2.
Examples of EMG activity in the elbow flexors (biceps brachii, BB), finger extensors (extensor digitorum communis, EDC), and sternocleidomastoid (SCM) muscles during the different trial conditions. The left panel shows responses during imagined movements (IM), and the right panel shows executed movements (EM). During EM control trials (no SAS) (bottom right plot), movement release was preceded by a suppression of EMG activity in BB (shaded gray box) and activation of EDC. Mental imagery of the same task (bottom left plot) was associated with tonic BB activity to maintain the elbow posture, but there was no suppression of BB or activation of EDC. The presentation of a SAS at −1500, −500, or −200 ms during the preparation phase for EMs evoked a rapid release of the weight that was accompanied by a burst of activity in the SCM muscle, suppression of BB activity, followed by the activation of the EDC muscle. During IMs, the activation of EDC is absent, but the SAS consistently produced a suppression of BB activity. Similarly, the presentation of a SAS alone (i.e., no warning or release tone) also produced BB EMG suppression and a SCM burst, but no EDC activity
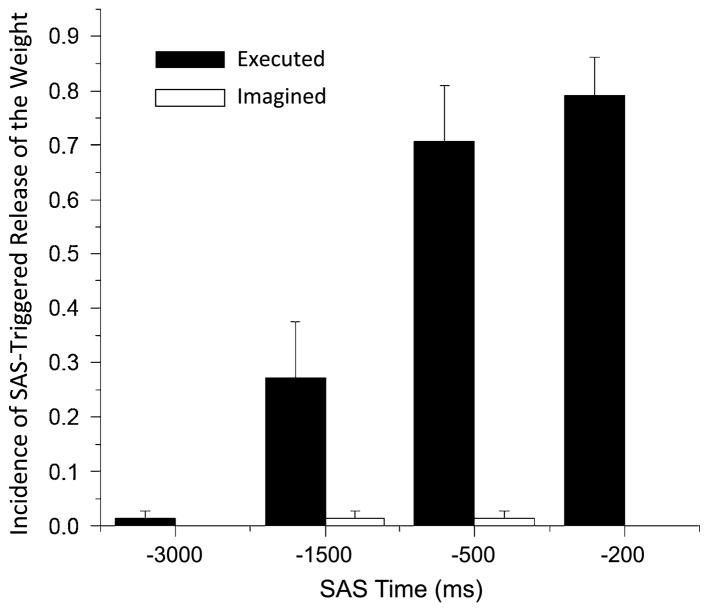
The incidence of early release of the weight by a SAS was markedly different across time points and between the EM and IM tasks (Fig. 3). The EM task was associated with a progressive increase in incidence of release from 24 % at −1500 ms, 71 % at −500 ms to 80 % at −200 ms. In contrast, only two trials of movement release were recorded across all subjects in the IM condition (both release trials were in the same subject, once at −1500 ms and −500 ms). In both task conditions, the presentation of a SAS at the time of the warning cue (control SAS condition) rarely evoked release of movement (only one trial across all subjects). ANOVA results showed a significant main effect of task (F(1,8) = 57.5, p < 0.001, ), time (F(3,24) = 46.2, p < 0.001, ), and a task by time interaction (F(3,24) = 62.4, p < 0.001, ). Post hoc analysis of the time effect showed that the incidence of release was significantly greater at the −1500, −500, and −200 ms SAS times (p < 0.014) compared to the SAS control (SAS alone at −3000 ms) condition and at −500 and −200 ms compared to the −1500 ms condition (p < 0.001), but the incidence was not significantly different between the −500 and −200 ms SAS conditions (p = 0.348). Analysis of the interaction effect showed that the incidence of early release was significantly greater in the EM compared to the IM tasks at −1500, −500, and −200 ms (p < 0.009) but not between the SAS control conditions (p = 0.347).
Fig. 3.
Average incidence of SAS-triggered release of the weight across subjects for both the executed and imagined movement tasks. Note the progressive increase in incidence for the executed task and the lack of movement release for the imagined task. Error bars are one standard error
The timing of EDC activation and BB suppression during control and SAS trials is summarized in Table 1. Statistical analysis of the EDC onset data was restricted to the control trials (non-SAS) and −500 and −200 ms SAS timing conditions since four out of nine subjects had no responses with the −1500 ms probe, and to the EM task since the finger extensors were rarely triggered by a SAS for the IM task. A one-way ANOVA with repeated measures showed a main effect of time on the onset latency of EDC EMG (F(2,16) = 30.5, p < 0.001, ). Post hoc tests showed that the onset latency was significantly shorter following a SAS at −500 and −200 ms than in control trials (p < 0.01).
Table 1.
Summary of EMG data (all values are mean ± 1 standard deviation)
| Executed
|
Imagined
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (non-SAS) | SAS timing
|
Control SAS | Control (non-SAS) | SAS timing
|
Control SAS | |||||
| −1500 ms | −500 ms | −200 ms | −3000 ms | −1500 ms | −500 ms | −200 ms | −3000 ms | |||
| Onset EDC EMG (ms) | 114 ± 19 | 97 ± 27a | 86 ± 21 | 75 ± 12 | – | – | – | – | – | – |
| Onset BB EMG suppression (ms) | 107 ± 21 | 100 ± 10 | 97 ± 17 | 86 ± 14 | 95 ± 17 | – | 95 ± 12 | 98 ± 16 | 103 ± 10 | 107 ± 18 |
| Duration BB suppression (ms) | 54 ± 18 | 51 ± 26 | 45 ± 23 | 46 ± 27 | 49 ± 24 | – | 50 ± 27 | 51 ± 27 | 50 ± 24 | 46 ± 28 |
| Magnitude BB suppressionb | 0.36 ± 0.13 | 0.35 ± 0.20 | 0.42 ± 0.19 | 0.52 ± 0.26 | 0.36 ± 0.13 | 1.14 ± 0.12 | 0.36 ± 0.17 | 0.39 ± 0.11 | 0.39 ± 0.13 | 0.37 ± 0.11 |
EDC extensor digitorum communis, BB biceps brachii, SAS startling acoustic stimulus
Mean based on five subjects
Ratio of (area under curve during suppression)/(area under curve during baseline)
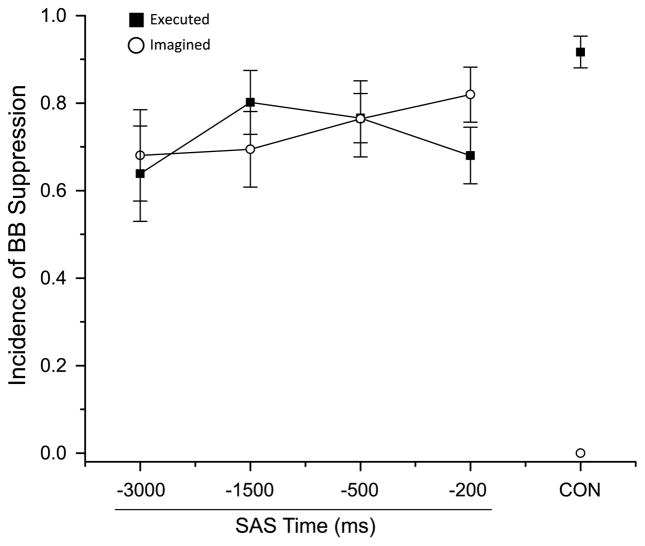
In contrast to the finger extensors, the presentation of a SAS consistently evoked a suppression of BB activity during both the EM and IM tasks (see Fig. 2, for example, trials). The average incidence of SAS-evoked suppression of BB activity is shown in Fig. 4. Note that the incidence of suppression was high across all SAS conditions for both tasks. Most importantly, a suppression of BB activity was also seen for the SAS control condition (SAS at −300 ms) during which subjects were holding the posture and awaiting the warning cue (i.e., prior to the preparation phase of the task). A repeated measures ANOVA showed there were no significant main effects of task (F(1,8) < 2.6, p > 0.61), time (F(3,24) < 1.3, p > 0.30), or interaction effects (F(3,24) < 1.8, p > 0.08) in the incidence, onset timing, duration, or magnitude of the BB suppression.
Fig. 4.
Average incidence of suppression of biceps brachii (BB) EMG across subjects for both the executed and imagined movement tasks. Note that BB suppression was observed for all SAS timings, including the SAS control condition (SAS at −3000 ms). CON = control trials without a SAS. Error bars are one standard error
There were also distinct differences in the excitability of startle reflex pathways between the EM and IM tasks and between the focal and postural responses. Much like the incidence of movement release, the preparation phase of the EM task was associated with a progressive increase in the incidence of a SAS-evoked burst of SCM activity. The average incidence of a SCM burst for the EM task was 39 ± 35, 47 ± 32, 76 ± 27, and 84 ± 21 % of trials for the SAS control, −1500, −500, and −200 ms conditions, respectively. In contrast, the incidence of an SCM burst in the IM task was lower for the SAS control condition (27 ± 37 %) and relatively constant during the preparation time period (40 ± 33, 40 ± 32, and 45 ± 32 % for the −1500, −500, and −200 ms conditions, respectively). There were significant main effects of task (F(1,8) = 25.975, p = 0.011, ), time (F(3,24) = 15.743, p = <0.001, ), and an interaction effect (F(3,24) = 4.161, p = <0.017, ) for the incidence of an SCM burst. The incidence was significantly higher when a SAS was applied at −200 and 500 ms compared with the −1500 ms and SAS control conditions (p < 0.016), and the incidence was higher at −1500 ms compared to SAS control (p = 0.016). Post hoc testing of the interaction effect showed that the incidence of a SCM burst was higher for the EM compared with the IM task at −500 and −200 ms and for SAS control condition (p < 0.05) but not at −1500 ms. For the EM task, the majority of SAS trials with an early onset of the EDC muscle, BB suppression, and release of the weight were accompanied by a burst of activity in the SCM muscle (77, 78, and 84 % of release trials for the −1500, −500, and −200 ms condition) and thus were consistent with a StartReact effect (Valls-Solé et al. 2008; Carlsen et al. 2012b). In contrast, the BB suppression evoked by a SAS during the IM task (without EDC activity or release of the weight) was accompanied by a SCM burst in only about one half of the trials (50, 48, 54, and 41 % of trials for the −1500, −500, −200 ms, and SAS control conditions, respectively). When trials with and without a SCM burst were separated, there was no significant difference in BB suppression onset time between conditions at each SAS time point (p > 0.13).
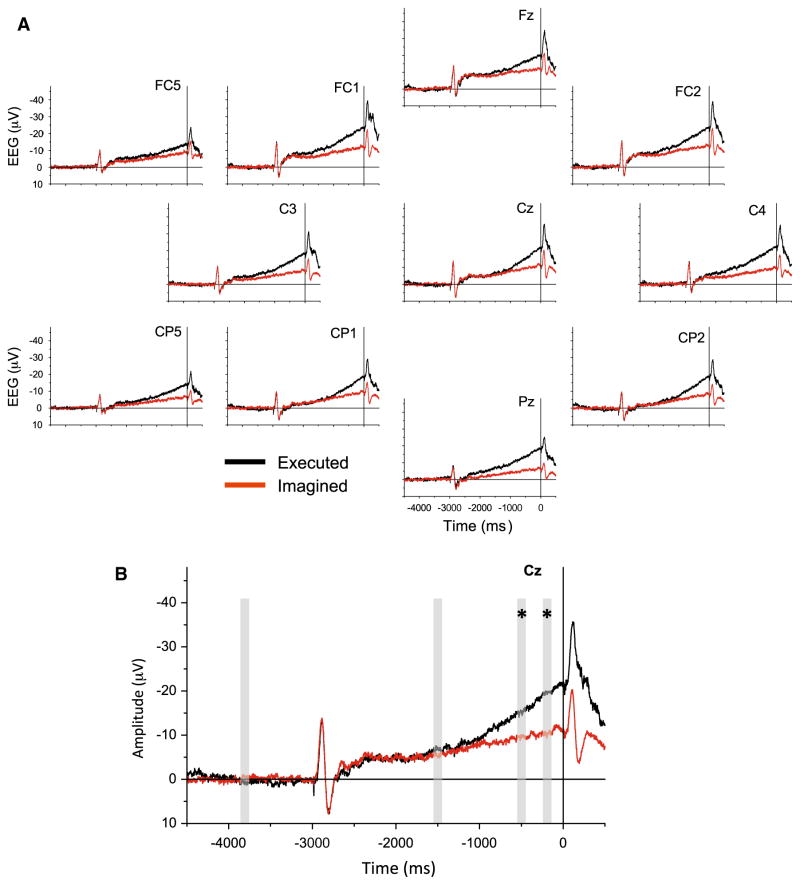
CNV associated with executed and imagined movement
The CNV waveform for the EM task was characterized by an initial auditory-evoked potential in response to the warning tone at −3000 ms, a subsequent period of sustained activity with a slow-developing negative slope, followed by a more rapid-rising negative potential beginning an average of 1750 ± 320 ms (mean ± 1 SD) prior to the onset of the finger extensor muscle activity (Fig. 4). The IM task was also associated with a slow-rising negative potential that was time locked to the onset of the release tone; however, the late increase in the slope of the negative potential that was seen during the EM task was absent. Both the EM and IM tasks showed a second auditory-evoked potential produced by the release tone followed by a decrease in the negative potential toward baseline. The EM and IM waveforms following the release tone were closely aligned suggesting that both tasks were completed with a similar time course. ANOVA of the area under the CNV curve at the vertex electrode (Cz) at the selected time points (baseline, −1500, −500, −200 ms) showed a significant main effect of time (F(3,24) = 18.256, p < 0.001, ), reflecting the increase in amplitude of the CNV throughout the preparatory time period. There was also a main effect of task (F(1,8) = 6.258, p = 0.037, ) indicating that the EM task resulted in larger amplitude CNV waveforms than the IM. A significant task × time interaction effect was also observed (F(3,24) = 8.225, p = 0.001, ). Post hoc testing showed that there were no differences in CNV amplitude between EM and IM at baseline and at −1500 ms, but the waveform for the EM task had a significantly higher area under the curve than the IMs at the −500 and −200 ms time points (p < 0.05; Fig. 4b). Similar findings of task (p < 0.008), time (p < 0.001), and task × time interaction effects (p < 0.001) were observed at the C3 and C4 electrodes with the exception that significant differences were also observed between tasks at −1500 ms (p < 0.05) at the C4 electrode.
Discussion
The main finding from this study was the observation that the presentation of a SAS during preparation for imagined movement rarely (<2 % of trials across subjects) evoked the release of the weight. This result is in marked contrast to the EM task during which a SAS evoked a premature release of the weight when the stimulus was presented between 1500 and 200 ms prior to the onset of the release tone. These findings provide evidence that executed and imagined movements are prepared differently. In addition, we observed that a SAS could evoke the release of the required postural response (BB EMG suppression), irrespective of the task, or timing of the SAS, suggesting that the timing and mechanisms of release of the focal and postural components of the task are prepared independently. Interpretation and implications of these findings are discussed below.
In keeping with previous studies (Carlsen and MacKinnon 2010; MacKinnon et al. 2013), we observed that the presentation of a SAS as early as 1500 ms before the release cue often resulted in the premature execution of the focal movement (hand opening and release of the weight) for the EM task. As the timing of the SAS probe approached the onset of the release tone, the incidence of movement release progressively increased in conjunction with an increased negativity of the CNV. The mean reaction times from SAS to finger extensor EMG onset were below 100 ms for each of the SAS probe timings and typically accompanied by a startle reflex suggesting that these movements were involuntarily released by fast conducting pathways that mediate the StartReact effect (Valls-Solé et al. 1999; Carlsen et al. 2004a; Kumru and Valls-Solé 2006) and do not reflect inadvertent voluntary reactions to the SAS tone (Carlsen et al. 2007). These findings demonstrate that, during the EM task, there was a progressive increase in cortical activity mediating the preparation and storage of the focal movement, that this motor output was ready as early as 1500 ms prior to the intended action, and that during the preparation phase, there was a decrease in the threshold by which sensory stimuli can trigger the release of the planned movement.
In contrast, a StartReact effect on the focal movement (release of the weight) was almost completely absent for the IM task. Across subjects, a SAS evoked the release of the weight in less than 2 % of all IM trials. The presence of a CNV that was time locked to the release tone and terminated at a time consistent with the completion of an actual release (as for the EM condition; Fig. 5) provides evidence that subjects performed the IM task as instructed. The most parsimonious explanation for the absence of SAS-evoked movement release during imagined movements is that there was insufficient cortical activation, as reflected in the CNV, for the SAS to trigger movement (Carlsen and MacKinnon 2010; Carlsen et al. 2012b). This explanation assumes that preparatory motor activity must reach a sufficient level, so that the excitatory input provided by the SAS exceeds the threshold required to trigger the initiation of movement. Indeed, the excitability of the startle reflex pathway progressively increased during the EM task, as evidenced by an increased incidence of a SCM burst during SAS trials, but did not during the IM task. Previous studies have shown that the level of startle reflex excitability closely parallels the StartReact effect (Carlsen and MacKinnon 2010). However, the amplitude of the CNV was higher at the Cz electrode during the IM task at −200 ms compared to the EM task at −1500 ms (paired test; p = 0.019; see Fig. 5), and the level of excitability of the startle reflex was comparable (SCM incidence: IM at −200 ms = 45 %, EM at −1500 = 47 %). If the cortical activity at these two time points reflects comparable processes, but with heightened excitability during the IM task, then the presentation of a SAS at −200 ms should have released the movement in at least 24 % of trials (similar to the EM incidence at −1500 ms). This suggests that the underlying neural processes between tasks at these points of time are different; during the EM task, activity at −1500 ms included components of readiness for motor output, while activity at −200 ms for the IM task did not.
Fig. 5.
a Grand average CNV waveforms at each scalp surface electrode for the executed (black lines) and imagined (red lines) movement tasks. The zero point on the time axis is the onset of the release tone. b Expansion of the waveform at electrode Cz. The gray bars indicate the 100 ms windows used to calculate areas under the CNV waveform centered on the −3800, −1500, −500, and −200 time points. Asterisk (*) indicates a significant difference between tasks (p < 0.05) at the marked time point
Another possible explanation for the absence of movement release during the IM task is that subjects actively suppressed movement output throughout the task to ensure that the weight was not released in response to the SAS or imperative tone. Suppression of motor excitability has been proposed as a mechanism of “impulse control” that prevents the inadvertent release of the intended response (Duque and Ivry 2009; Duque et al. 2010; Confais et al. 2012). We consider this possibility to be unlikely for the following reasons. First, care was taken to avoid this confound by providing specific instructions for subjects to imagine the kinesthetic sense of movement release rather than the suppression of movement. Second, an active suppression of motor output, as occurs during go/no–go tasks, is associated with an abrupt suppression of the slow-rising negative potential at the time of movement cancelation (Smith et al. 2013). As noted above, the CNV associated with the IM task showed a rising slope throughout the preparatory period and terminated at a time appropriate with the imagined release of the weight (i.e., after the release cue), similar to the data for the executed movement.
The results of this experiment are, in many respects, congruent with those reported by Maslovat et al. (2013) who used a similar experimental paradigm to study the preparation of imagined movement (Maslovat et al. 2013), but differences in methodology and analysis lead to a different interpretation of the findings. Maslovat et al. showed that the presentation of a SAS at the same time as the imperative “go” stimulus rarely (8 % of trials) triggered the full motor response (release of a switch by extending the wrist), but a partial release was commonly observed (21 % of trials). The presence of partial responses was interpreted to suggest that the neural substrates mediating executed and imagined movements are similar, but lowered excitability during motor imagery attenuates the StartReact effect resulting in a “leaking out” of the planned motor output. However, it is important to note that partial movements were also commonly seen during imagery trials without a SAS (14 % of trials; 7 of 16 subjects), demonstrating that many participants executed the movement during imagery. In subjects that did not show EMG activity during imagery, the presentation of a SAS never evoked a full or partial response. Without EEG recordings or a comparable probe of imagery-related cortical activity, it cannot be discerned if the lack of a StartReact reflected an absence of motor preparation or non-compliance with the task instructions. In the present study, we controlled for these potential confounds by recording the imagination-related CNV and excluded all IM trials that showed EMG activity above baseline levels (from −4000 to +500 ms for control trials; from −4000 to 50 ms after the SAS for SAS trials). When these factors were controlled for, we found that a StartReact effect during the preparatory phase of motor imagery was very rare, suggesting that in the vast majority of trials and subjects, threshold for release of movement is either very high or imagery does not normally involve the preparation of motor output for the focal task.
An unexpected and novel finding from this experiment was the observation that the SAS evoked a suppression of BB activity during all task and timing conditions. This includes trials when the SAS was presented while subjects awaited the warning cue and were holding the required posture of the arm (Figs. 2, 4). This demonstrates that triggering of the intended postural response was unrelated to the presence of movement- or imagination-related preparation. An auditory startle reflex is usually accompanied by the activation of the elbow flexors (Brown et al. 1991), so the suppression of BB activity is not easily explained by classic startle pathways; however, testing of the startle reflex is not usually conducted in the context of a postural stability task. Nonetheless, the fact that the magnitude and duration of the BB suppression were not significantly different from the suppression observed during executed movements provides evidence that the release was linked to the planned movement. The observation that the required postural response was frequently evoked by the SAS, even during the hold period between trials, whereas the focal task was not suggests that the neural mechanisms and pathways by which the stimulus accesses the focal and postural components of a task may be different. Studies of focal movements requiring either voluntary activation or inhibition of muscle activity have consistently shown that the rapid release of the planned action by a startle is usually accompanied by SCM activation (Valls-Solé et al. 1999; Carlsen et al. 2004a, 2012a, b). In contrast, the triggering of BB suppression during the IM task was not requisitely tied to a startle reflex. Approximately one half of the trials with a SAS-evoked BB suppression did not show a SCM burst. Several studies have shown that context-relevant reflex behaviors, such as postural responses, can result from perturbations (Gahery et al. 1981; Cordo and Nashner 1982), even if no focal movement is made (Aruin et al. 2001). In the present study, the SAS can be considered to be a perturbation that evokes BB suppression to maintain the required postural set, that is, the stabilization of the arm and elbow angle throughout the task. In this manner, the BB suppression may reflect a default state of postural preparation. An alternate hypothesis is that the BB suppression is part of the stereotypical response to SAS when holding an object. In support of this idea, a recent study found that the subjects persist in holding an object in the face of a perturbation that causes a conflict between holding and releasing the object (Bateni et al. 2004). Further experiments are necessary to distinguish between these two explanations.
In summary, the results of this experiment provide evidence that preparatory cortical activity, subcortical excitability (both the startle reflex and the StartReact), and level of preparation of the focal planned movement are distinctly different between executed and imagined movements. This has important ramifications for applications that rely upon the assumption that neuronal activity associated with mental imagery of a task involves similar preparatory activity to that seen for executed movements. These applications include brain–machine interfaces (Mason et al. 2007), functional neuroimaging of whole-body or lower limb movement tasks (Malouin et al. 2003; Bakker et al. 2007, 2008; la Fougere et al. 2010; Snijders et al. 2011), neurorehabilitation (Dickstein et al. 2004; Jackson et al. 2004; Butler and Page 2006; Dunsky et al. 2006; Lotze and Cohen 2006; Sharma et al. 2006; Braun et al. 2008), and enhancement of sports performance (Kearns and Crossman 1992; Gentili et al. 2006; Fontani et al. 2007; Olsson et al. 2008). Our data may explain why these approaches have met with limited success (Holmes and Calmels 2008). Instructing individuals to attempt to move, despite an inability to do so (e.g., in spinal cord injured patients), may provide a more effective method to recapitulate normal motor preparatory processes than strict kinesthetic imagery, especially in the fields of neurorehabilitation and training for brain–computer interface devices.
Acknowledgments
We thank the volunteers for these experiments, and the technical assistance from Mr. Di Zhang. J.E. was supported by a Grant from NIH T32 HD057845 and ANC by a Grant from the Natural Sciences and Engineering Research Council of Canada (PDF-357177).
Contributor Information
Jeremy S. Eagles, Department of Physical Therapy and Human Movement, Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Anthony N. Carlsen, Department of Physical Therapy and Human Movement, Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA. School of Human Kinetics, Faculty of Health Sciences, University of Ottawa, Ottawa, Canada
Colum D. MacKinnon, Email: cmackinn@umn.edu, Department of Physical Therapy and Human Movement, Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA. Department of Neurology, University of Minnesota, 717 Delaware St., S.E., Minneapolis, MN 55455, USA
References
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol. 2012;590:919–936. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin AS, Shiratori T, Latash ML. The role of action in postural preparation for loading and unloading in standing subjects. Exp Brain Res. 2001;138:458–466. doi: 10.1007/s002210100729. [DOI] [PubMed] [Google Scholar]
- Bakker M, de Lange FP, Stevens JA, Toni I, Bloem BR. Motor imagery of gait: a quantitative approach. Exp Brain Res. 2007;179:497–504. doi: 10.1007/s00221-006-0807-x. [DOI] [PubMed] [Google Scholar]
- Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41:998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Barclay-Goddard RE, Stevenson TJ, Poluha W, Thalman L. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst Rev. 2011;5:1–47. doi: 10.1002/14651858.CD005950.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateni H, Zecevic A, McIlroy WE, Maki BE. Resolving conflicts in task demands during balance recovery: does holding an object inhibit compensatory grasping? Exp Brain Res. 2004;157:49–58. doi: 10.1007/s00221-003-1815-8. [DOI] [PubMed] [Google Scholar]
- Braun S, Kleynen M, Schols J, Schack T, Beurskens A, Wade D. Using mental practice in stroke rehabilitation: a framework. Clin Rehabil. 2008;22:579–591. doi: 10.1177/0269215508090066. [DOI] [PubMed] [Google Scholar]
- Braun S, Kleynen M, van Heel T, Kruithof N, Wade D, Beurskens A. The effects of mental practice in neurological rehabilitation; a systematic review and meta-analysis. Front Hum Neurosci. 2013;7:390. doi: 10.3389/fnhum.2013.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114(Pt 4):1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Page SJ. Mental practice with motor imagery: evidence for motor recovery and cortical reorganization after stroke. Arch Phys Med Rehabil. 2006;87:S2–S11. doi: 10.1016/j.apmr.2006.08.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldara R, Deiber MP, Andrey C, Michel CM, Thut G, Hauert CA. Actual and mental motor preparation and execution: a spatiotemporal ERP study. Exp Brain Res. 2004;159:389–399. doi: 10.1007/s00221-004-2101-0. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, MacKinnon CD. Motor preparation is modulated by the resolution of the response timing information. Brain Res. 2010;1322:38–49. doi: 10.1016/j.brainres.2010.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004b;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Almeida QJ, Franks IM. Startle decreases reaction time to active inhibition. Exp Brain Res. 2012a;217:7–14. doi: 10.1007/s00221-011-2964-9. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol. 2012b;123:21–33. doi: 10.1016/j.clinph.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT, Galdo-Alvarez S, Lastra-Barreira C. Equivalent is not equal: primary motor cortex (MI) activation during motor imagery and execution of sequential movements. Brain Res. 2008;1226:134–143. doi: 10.1016/j.brainres.2008.05.089. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Confais J, Kilavik BE, Ponce-Alvarez A, Riehle A. On the anticipatory precue activity in motor cortex. J Neurosci. 2012;32:15359–15368. doi: 10.1523/JNEUROSCI.1768-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Carlsen AN, Chua R, Franks IM. Temporal uncertainty does not affect response latencies of movements produced during startle reactions. Exp Brain Res. 2006;171:278–282. doi: 10.1007/s00221-006-0459-x. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL, Phillips JG. Movement-related potentials associated with movement preparation and motor imagery. Exp Brain Res. 1996;111:429–436. doi: 10.1007/BF00228732. [DOI] [PubMed] [Google Scholar]
- Decety J. Analysis of actual and mental movement times in graphic tasks. Acta Psychol (Amst) 1993;82:367–372. doi: 10.1016/0001-6918(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M. Mentally simulated movements in virtual reality: does Fitts’s law hold in motor imagery? Behav Brain Res. 1995;72:127–134. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M, Prablanc C. The timing of mentally represented actions. Behav Brain Res. 1989;34:35–42. doi: 10.1016/s0166-4328(89)80088-9. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Dunsky A, Marcovitz E. Motor imagery for gait rehabilitation in post-stroke hemiparesis. Phys Ther. 2004;84:1167–1177. [PubMed] [Google Scholar]
- Dunsky A, Dickstein R, Ariav C, Deutsch J, Marcovitz E. Motor imagery practice in gait rehabilitation of chronic post-stroke hemiparesis: four case studies. Int J Rehabil Res. 2006;29:351–356. doi: 10.1097/MRR.0b013e328010f559. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontani G, Migliorini S, Benocci R, Facchini A, Casini M, Corradeschi F. Effect of mental imagery on the development of skilled motor actions. Percept Mot Skills. 2007;105:803–826. doi: 10.2466/pms.105.3.803-826. [DOI] [PubMed] [Google Scholar]
- Frank C, Land WM, Popp C, Schack T. Mental representation and mental practice: experimental investigation on the functional links between motor memory and motor imagery. PLoS ONE. 2014;9:e95175. doi: 10.1371/journal.pone.0095175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahery Y, Ioffe ME, Massion J, Polit A. Postural support for local movements in cats and dogs. Zh Vyssh Nerv Deiat Im I P Pavlova. 1981;31:232–241. [PubMed] [Google Scholar]
- Gentili R, Papaxanthis C, Pozzo T. Improvement and generalization of arm motor performance through motor imagery practice. Neuroscience. 2006;137:761–772. doi: 10.1016/j.neuroscience.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, et al. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Green JB, Bialy Y, Sora E, Thatcher RW. An electroencephalographic study of imagined movement. Arch Phys Med Rehabil. 1997;78:578–581. doi: 10.1016/s0003-9993(97)90421-4. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex. 2008;18:2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- Holmes P, Calmels C. A neuroscientific review of imagery and observation use in sport. J Mot Behav. 2008;40:433–445. doi: 10.3200/JMBR.40.5.433-445. [DOI] [PubMed] [Google Scholar]
- Hugon M, Massion J, Wiesendanger M. Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflugers Arch. 1982;393:292–296. doi: 10.1007/BF00581412. [DOI] [PubMed] [Google Scholar]
- Ietswaart M, Johnston M, Dijkerman HC, Joice S, Scott CL, MacWalter RS, Hamilton SJ. Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain. 2011;134:1373–1386. doi: 10.1093/brain/awr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Doyon J, Richards CL, Malouin F. The efficacy of combined physical and mental practice in the learning of a foot-sequence task after stroke: a case report. Neurorehabil Neural Repair. 2004;18:106–111. doi: 10.1177/0888439004265249. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG. Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp Brain Res. 2002;147:98–107. doi: 10.1007/s00221-002-1220-8. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kearns DW, Crossman J. Effects of a cognitive intervention package on the free-throw performance of varsity basketball players during practice and competition. Percept Mot Skills. 1992;75:1243–1253. doi: 10.2466/pms.1992.75.3f.1243. [DOI] [PubMed] [Google Scholar]
- Kho AY, Liu KP, Chung RC. Meta-analysis on the effect of mental imagery on motor recovery of the hemiplegic upper extremity function. Aust Occup Ther J. 2014;61:38–48. doi: 10.1111/1440-1630.12084. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Mathews S, Dean PJ, Sterr A. On the equivalence of executed and imagined movements: evidence from lateralized motor and nonmotor potentials. Hum Brain Mapp. 2009;30:3275–3286. doi: 10.1002/hbm.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranczioch C, Mathews S, Dean P, Sterr A. Task complexity differentially affects executed and imagined movement preparation: evidence from movement-related potentials. PLoS ONE. 2010;5:e9284. doi: 10.1371/journal.pone.0009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Valls-Solé J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Lotze M, Cohen LG. Volition and imagery in neurorehabilitation. Cogn Behav Neurol. 2006;19:135–140. doi: 10.1097/01.wnn.0000209875.56060.06. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Allen DP, Shiratori T, Rogers MW. Early and unintentional release of planned motor actions during motor cortical preparation. PLoS ONE. 2013;8:e63417. doi: 10.1371/journal.pone.0063417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslovat D, Chua R, Hodges NJ. When unintended movements “leak” out: a startling acoustic stimulus can elicit a prepared response during motor imagery and action observation. Neuropsychologia. 2013;51:838–844. doi: 10.1016/j.neuropsychologia.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Mason SG, Bashashati A, Fatourechi M, Navarro KF, Birch GE. A comprehensive survey of brain interface technology designs. Ann Biomed Eng. 2007;35:137–169. doi: 10.1007/s10439-006-9170-0. [DOI] [PubMed] [Google Scholar]
- Olsson CJ, Jonsson B, Nyberg L. Internal imagery training in active high jumpers. Scand J Psychol. 2008;49:133–140. doi: 10.1111/j.1467-9450.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kennedy R, Palmer S, et al. Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol. 2011;106:915–924. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Schack T, Essig K, Frank C, Koester D. Mental representation and motor imagery training. Front Hum Neurosci. 2014;8:328. doi: 10.3389/fnhum.2014.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a back-door to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR. Motor and visual imagery as two complementary but neurally dissociable mental processes. J Cogn Neurosci. 2001;13:910–919. doi: 10.1162/089892901753165827. [DOI] [PubMed] [Google Scholar]
- Smith JL, Jamadar S, Provost AL, Michie PT. Motor and non-motor inhibition in the Go/NoGo task: an ERP and fMRI study. Int J Psychophysiol. 2013;87:244–253. doi: 10.1016/j.ijpsycho.2012.07.185. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516(Pt 3):931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wriessnegger SC, Steyrl D, Koschutnig K, Muller-Putz GR. Short time sports exercise boosts motor imagery patterns: implications of mental practice in rehabilitation programs. Front Hum Neurosci. 2014;8:469. doi: 10.3389/fnhum.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]