Abstract
Background and Objective
Serious GI adverse events in the outpatient setting were examined by polypectomy technique, endoscopist volume, and facility type (ambulatory surgery center and hospital outpatient department).
Design
Retrospective follow-up study.
Setting
Ambulatory surgery and hospital discharge datasets from Florida (1997-2004) were used.
Patients
A total of 2,315,126 outpatient colonoscopies performed in patients of all ages and payers were examined.
Main Outcome
Thirty-day hospitalizations because of colonic perforations and GI bleeding, measured as cumulative and specific outcomes, were investigated.
Results
Compared with simple colonoscopy, the adjusted risks of cumulative adverse events were greater with the use of cold forceps (1.21 [95% CI, 1.01-1.44]), ablation (3.75 [95% CI, 2.97-4.72]), hot forceps (5.63 [95% CI, 4.97-6.39]), snares (7.75 [95% CI, 6.95-8.64]), or complex colonoscopy (8.83 [95% CI, 7.70-10.12]). Low-volume endoscopists had higher risks of adverse events (1.18 [95% CI, 1.07-1.30]). A higher risk of adverse events was associated with procedures performed in ambulatory surgery centers (1.27 [95% CI, 1.16-1.40]). Important findings were also reported for the analyses stratified by specific outcomes and procedures.
Limitation
The study was constrained by limitations inherent in administrative data pertaining to a single state.
Conclusions
As the complexity of polypectomy increases, a higher risk of adverse events is reported. Using lower risk procedures when clinically appropriate or referring patients to high-volume endoscopists can reduce the rates of perforations and GI bleeding. Given the large number of colonoscopies performed in the United States, it is critical that the rates of adverse events be considered when choosing procedures.
Colorectal cancer (CRC) is the second leading cause of cancer mortality and is among the most common cancers in the United States.1 CRC is preventable through removal of premalignant polyps.2 CRC screening with colonoscopy procedures to detect and remove polyps is expected to surpass 14 million procedures per year.3 Although a screening colonoscopy is a relatively safe procedure, colonoscopy with cautery is associated with a ninefold increased risk of colonic perforations and GI bleeding compared with colonoscopies without cautery.4
Endoscopists’ use of polypectomy techniques is highly variable.5 There is neither agreement on the best practice in terms of a specific technique nor clear empirical evidence to favor one technique over another.5,6 Previous research is limited by insufficient data to identify specific polypectomy techniques,4 aggregation of colonos-copies with any form of cautery, and their procedure codes in a single category.7,8 The lack of data on a specific polypectomy and its potential contribution to serious adverse events leaves a substantial gap in the literature and an absence of practical guidance for patients and endoscopists.
In addition, a growing literature on inpatient care has linked provider volume to patient outcomes over multiple surgical procedures and conditions.9 However, the relationship between colonoscopy provider volume and patient outcomes is less understood, leading to a recent call for additional research.10 A Canada-based study reported that endoscopist specialty (ie, gastroenterology, general surgery, or primary care) was not associated with an increased risk of adverse events, but low-volume providers had a higher risk of adverse events than did high-volume providers.8 Because most outpatient colonoscopies are provided at ambulatory surgery centers (ASCs) or hospital-based outpatient departments (HOPDs), research comparing the rates of adverse events in these facilities is also needed.11
This study used a large, encounter-level, and all-payer dataset with patient and physician identifiers for all Florida ASCs, HOPDs, and hospitals to identify hospitalizations for serious GI adverse events within 30 days after colonos-copy. These data cover the entire population of Florida from 1997 to 2004. The rates of serious GI risks associated with each specific biopsy or polypectomy technique were estimated. The relationship between provider volume and the risk of serious adverse events was examined. Finally, the rates of adverse events after colonoscopy between ASCs and HOPDs were compared.
METHODS
Data sources
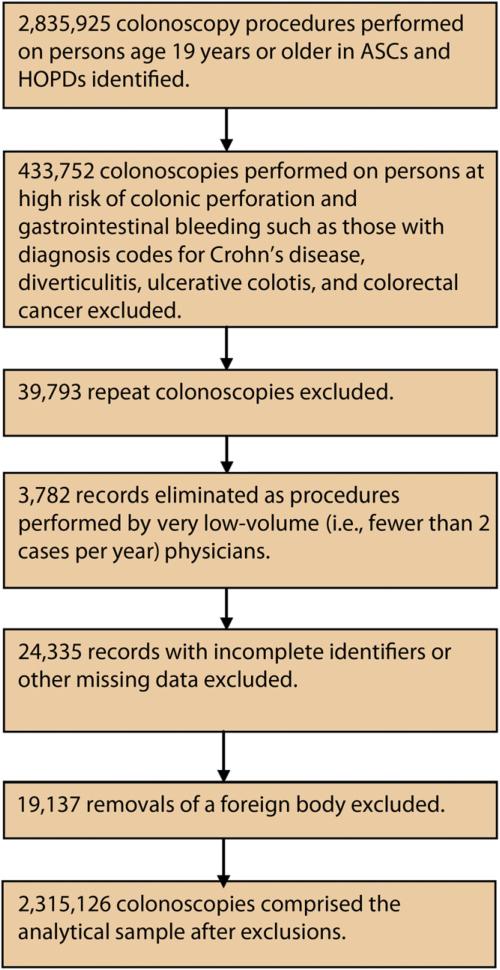
Ambulatory surgery and inpatient hospital discharge datasets were obtained from the Florida Agency for Health Care Administration for 1997 through 2004. Both datasets were at the encounter level and included unique patient and physician identifiers, primary and secondary diagnoses as classified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), primary and secondary procedure codes based on Current Procedural Terminology (CPT), payer types, facility types, dates of outpatient procedures and hospitalizations, and patient demographic characteristics. Colonoscopy procedures were linked to subsequent hospitalizations to identify all hospital admissions for serious adverse events within 30 days. Figure 1 describes how the analytical sample of 2,315,126 was derived.
Figure 1.
Analytical sample. ASC, ambulatory surgery center; HOPD, hospital outpatient department.
Serious adverse events
The primary outcome was a cumulative measure of hospitalizations for colonic perforation and GI bleeding, including cases of acute posthemorrhagic anemia and blood transfusion within 30 days of a colonoscopy.7 In addition, specific outcomes for colonic perforation and GI bleeding, including cases of acute posthemorrhagic anemia and blood transfusions within 30 days of a colonos-copy, were independently analyzed. Primary diagnosis codes in the inpatient hospital discharge dataset were used to identify colonic perforations (ICD-9-CM: 569.83, 998.2),7 GI bleeding (ICD-9-CM: 578, 578.1, 578.9, 998.1),7 acute posthemorrhagic anemia (ICD-9-CM: 285.1), and blood transfusion (ICD-9-CM: 280-284.9, 285.2-285.9, 99.03, 99.04).7
Colonoscopy procedures
Six colonoscopy procedure categories were constructed by using the primary CPT codes. Colonoscopy without polypectomy was categorized as simple colonos-copy (the reference category), which combined diagnostic and screening colonoscopy (CPT 45378, G0105, and G0121), and colonoscopy with single or multiple biopsies (CPT 45380) (cold biopsy forceps).12 Three categories with polypectomy were identified: ablation (CPT 45383), polypectomy by hot biopsy forceps or bipolar cautery forceps (CPT 45384), and snaring (CPT 45385).12 Finally, the sixth category, the complex colonoscopy, was identified in which multiple colonoscopy procedures were performed, and primary and secondary CPT codes with any form of cautery (eg, CPT 45380, 45383, 45384, and 45385) were billed during the same session.12
Endoscopist volume, facility type, and patient characteristics
The annual colonoscopy volume for each physician was calculated as the total number of primary colonos-copy procedures in each year of the study. Physicians were then assigned to low- (<300 cases per year) or high-(≥300 cases per year) volume categories based on annual volume of all colonoscopy procedures. Provider volume was allowed to vary from year to year. The high-volume category was used as the reference. A dichotomous variable representing facility type was included, with HOPDs as the reference.
Patient age was categorized as 19 to 49 years (referent), 50 to 64, 65 to 74, 75 to 84, and 85 years and older. Race/ethnicity was specified as white (referent), Hispanic, black or African American, or other (including unknowns). Sex was included as a binary variable (male as the reference). Health insurance types were categorized as Medicare (referent), Medicare health maintenance organization (HMO), Medicaid, Medicaid HMO, commercial indemnity, commercial HMOs, commercial preferred provider organizations, self-pay or charity, and other. Diagnostic cost groups/hierarchical condition categories, which use all available ICD-9-CM diagnostic codes to categorize patients, provided risk scores and indicated a greater severity of illness among patients with higher scores.13-15 Finally, unobserved changes over time common for both ASCs and HOPDs (eg, changes in practice guidelines, new policy recommendations) were controlled by including a set of dummy variables for each year between 1997 and 2004.
Statistical analysis
Patient and provider characteristics were examined, stratifying by colonoscopy procedures. Unadjusted rates for cumulative and specific GI adverse events were calculated by dividing the total numbers of adverse events by the total number of colonoscopies in the sample. Additionally, the unadjusted rates of adverse events for each procedure, and by endoscopist volume and facility type were calculated.
Multivariate logistic regression analyses were used to create predictive models of cumulative and specific adverse events. These models detected those variables independently associated with the adverse events of interest, after adjusting for the contributions of the other variables. Odds ratios were estimated to evaluate the magnitude and direction of the effect for the key parameters of interest. The models also adjusted for clustering of outcomes in the same physician, because patients treated by the same provider may have experienced similar outcomes because of similar care practices and processes of care delivery, and estimated robust confidence intervals for the odds ratios.
Wald tests of linear restrictions were conducted to evaluate different polypectomy techniques. In addition, logistic regression models, stratified by procedures, were used to predict the adjusted, procedure-specific adverse events for endoscopist volume and facility type.
All data were collected in 2006, and the current analysis was conducted in 2012. There is a time lag of several years for organizing claims data into analytical files and making them available to researchers. We obtained these data from a contract with the Florida Agency for Health Care Administration to study comparative effectiveness of ASCs and HOPDs. Once that analysis was complete, the research team turned to other topics of interest including the one presented here. When using claims data, a lag of several years is not unusual.
For individual statistical procedures, P < .05 was viewed as significant. It is recognized that there was multiple testing of outcome data arising from individual patients’ data records. As the univariate tests were exploratory, meant to highlight differences, their significant results were not subjected to any corrections for multiple testing. The results from the multivariate analyses are most definitive. There are no corrections for multiple testing of their results, as these findings are to be taken as suggestive, only because of the source and nature of the data, with their inherent limitations. The study was approved by the institutional review board of Virginia Commonwealth University.
RESULTS
Descriptive analyses
Among the more than 2 million colonoscopies in the sample, 44.38% were simple colonoscopies, 12.64% involved the use of cold biopsy forceps, 2.25% were ablations, 14.95% involved the use of hot biopsy forceps, 17.41% involved the use of snaring, and 8.37% were complex colonoscopies (Table 1). Low-volume endoscopists provided, on average, 179 simple colonoscopies, 177 colonoscopies with cold biopsy forceps, 180 colonosco-pies with ablation, 183 colonoscopies with hot biopsy forceps, 188 colonoscopies with snaring, and 188 complex colonoscopies per year, whereas high-volume providers averaged 683 simple colonoscopies, 653 colonoscopies with cold biopsy forceps, 615 colonoscopies with ablation, 704 colonoscopies with hot biopsy forceps, 693 colonos-copies with snaring, and 703 complex colonoscopy annually (Table 1). Physicians at ASCs provided higher proportions of simple colonoscopies, colonoscopies with cold and hot biopsy forceps and snares, and lower proportions of colonoscopies with ablation and complex colonosco-pies than physicians in HOPDs (Table 1).
TABLE 1.
Patient and provider characteristics by outpatient colonoscopy procedures
| No polypectomy |
With polypectomy |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Simple colonoscopy (no biopsy) | Cold biopsy forceps (single or multiple) | Ablation | Hot biopsy forceps or cautery | Snare | Complex colonoscopy (biopsy and polypectomy) |
| Total no. (%) | 1,027,515 (44.38) | 292,667 (12.64) | 52,024 (2.25) | 346,150 (14.95) | 403,077 (17.41) | 193,693 (8.37) |
| Patient characteristics | ||||||
| Age categories, y, no. (%) | ||||||
| 19-49 | 211,157 (20.55) | 79,643 (27.21) | 4753 (9.14) | 37,460 (10.82) | 41,753 (10.36) | 21,269 (10.98) |
| 50-64 | 396,560 (38.59) | 92,588 (31.64) | 15,731 (30.24) | 120,465 (34.8) | 142,148 (35.27) | 61,304 (31.65) |
| 65-74 | 240,792 (23.43) | 68,251 (23.32) | 16,858 (32.4) | 110,036 (31.79) | 126,111 (31.29) | 62,187 (32.11) |
| 75-84 | 154,680 (15.05) | 44,732 (15.28) | 12,781 (24.57) | 68,876 (19.9) | 81,009 (20.1) | 42,512 (21.95) |
| >85 | 24,326 (2.37) | 7453 (2.55) | 1901 (3.65) | 9313 (2.69) | 12,056 (2.99) | 6421 (3.32) |
| Sex, no. (%) | ||||||
| Male | 411,558 (40.05) | 114,401 (39.09) | 27,213 (52.31) | 180,643 (52.19) | 221,214 (54.88) | 105,664 (54.55) |
| Female | 615,957 (59.95) | 178,266 (60.91) | 24,811 (47.69) | 165,507 (47.81) | 181,863 (45.12) | 88,029 (45.45) |
| Race, no. (%) | ||||||
| White | 778,400 (75.76) | 236,897 (80.94) | 43,913 (84.41) | 277,749 (80.24) | 321,672 (79.8) | 165,145 (85.26) |
| Hispanic | 98,072 (9.54) | 17,513 (5.98) | 2875 (5.53) | 26,687 (7.71) | 27,776 (6.89) | 9484 (4.9) |
| Black/African American | 72,992 (7.1) | 14,667 (5.01) | 2291 (4.4) | 15,207 (4.39) | 19,831 (4.92) | 7425 (3.83) |
| Other/unknown | 78,051 (7.6) | 23,590 (8.06) | 2945 (5.66) | 26,507 (7.66) | 33,798 (8.38) | 11,639 (6.01) |
| Risk score, mean (SD) | 1.29 (0.68) | 1.36 (0.73) | 1.49 (0.68) | 1.43 (0.65) | 1.44 (0.67) | 1.53 (0.71) |
| Insurance types, no. (%) | ||||||
| Medicare | 342,422 (33.33) | 104,664 (35.76) | 25,409 (48.84) | 153,782 (44.43) | 173,447 (43.03) | 92,364 (47.69) |
| Medicare HMO | 36,524 (3.55) | 9054 (3.09) | 2376 (4.57) | 18,383 (5.31) | 19,223 (4.77) | 10,577 (5.46) |
| Medicaid | 12,632 (1.23) | 4040 (1.38) | 393 (0.76) | 2632 (0.76) | 3003 (0.75) | 1350 (0.7) |
| Medicaid HMO | 5001 (0.49) | 1522 (0.52) | 180 (0.35) | 1213 (0.35) | 1140 (0.28) | 766 (0.4) |
| Indemnity | 214,836 (20.91) | 65,293 (22.31) | 7686 (14.77) | 59,195 (17.1) | 73,289 (18.18) | 32,540 (16.8) |
| Commercial HMO | 207,815 (20.23) | 51,025 (17.43) | 7803 (15) | 52,389 (15.13) | 65,827 (16.33) | 25,683 (13.26) |
| Commercial PPO | 168,554 (16.4) | 44,538 (15.22) | 6527 (12.55) | 46,552 (13.45) | 52,866 (13.12) | 24,402 (12.6) |
| Self-pay/charity | 15,677 (1.53) | 4624 (1.58) | 503 (0.97) | 4523 (1.31) | 4876 (1.21) | 2165 (1.12) |
| Other payers | 24,054 (2.34) | 7907 (2.7) | 1147 (2.2) | 7481 (2.16) | 9406 (2.33) | 3846 (1.99) |
| Provider characteristics | ||||||
| Endoscopist volume, mean (SD) | ||||||
| <300 | 179 (81) | 177 (82) | 180 (80) | 183 (81) | 188 (79) | 188 (77) |
| ≥300 | 683 (313) | 653 (305) | 615 (263) | 704 (374) | 693 (329) | 703 (390) |
| Facility type, no. (%) | ||||||
| ASC | 573,670 (55.83) | 177,131 (60.52) | 21,951 (42.19) | 186,310 (53.82) | 222,696 (55.25) | 94,247 (48.66) |
| HOPD | 453,845 (44.17) | 115,536 (39.48) | 30,073 (57.81) | 159,840 (46.18) | 180,381 (44.75) | 99,446 (51.34) |
SD, Standard deviation; HMO, health maintenance organization; PPO, preferred provider organization; ASC, ambulatory surgery center; HOPD, hospital outpatient department.
Table 2 reports procedure and patient characteristics by endoscopist volume and facility type. Low-volume endoscopists treated slightly higher proportions of elderly (>85 years of age), patients with higher severity scores, minorities, patients covered by Medicare HMO, Medicaid, self-pay/charity, and other payers.
TABLE 2.
Outpatient colonoscopy procedure and patient characteristics by provider types
| Endoscopist volume |
Facility type |
|||
|---|---|---|---|---|
| <300 | ≥300 | ASC | HOPD | |
| Procedure | ||||
| Simple colonoscopy | 242,955 (41.70) | 784,560 (45.28) | 573,670 (44.96) | 453,845 (43.68) |
| Cold biopsy forceps | 78,834 (13.53) | 213,833 (12.34) | 177,131 (13.88) | 115,536 (11.12) |
| Polypectomy by ablation | 17,439 (2.99) | 34,585 (2.00) | 21,951 (1.72) | 30,073 (2.89) |
| Polypectomy by hot biopsy forceps or bipolar cautery | 97,432 (16.72) | 248,718 (14.36) | 186,310 (14.6) | 159,840 (15.38) |
| Polypectomy by snare | 91,309 (15.67) | 311,768 (18.00) | 222,696 (17.45) | 180,381 (17.36) |
| Complex colonoscopy | 54,645 (9.38) | 139,048 (8.03) | 94,247 (7.39) | 99,446 (9.57) |
| Patient characteristics | ||||
| Age, y | ||||
| 19-49 | 106,835 (18.34) | 289,200 (16.69) | 194,408 (15.24) | 201,627 (19.40) |
| 50-64 | 187,788 (32.23) | 641,008 (37.00) | 445,121 (34.88) | 383,675 (36.92) |
| 65-74 | 160,243 (27.50) | 463,992 (26.78) | 369,189 (28.93) | 255,046 (24.54) |
| 75-84 | 109,174 (18.74) | 295,416 (17.05) | 234,769 (18.40) | 169,821 (16.34) |
| >85 | 18,574 (3.19) | 42,896 (2.48) | 32,518 (2.55) | 28,952 (2.79) |
| Sex | ||||
| Female | 310,138 (53.23) | 944,295 (54.50) | 694,127 (54.40) | 560,306 (53.92) |
| Race | ||||
| White | 436,163 (74.86) | 1,387,613 (80.09) | 990,628 (77.64) | 833,148 (80.18) |
| Hispanic | 53,418 (9.17) | 128,989 (7.45) | 96,269 (7.54) | 86,138 (8.29) |
| Black/African American | 37,183 (6.38) | 95,230 (5.50) | 57,568 (4.51) | 74,845 (7.20) |
| Other/unknown | 55,850 (9.59) | 120,680 (6.97) | 131,540 (10.31) | 44,990 (4.33) |
| Risk score | ||||
| ≥1 | 424,552 (72.87) | 1,221,831 (70.52) | 885,271 (69.38) | 761,112 (73.25) |
| Insurance type | ||||
| Medicare | 228,503 (39.22) | 663,585 (38.3) | 533,864 (41.84) | 358,224 (34.47) |
| Medicare HMO | 30,808 (5.29) | 65,329 (3.77) | 28,020 (2.20) | 68,117 (6.56) |
| Medicaid | 9185 (1.58) | 14,865 (0.86) | 10,882 (0.85) | 13,168 (1.27) |
| Medicaid HMO | 3386 (0.58) | 6436 (0.37) | 2164 (0.17) | 7658 (0.74) |
| Indemnity | 93,389 (16.03) | 359,450 (20.75) | 356,018 (27.9) | 96,821 (9.32) |
| Commercial HMO | 95,798 (16.44) | 314,744 (18.17) | 178,455 (13.99) | 232,087 (22.33) |
| Commercial PPO | 89,555 (15.37) | 253,884 (14.65) | 124,918 (9.79) | 218,521 (21.03) |
| Self-pay/charity | 11,453 (1.97) | 20,915 (1.21) | 10,600 (0.83) | 21,768 (2.09) |
| Other payers | 20,537 (3.52) | 33,304 (1.92) | 31,084 (2.44) | 22,757 (2.19) |
ASC, Ambulatory surgery center; HOPD, hospital outpatient department; HMO, health maintenance organization; PPO, preferred provider organization.
Patients undergoing colonoscopy in HOPDs tended to be younger (19-49 years of age), ethnic minorities, have higher severity scores. ASCs treated disproportionally higher percentages of patients with private indemnity and traditional Medicare insurance plans than did HOPDs.
Table 3 reports the unadjusted rates of adverse events resulting in a hospitalization within 30 days after colonoscopy. The unadjusted rate of cumulative GI adverse events was 1.98 (95% CI, 1.93-2.04) per 1000 procedures, the rate of GI bleeding was 1.65 (95% CI, 1.60-1.70) per 1000 procedures, and the rate of colonic perforations was 033 (95% CI, 0.33-0.36) per 1000 procedures. Procedure-specific rates of cumulative adverse events progressively increased from simple (0.33; 95% CI, 0.31-0.36) to complex colonoscopy (5.00; 95% CI, 4.69-5.32) per 1000 procedures (Table 3). The rates of cumulative adverse events per 1000 procedures were 2.27 (95% CI, 2.15-2.40) for low-volume and 1.89 (95% CI, 1.82-1.95) for high-volume endoscopists. The rate of cumulative adverse events was higher for ASCs (2.08; 95% CI, 2.00-2.26) than for HOPDs (1.87; 95% CI, 1.79-1.95) per 1000 procedures.
TABLE 3.
Unadjusted rates of colonoscopy-related adverse events per 1000 outpatient procedures and univariate associations among polypectomy technique, endoscopist volume, and facility type
| Rates (95% CI) | No. of adverse events | No. of procedures | |
|---|---|---|---|
| Cumulative GI adverse events | 1.98 (1.93-2.04) | 4595 | 2,315,126 |
| GI bleeding | 1.65 (1.60-1.70) | 3822 | 2,315,126 |
| Colonoscopic perforation | 0.33 (0.31-0.36) | 773 | 2,315,126 |
| Simple colonoscopy | 0.49 (0.45-0.54) | 507 | 1,027,515 |
| Cold biopsy forceps | 0.60 (0.52-0.70) | 176 | 292,667 |
| Polypectomy by ablation | 2.15 (1.77-2.59) | 112 | 52,024 |
| Polypectomy by hot biopsy or bipolar cautery forceps | 3.15 (2.96-3.34) | 1089 | 346,150 |
| Polypectomy by snares | 4.32 (4.12-4.53) | 1743 | 403,077 |
| Complex colonoscopy | 5.00 (4.69-5.32) | 968 | 193,693 |
| Endoscopist volume | |||
| <300 | 2.27 (2.15-2.40) | 1324 | 582,614 |
| ≥300 | 1.89 (1.82-1.95) | 3271 | 1,732,512 |
| Facility type | |||
| ASC | 2.08 (2.00-2.16) | 2653 | 1,276,005 |
| HOPD | 1.87 (1.79-1.95) | 1942 | 1,039,121 |
CI, Confidence interval; ASC, ambulatory surgery center; HOPD, hospital outpatient department.
Multivariate analyses
Table 4 presents estimates from multivariate logistic regressions. Compared with simple colonoscopy, the odds ratios for cumulative adverse events were significantly greater after colonoscopies with cold biopsy forceps (1.21; 95% CI, 1.01-1.44), ablation (3.75; 95% CI, 2.97-4.72), hot biopsy forceps (5.63; 95% CI, 4.97-6.39), and snaring (7.75; 95% CI, 6.95-8.64) and complex colonoscopies (8.83; 95% CI, 7.70-10.12). Additionally, the odds ratios for GI bleeding were significantly greater after colonoscopies with cold biopsy forceps (1.84; 95% CI, 1.47-2.30], ablation (6.82; 95% CI, 5.25-8.85), hot biopsy forceps (10.90; 95% CI, 9.33-12.74), and snaring (15.13; 95% CI, 13.18-17.35) and after complex colonoscopies (16.97; 95% CI, 14.38-20.02). The odds ratios for colonic perfo-rations were lower for colonoscopies with cold biopsy forceps (ie, 0.66; 95% CI, 0.50-0.87), but greater for colonoscopies with snaring (1.56; 95% CI, 1.29-1.89) and complex colonoscopies (1.99; 95% CI, 1.56-2.54) compared with simple colonoscopies.
TABLE 4.
Odds ratios (95% confidence intervals) from multivariate logistic regression analyses of colonoscopy-related adverse events for various factors, with modeling that recognized clustering of outcomes by endoscopist
| Cumulative GI adverse events | GI bleeding | Colonic perforation | |
|---|---|---|---|
| Cold biopsy forceps | 1.21 (1.01-1.44) | 1.84 (1.47-2.30) | 0.66 (0.50-0.87) |
| Polypectomy by ablation | 3.75 (2.97-4.72) | 6.82 (5.25-8.85) | 1.20 (0.73-1.98) |
| Polypectomy by hot biopsy or bipolar cautery forceps | 5.63 (4.97-6.39) | 10.90 (9.33-12.74) | 1.22 (0.97-1.55) |
| Polypectomy by snares | 7.75 (6.95-8.64) | 15.13 (13.18-17.35) | 1.56 (1.29-1.89) |
| Complex colonoscopy | 8.83 (7.70-0.12) | 16.97 (14.38-20.02) | 1.99 (1.56-2.54) |
| Endoscopist volume <300 | 1.18 (1.07-1.30) | 1.13 (1.02-1.25) | 1.49 (1.23-1.80) |
| ASC | 1.27 (1.16-1.40) | 1.11 (1.01-1.23) | 2.67 (2.21-3.22) |
| Risk score | 1.34 (1.19-1.50) | 1.25 (1.11-1.42) | 1.80 (1.39-2.34) |
| Age 50-64 | 1.13 (0.99-1.28) | 1.08 (0.94-1.25) | 1.38 (1.01-1.87) |
| Age 65-74 | 1.31 (1.12-1.53) | 1.22 (1.03-1.45) | 1.80 (1.24-2.62) |
| Age 75-84 | 1.81 (1.54-2.13) | 1.71 (1.43-2.05) | 2.36 (1.61-3.48) |
| Age >85 | 2.43 (2.00-2.95) | 2.34 (1.90-2.88) | 2.88 (1.75-4.72) |
| Female | 0.74 (0.69-0.79) | 0.65 (0.61-0.70) | 1.33 (1.15-1.55) |
| Hispanic | 1.18 (1.05-1.33) | 1.23 (1.08-1.39) | 0.99 (0.75-1.31) |
| Black or African American | 1.23 (1.07-1.42) | 1.32 (1.13-1.53) | 0.86 (0.60-1.25) |
| Other or unknown race | 0.98 (0.87-1.12) | 1.00 (0.87-1.14) | 0.90 (0.68-1.20) |
| Medicare HMO | 1.08 (0.93-1.26) | 1.04 (0.88-1.24) | 1.34 (0.99-1.82) |
| Medicaid | 1.35 (1.00-1.81) | 1.28 (0.91-1.79) | 1.74 (0.91-3.33) |
| Medicaid HMO | 1.13 (0.68-1.88) | 1.22 (0.74-2.01) | 0.57 (0.08-3.90) |
| Indemnity | 0.90 (0.80-1.00) | 0.90 (0.79-1.03) | 0.88 (0.66-1.16) |
| Commercial HMO | 1.00 (0.88-1.12) | 0.97 (0.86-1.11) | 1.11 (0.84-1.47) |
| Commercial PPO | 0.99 (0.87-1.14) | 0.96 (0.83-1.11) | 1.19 (0.87-1.64) |
| Self pay/charity | 1.25 (0.97-1.62) | 1.28 (0.98-1.68) | 1.00 (0.45-2.28) |
| Other payers | 1.01 (0.81-1.25) | 0.92 (0.71-1.20) | 1.45 (0.91-2.31) |
| 1998 | 1.06 (0.92-1.23) | 1.00 (0.86-1.18) | 1.43 (0.99-2.07) |
| 1999 | 0.88 (0.75-1.02) | 0.84 (0.71-1.00) | 1.08 (0.73-1.59) |
| 2000 | 1.06 (0.92-1.22) | 1.03 (0.86-1.20) | 1.22 (0.86-1.73) |
| 2001 | 0.96 (0.83-1.12) | 0.94 (0.80-1.11) | 1.08 (0.75-1.57) |
| 2002 | 0.91 (0.78-1.05) | 0.86 (0.73-1.01) | 1.20 (0.85-1.71) |
| 2003 | 0.95 (0.82-1.10) | 0.93 (0.79-1.09) | 1.06 (0.75-1.52) |
| 2004 | 0.89 (0.77-1.03) | 0.85 (0.72-0.99) | 1.15 (0.81-1.62) |
ACS, Ambulatory surgery center; HMO, health maintenance organization; PPO, preferred provider organization.
Low-volume endoscopists had higher odds ratios for cumulative adverse events (ie, 1.18 [95% CI, 1.05-1.27]), GI bleeding (ie, 1.13 [95% CI, 1.02-1.25]), and perforations (ie, 1.49 [95% CI, 1.23-1.80]) compared with high-volume providers. Greater odds ratios for cumulative, GI bleeding, and perforation adverse events were associated with procedures performed at ASCs compared with HOPDs (ie, 1.27 [95% CI, 1.16-1.30]; 1.11 [95% CI, 1.01-1.23]; and 2.67 [95% CI, 2.24-3.22], respectively).
In addition, a greater severity was associated with increased odds ratios for GI adverse events (1.34 [95% CI, 1.19-1.40] for cumulative, 1.25 [95% CI, 1.11-1.42] for GI bleeding, and 1.80 [95% CI, 1.39-2.34] for perforation). Increased odds ratios for cumulative adverse events were positively associated with older age groups of 65 to 74, 75 to 84 and those older than 85 years of age (1.31 [95% CI, 1.12-1.53], 1.81 [95% CI, 1.54-2.13], and 2.43 [95% CI, 2.00-2.95], respectively) (Table 4). Compared with white patients, Hispanic and black or African American patients had increased odds ratios for cumulative adverse events (1.18 [95% CI, 1.05-1.33] and 1.23 [95% CI, 1.07-1.42], respectively) and GI bleeding (1.23 [95% CI, 1.08-1.39] and 1.32 [95% CI, 1.13-1.53], respectively). In addition, Medicaid coverage was associated with an increased odds ratio for cumulative adverse events (1.35 [95% CI, 1.00-1.81]) relative to those covered by Medicare (Table 4).
In a stratified multivariate analysis, low-volume endoscopists had higher risks of cumulative adverse events associated with simple colonoscopies (1.45 [95% CI, 1.17-1.81]) and colonoscopies with cold biopsy forceps (1.58 [95% CI, 1.11-2.25]) compared with high-volume providers. Higher odds ratios for cumulative adverse events at ASCs relative to HOPDs were observed for simple colonoscopies (2.42 [95% CI, 1.94-3.01]) and for colonoscopies with snaring (1.43 [95% CI, 1.26-1.61]), respectively (Table 5). In addition, low-volume endoscopists performed worse than high-volume endoscopists in terms of colonic perforations for simple colonoscopies (1.56 [95% CI, 1.16-2.08]), colonoscopies with cold biopsy forceps (1.88 [95% CI, 1.04-3.41]), and colonoscopies with hot biopsy forceps (1.77 [95% CI, 1.14-2.75]). ASCs had higher perforation risks than HOPDs for simple colonoscopies (5.00 [95% CI, 3.47-7.22]), colonoscopies with cold biopsy forceps (2.17 [95% CI, 1.19-3.96]), colonoscopies with hot biopsy forceps (1.66 [95% CI, 1.06-2.62]), and colonoscopies with snaring (2.25 [95% CI, 1.58-3.19]) and complex colonoscopies (1.93 [95% CI, 1.30-2.87]).
TABLE 5.
Odds ratios (95% confidence intervals) from multivariate logistic regression analyses of colonoscopy-related adverse events for endoscopist volume and facility type, stratified by procedure
| No polypectomy |
With polypectomy |
||||||
|---|---|---|---|---|---|---|---|
| Adverse events | Provider characteristics | Simple colonoscopy (n = 1,027,515) | Cold biopsy forceps (n = 292,667) | Ablation (n = 52,024) | Hot biopsy or cautery forceps (n = 346,150) | Snare (n = 403,077) | Complex colonoscopy (n = 193,693) |
| Endoscopist volume | |||||||
| Cumulative GI adverse events | ≥300 | 1 | 1 | 1 | 1 | 1 | 1 |
| <300 | 1.45 (1.17-1.81) | 1.58 (1.11-2.25) | 0.80 (0.50-1.29) | 1.17 (0.98-1.40) | 1.12 (0.99-1.29) | 1.13 (0.94-1.35) | |
| HOPD | 1 | 1 | 1 | 1 | 1 | 1 | |
| ASC | 2.42 (1.94-3.01) | 1.36 (0.93-2.00) | 0.62 (0.37-1.03) | 1.05 (0.88-1.23) | 1.43 (1.26-1.61) | 1.01 (0.83-1.22) | |
| Endoscopist volume | |||||||
| ≥300 | 1 | 1 | 1 | 1 | 1 | 1 | |
| GI bleeding | <300 | 1.34 (0.98-1.82) | 1.45 (0.94-2.24) | 0.69 (0.40-1.16) | 1.11 (0.92-1.34) | 1.13 (0.98-1.30) | 1.11 (0.92-1.34) |
| HOPD | 1 | 1 | 1 | 1 | 1 | 1 | |
| ASC | 1.29 (0.95-1.75) | 1.12 (0.70-1.78) | 0.48 (0.28-0.82) | 0.99 (0.83-1.17) | 1.36 (1.20-1.55) | 0.93 (0.75-1.14) | |
| Endoscopist volume | |||||||
| ≥300 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Colonic perforation | <300 | 1.56 (1.16-2.08) | 1.88 (1.04-3.41) | 1.78 (0.72-4.44) | 1.77 (1.14-2.75) | 1.15 (0.78-1.70) | 1.28 (0.81-2.00) |
| HOPD | 1 | 1 | 1 | 1 | 1 | 1 | |
| ASC | 5.00 (3.47-7.22) | 2.17 (1.19-3.96) | 1.95 (0.83-4.58) | 1.66 (1.06-2.62) | 2.25 (1.58-3.19) | 1.93 (1.30-2.87) | |
HOPD, Hospital outpatient department; ASC, ambulatory surgery center.
DISCUSSION
Analyses of all-encounter, all-payer data covering the entire population of Florida for a 7-year period provide an important advancement over previous studies that examined a limited number of facilities or health systems,4,16,17 used restricted datasets such as Medicare7 or Medicaid-only data,18 or were non-U.S. based.8,19 As the complexity of polypectomy increases, a higher risk of adverse events was found in this study.
Simple colonoscopy without cautery is associated with the fewest cumulative and GI bleeding adverse events. The use of cold biopsy forceps is associated with the next fewest cumulative and GI events, but may be limited to potentially removing small polyps or performing a biopsy on a large lesion.12 Perhaps the majority of GI bleeds caused by the use of cold biopsy forceps occur immediately after the procedure and are more likely to be capillary and thus clinically insignificant. This study's findings demonstrate that some small risk of clinically significant GI bleeding is associated with the use of cold biopsy forceps. This may occur in patients with previous use of anticoagulant agents or other forms of coagulopathy and in cases of GI hemorrhages potentially unrelated to biopsy that were not fully adjusted for in our study because of limitations inherent in administrative data. However, the risk of significant GI bleeding after the use of cold biopsy forceps is much lower compared with the risks of hemorrhage associated with other polypectomy techniques. Moreover, the study findings show that there is a lower risk of perforation with colonoscopies with cold biopsy forceps compared with simple colonoscopies. As such, cold biopsy forceps for removal of small polyps may be preferred over hot biopsy forceps.
In terms of colonoscopy with polypectomy, the option associated with the fewest cumulative and GI bleeding adverse events is polypectomy by ablation. Ablation, however, is associated with an approximately 6 times higher risk of GI bleeding compared with cold biopsy forceps. This evidence is consistent with recommendations to use ablation infrequently and only to remove small polyps that are not amenable to removal by snaring.12 Both hot biopsy forceps and snaring are commonly used by endoscopists for polyp removal. Snaring (hot or cold) is believed to be more effective than forceps (hot or cold) for removal of both small and large polyps.5,6 This study's findings show that cumulative and specific risks of adverse events are less likely after cold or hot forceps relative to snaring because of the probable use of snares for larger polyps. The findings are in line with results from a survey of endoscopists that showed that forceps techniques (hot or cold) dominated removal of polyps 1 to 3 mm in size and hot snaring was primarily used for polyps 7 to 9 mm in diameter compared with other polypectomy methods.5 In addition, when complex colonoscopy procedures are undertaken to remove potentially large and/or multiple polyps using several polypectomy techniques during the same session, the likelihood of serious adverse effects significantly increases relative to any single polypectomy technique.
Cold or hot biopsy forceps, cold or hot snares, and mixed polypectomy methods were used interchangeably for polyps 4 to 6 mm in size.5 Endoscopists should weigh the risks and benefits associated with each polypectomy method in clinical situations (eg, for polyps 4-6 mm in size) when different polypectomy techniques are at their discretion. Moreover, our study's administrative data did not include information on polyp size, type, or multiplicity and also did not distinguish between cold and hot snaring. More research is needed to better understand differences in risks of serious adverse events with cold and hot snaring relative to cold and hot forceps, while controlling for polyp size, type, and multiplicity.
Consistent with previous studies,7,20 this study found that the odds ratios for cumulative and specific adverse events increased with patient age after adjusting for polypectomy techniques, endoscopist volume, and patient severity of illness. As such, endoscopists need to balance potential colonoscopy-related risks of adverse events against an individual's benefit from CRC detection and prevention, especially for the patients older than 75 years, given their remaining life expectancy.20
Compared with high-volume endoscopists, low-volume endoscopists had increased overall risks of adverse events and higher perforation risks for simple colonoscopies and colonoscopies with cold and hot forceps. Low-volume endoscopists may have less experience and/or a specialty other than gastroenterology. Elderly and minority patients and those covered by Medicaid and other less generous insurance plans were more likely to have a colonoscopy performed by a low-volume endoscopist than their white, younger, and privately insured counterparts. Previous research found that black and Medicaid populations are more likely to receive care from physicians or physician groups who are less trained and have less access to important clinical resources.21,22 Differences in endoscopist experience may account for disparities in outcomes among these patients.
Positive volume
Outcome effects were found for endoscopists who performed more than 300 colonoscopies per year. A study based in Canada demonstrated that endoscopist volume below of a threshold of approximately 300 colonoscopies per year was significantly associated with increased risks of adverse events.8 In addition, the European Guidelines for Quality Assurance in Colorectal Cancer Screening include standards for endoscopist volume, suggesting that each endoscopist should perform at least 300 procedures per year to ensure a sufficient sample size to assess competence.23 However, the standards for performance of colonoscopy set by specialty societies in the United States are neither uniformly adopted nor enforced, and a minimum number of colonoscopy procedures performed per year is not a compulsory requirement in the majority of GI endoscopy centers.24 Perhaps this practice should be reconsidered in light of our study's findings.
In this regard, implications for health care policy were identified. First, consistent with the recommendations of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable in the United States, information concerning endoscopist volume could be made available for public reporting and used by primary care physicians and patients to select high-volume endoscopists, regardless of their specialty and training.24 A measure of endoscopist volume would be easy to implement, monitor, and report. Second, implications for medical education were identified. Specifically, high-volume endoscopists who are skilled in all types of polypectomy should participate in clinical training of medical students, gastroenterology fellows, and residents to ensure the development of endoscopic proficiency and procedure-specific competence in different polyp removal techniques.
Outpatient colonoscopies provided at ASCs were associated with the higher risks of adverse events. Although the overall differences were relatively small and arguably clinically insignificant, endoscopists at ASCs tend to select healthier patients and thus would be expected to perform better than endoscopists at HOPDs.25 One plausible explanation of the observed differences is that ASCs have a less stringent quality regulation, oversight, and reporting system in contrast to HOPDs’ requirements.26 Although there have been efforts to improve the quality of care in ASC settings, these efforts may not immediately translate into better health outcomes or guarantee that quality improvement procedures are applied successfully at ASCs. In a recent report to Congress, the Department of Health and Human Services recommended expanding ASC industry oversight and developing procedure-specific quality measures for ASCs to facilitate comparison of quality indicators across settings (specifically with HOPDs) and payers.27 This study's findings suggest that tracking 30-day adverse events as a comparative, colonoscopy-specific quality indicator for ASCs and HOPDs is feasible and could be facilitated through the use of administrative data.
The study has limitations. A lack of access to patients’ medical records to fully adjust for the quality of bowel preparation or previous use of medications (eg, anticoagulants or antibiotics), especially by elderly patients, was a limitation. We were unable to exclude colonoscopies with biopsies that were used for reasons other than polyp removal (eg, evaluation for microscopic colitis in patients with diarrhea) from the analyses. The inclusion of these colonoscopies would tend to underestimate the rate of adverse events. In addition, data were not available to include adverse events associated with 30-day emergency department visits and colonoscopies provided in physician offices. The study's data did not distinguish among cases of GI bleeding potentially unrelated to polypectomy (eg, bleeding from hemorrhoids, diverticula, caused by radiation proctitis, or other entities). The data did not capture the few patients with colonoscopy-related adverse events who were hospitalized outside the state of Florida. The data did not include information on endoscopists’ specialty, experience, age, or affiliation with academic centers, which may potentially correlate with volume and affect the rate of adverse events. There was also no information on lesion type, polyp size, and multiplicity in the dataset. As such, there may be potential confounding among lesion type/size, multiple polyps, and choice of polyp removal technique. Nevertheless, several different types of colonoscopy and polypectomy procedures, and their combination, were used, which may provide some control for differences in polyp type, size, and multiple polyps. More research is needed to better understand which type of polypectomy (eg, either piecemeal or total polyp removal, with or without saline solution injection, and with or without clips being placed after polyp removal) is a preferred option when lesion type and size are quantifiable and information on endoscopists’ specialty, experience, age, and affiliation with academic centers is available. Finally, although the study is population based, it is restricted to a single state and uses data for the period 1997 to 2005, which may not reflect current changes in polypectomy practice. As such, research using newer datasets from additional states is warranted.
Notwithstanding these limitations, this research has several important implications for clinical practice and policy. First, specific risks of serious colonoscopy-related adverse events associated with each specific type of polypectomy technique were documented. Second, we found that the risks of adverse events were higher for procedures performed by low-volume endoscopists; this issue could be addressed by monitoring provider volume and primary care physicians’ referring patients to experienced endoscopists and by using high-volume endoscopists proficient in a variety of polypectomy techniques to teach procedure-specific competence for the future generation of endoscopists. Requiring higher volume endoscopists will disproportionately benefit minority, low-income, and elderly populations in which disparities in health outcomes are known to exist. Finally, in clinical situations in which several polypectomy techniques are considered clinically appropriate, the endoscopist should consider the risks of adverse events associated with each technique when choosing a particular method of polyp removal. In addition, the immediate risks and 30-day postpolypectomy adverse events must be balanced with the risk of incomplete polypectomy, resulting in cancers in the distant future. Endoscopists should take into consideration the ability of a particular polypectomy technique in ensuring complete removal of polyps to avoid the need for repeat colonoscopies, increase the surveillance interval for subsequent colonoscopies, and potentially reduce the risk of CRC. Given the large number of colonoscopies performed each year in the United States (approximately 14 million procedures),2 a reduction in the rates of adverse events and the risk of CRC will substantially reduce patient morbidity, mortality, and health care costs.
Take-home Message.
As the complexity of polypectomy increases, a higher risk of adverse events is reported. Using lower risk procedures when clinically appropriate can reduce the rates of perforations and GI bleeding.
ACKNOWLEDGMENTS
The authors thank Dr Theodore R. Levin, research scientist at the Kaiser Permanente Northern California Division of Research, gastroenterologist at Kaiser Permanente Medical Center, Walnut Creek, and assistant clinical professor of medicine, University of California, San Francisco, and Dr Doumit BouHaidar, Associate Professor of Medicine, Director of Advanced Therapeutic Endoscopy, Division of Gastroenterology, Virginia Commonwealth University, for reviewing earlier drafts of the manuscript and providing valuable clinical commentary.
This study (Dr Chukmaitov) is supported by the Virginia Commonwealth University Massey Cancer Center pilot grant.
Abbreviations
- ASC
ambulatory surgery center
- CPT
Current Procedural Terminology
- CRC
colorectal cancer
- HMO
health maintenance organization
- HOPD
hospital outpatient department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
Footnotes
DISCLOSURE: The authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report, July 8, 2011. [November 21, 2011];Vital signs: colorectal cancer screening, incidence, and mortality - United States, 2002-2010. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6026a4.htm. [PubMed]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Levin TR, Zhao W, Conell C, et al. Adverse events of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc. 2004;60:414–8. doi: 10.1016/s0016-5107(04)01808-5. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 7.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–57. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145–61. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]
- 10.Ko CW, Dominitz JA. Adverse events of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am. 2010;20:659–71. doi: 10.1016/j.giec.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Medicare Payment Advisory Commission . Medicare Payment Advisory Commission Report to Congress: Medicare Payment Policy. MedPAC; Washington, DC: 2004. [February 29, 2012]. Available at: http://www.medpac.gov/documents/Mar04_Entire_reportv3.pdf. [Google Scholar]
- 12.HSS Inc [February 29, 2012];Understanding how to code colonoscopies. Available at: http://health-information.advanceweb.com/Article/Understanding-How-to-Code-Colonoscopies.aspx.
- 13.RiskSmart Stand Alone [computer program]. Version 2.1. DxCG Inc; Boston, MA: 2005. [Google Scholar]
- 14.Maciejewski ML, Liu CF, Derleth A, et al. The performance of administrative and self-reported measures for risk adjustment of Veterans Affairs expenditures. Health Serv Res. 2005;40:887–904. doi: 10.1111/j.1475-6773.2005.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chukmaitov A, Harless DW, Menachemi N, et al. How well does diagnosis-based risk-adjustment work for comparing ambulatory clinical outcomes? Health Care Manag Sci. 2009;12:420–33. doi: 10.1007/s10729-009-9101-3. [DOI] [PubMed] [Google Scholar]
- 16.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418–22. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 17.Rathgaber SW, Wick TM. Colonoscopy completion and complication rates in a community gastroenterology practice. Gastrointest Endosc. 2006;64:556–62. doi: 10.1016/j.gie.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 19.Viiala CH, Zimmerman M, Cullen DJ, et al. Complication rates of colonoscopy in an Australian teaching hospital environment. Intern Med J. 2003;33:355–9. doi: 10.1046/j.1445-5994.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- 20.Rutter CM, Johnson E, Miglioretti DL, et al. Adverse events after screening and follow-up colonoscopy. Cancer Causes Control. 2012;23:289–96. doi: 10.1007/s10552-011-9878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham HH, Schrag D, Hargraves JL, et al. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294:473–81. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 22.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 23.European Commission [February 29, 2012];European guidelines for quality assurance in colorectal cancer screening. Available at: http://www.uegf.org/eu_affairs/eu_news/CRC_guidelines_publication%20EU_2011.pdf.
- 24.Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757–66. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Casalino L, Devers K, Brewster L. Focused factories? Physician-owned specialty facilities. Health Affairs (Millwood) 2003;22:56–67. doi: 10.1377/hlthaff.22.6.56. [DOI] [PubMed] [Google Scholar]
- 26.Office of Inspector General [September 27, 2011];Quality oversight of ASCs: a system in neglect. Available at: http://oig.hhs.gov/oei/reports/oei-01-00-00450.pdf.
- 27.U.S. Department of Health and Human Services [February 27, 2012];Report to Congress: Medicare Ambulatory Surgical Center Value-Based Purchasing Implementation Plan. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/Downloads/C_ASC_RTC-2011.pdf.