Abstract
Apigenin, a natural flavonoid, found in several plants, fruits, vegetables, herbs, and spices, is known to have anti-oxidant and anti-inflammatory properties that are evident in the use of these substances for centuries as medicinal approaches to treat asthma, insomnia, Parkinson’s disease, neuralgia, and shingles. However, there is a considerable dearth of information regarding its effect on immune cells, especially dendritic cells (DC) that maintain the critical balance between an immunogenic and tolerogenic immune response, in an immunospecialized location like the central nervous system (CNS). In this paper we looked at the anti-inflammatory properties of Apigenin in restoration of immune function and the resultant decrease in neuroinflammation. In vivo, a significant reduction in severity of experimental autoimmune encephalomyelitis (EAE) progression and relapse was observed in C57BL/6 (progressive) and SJL/J (relapse-remitting) mouse models of multiple sclerosis upon treatment with Apigenin. Apigenin treated EAE mice show decreased expression of α4 integrin and CLEC12A on splenic DCs and an increased retention of immune cells in the periphery compared to untreated EAE mice. This correlated consequently with immunohistochemistry findings of decreased immune cell infiltration and reduced demyelination in the CNS. These results indicate a protective role of Apigenin against the neurodegenerative effects resulting from the entry of DC stimulated pathogenic T cells into the CNS thus implicating a potential therapy for neuroinflammatory disease.
Keywords: Flavonoid, Apigenin, Multiple sclerosis, EAE, Immune cells, Dendritic cells
Introduction
Flavones are a major subclass of naturally occurring low molecular weight plant products known as flavonoids that are ubiquitous in several plants, vegetables, and fruits (Lefort and Blay 2013; Nijveldt et al. 2001; Ross and Kasum 2002). Of these, Apigenin is commonly found in parsley and dried flowers of chamomile as well as in other plants, fruits, vegetables, herbs, and spices (Lefort and Blay 2013). The beneficial properties of Apigenin include antioxidant, anti-inflammatory, and anti-carcinogenic effects (Lefort and Blay 2013; Patel et al. 2007; Rice-Evans 2001; Shukla and Gupta 2010). Its chemopreventive effects against tumor have been reported in a variety of cancer cell lines such as leukemia, ovarian, prostate, colon, and lung (Miean and Mohamed 2001). Furthermore, Apigenin from plants have been utilized as medicine for centuries to treat diseases such as asthma, insomnia, Parkinson’s, neuralgia, and shingles (Lefort and Blay 2013) suggesting its potential use in the treatment of both peripheral and central nervous system (CNS) disorders. However, very little is known of the use of Apigenin as a potential treatment for neuroinflammatory disorders like multiple sclerosis (MS) that affects approximately 400,000 people in the United States alone.
Several theories have been put forth regarding the mechanism of action of Apigenin in the execution of its anti-inflammatory activities. Apigenin has been shown to block LPS-induced lethality in vivo and the expression of proinflammatory cytokines by inactivating NF-κB through the suppression of p65 phosphorylation (Nicholas et al. 2007). Inhibition of cycloxygenase-2 (COX-2) enzyme and monocyte adherence to human umbilical vein endothelium is shown to be affected by Apigenin through down-regulation of cellular adhesion molecules such as VCAM-1, ICAM-1, and E-selectin (Lee et al. 2007; Liang et al. 2001). Since, these molecules play a critical role in controlling leukocyte migration across the endothelial cells including those of blood–brain barrier (BBB), which regulate CNS homeostasis, Apigenin has the potential to inhibit immune cells’ entry into the CNS and prevent neuroinflammation. Apigenin has also been reported to down-regulate the expression of inducible form of nitric oxide synthase (iNOS), production of nitric oxide (NO) and prostaglandin E2 (PGE2) in microglial cells and macrophages thus showing potential neuroprotective effects (Ha et al. 2008). The effect of Apigenin on immune cell (particularly dendritic cells) activation, maturation, and migration across the BBB remains to be elucidated.
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that lie at a crucial junction of linking innate with adaptive immunity (Steinman and Cohn 1973). DCs play a prominent role in maintaining central and peripheral tolerance, the failure of which can lead to various autoimmune and neuroinflammatory diseases (Manuel et al. 2007; Wu and Laufer 2007). In this respect, it has been recently established that DCs can migrate into diverse regions of the CNS (reviewed in (Sagar et al. 2012a)). An increased frequency of both plasmacytoid and myeloid DCs (pDC and mDC) have been reported in the cerebrospinal fluid (CSF) of MS patients with the latter subpopulation having a more mature phenotype in the CSF compared to that in the blood (Pashenkov et al. 2001). Studies have shown brain DCs to activate myelin oligodendrocyte glycoprotein (MOG)-specific T cells in the presence of MOG peptide suggesting its importance in antigen presentation during experimental autoimmune encephalomyelitis (EAE), a demyelinating disease model of MS (Anandasabapathy et al. 2011; Liu and Nussenzweig 2010). Infiltration of DCs and T cells into the CNS result in the production of pro-inflammatory cytokines such as TNF-α, IL-6, IL-17, IL-1β and IFNγ, which result in the neurodegenerative effects associated with neuroinflammatory diseases. Apigenin has been previously shown to inhibit the cell surface expression of co-stimulatory molecules and aspects of DC functionality such as the production of pro-inflammatory cytokines and T cell differentiation (Sagar et al. 2012b; Yoon et al. 2006). Additionally, DCs exhibit the most efficient transmigration in response to neuroinflammatory signals (i.e., chemokine CCL2) both in vitro and in vivo (Sagar et al. 2012b). Since both integrins and lectins (a family of adhesion proteins found on DCs) are involved in DC-CNS trafficking (Rice et al. 2005; Sagar et al. 2012a, b) targeting these specific receptor interactions between DCs and the endothelial cells of the brain can lead to reduced transmigration into the CNS (Nico et al. 1998; Plattner et al. 2010).
Based on the previously documented anti-inflammatory action of Apigenin on DCs and other immune cells, in the present study we examine the effects of Apigenin on EAE disease severity and relapse. In mice induced with EAE (active or relapse-remitting) Apigenin treatment significantly reduced disease severity and relapse. Immune cells, especially DCs and T cells were seen to accumulate in the periphery of the mice treated with Apigenin. Correspondingly, immune infiltration into the CNS was significantly reduced with Apigenin treatment. A significant reduction in the cell surface expression of MHC class II molecules and CD86 on DCs indicated a shift from a pro-inflammatory to a more tolerogenic phenotype. Furthermore, a significant reduction in the number of Th17 cells and concomitant increase in the regulatory T cell phenotype was seen in the Apigenin treated mice indicating restoration of immune balance. Furthermore, a decrease in cell surface expression of α4 integrin (adhesion to BBB) and CLEC12A (antigen uptake) on DCs was observed on Apigenin treatment. Thus, Apigenin can help reduce the marked neuroinflammation and its associated clinical pathologies in MS through possible regulation of DC and other immune cell entry in to the CNS, and their subsequent activation and proinflammatory functions.
Materials and Methods
Mice
Female C57/BL/6 mice (6–8 weeks old) were purchased from the National Cancer Institute (Rockville, MD, USA) and were maintained and bred at the UAB mouse facility (Birmingham, AL, USA) and treated in accordance with the National Institute of Health (NIH) and the University of Alabama Animal Care and Use Committee guidelines. Female SJL/J mice (6–8 weeks old) were purchased from the National Cancer Institute and housed at the AAALAC-accredited University of South Carolina, School of Medicine (Columbia, SC, USA). The SJL/J mice were treated in accordance to NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of South Carolina.
EAE Induction
Progressive EAE was induced in the C57BL/6 mice (8–12 weeks) according to a previously described protocol (Axtell et al. 2004; Naves et al. 2013). Briefly, an immunization mixture of 150 μg myelin oligodendrocyte protein (MOG38–49) (CPC Scientific), 500 μg Mycobacterium tuberculosis and incomplete Freund’s adjuvant was injected subcutaneously in the flank region on day 0 followed by an intraperitoneal (i.p.) administration of 200 ng pertussis toxin on day 0 and 400 ng pertussis toxin on day 2. Relapse-remitting EAE (RR-EAE) was induced in female SJL/J (6–8 weeks old) by subcutaneous injection of 50 μg PLP139–151 peptide emulsified in complete Freund’s Adjuvant (CFA; BD Diagnostics Systems, Franklin Lakes, NJ, USA) containing killed Mycobacterium tuberculosis (400 μg/ml). Clinical scores (0, no symptoms; 1, loss of tail tone; 2, flaccid tail; 3, partial paralysis of hind limbs; 4, complete hind limb paralysis; 5, moribund; 6, death) were recorded every day. The mean score was calculated for each group.
Apigenin Treatment
In the progressive EAE group, C57BL/6 mice were randomized into groups receiving Apigenin or vehicle alone. In the treatment group Apigenin (R&D Systems) dissolved in PBS was administered (i.p.) post-disease onset on day 17 for 5 consecutive days. Mice were sacrificed on day 30 and spleen, cervical lymph nodes, brain and spinal cord were harvested. In the SJL/J mice Apigenin dissolved in distilled water containing 5 % sodium carboxyl methyl cellulose (CMC-Na) was administered by oral gavage at the onset of EAE at a dose of 40 mg/kg body weight, every day till day 15. Shortly after first relapse mice were sacrificed on day 42 and spleen, cervical lymph nodes, brain and spinal cord were harvested.
Histological Analysis of Demyelination and Immune Cell Infiltration
Following mice sacrifice on day 28 and 42 for progressive EAE and RR-EAE respectively, the animals were perfused with 10 mL of heparinized PBS, and spinal cord was removed and fixed with 10 % formalin. Paraffin blocks were prepared and microtome sections (10 μm) were generated, followed by immunohistochemical staining of tissue sections. Spinal cord sections were deparaffinized and hydrated prior to staining with Luxol Fast Blue/Cresyl Violet (Novaultra, Woodstock, MD, USA) and hematoxylin and eosin (Polyscientific, Bayshore, NY, USA).
Quantification of Immune Cells and Stimulation with Antigenic Peptides
Spleen and cervical lymph nodes were excised prior to perfusion on day 28 and 42 for progressive EAE and RR-EAE respectively. Spleen and lymph nodes were homogenized separately into a single-cell suspension and subjected to red blood cell lysis. Cells were stained with anti-CD11c, anti-CD11b, anti-CD68, anti-CD45R, anti-CD4, and anti-CD8α (Biolegend) antibodies and 30,000 cells acquired for quantification. Cells from blood of SJL/J mice were also quantified in a similar fashion.
Splenocytes and lymphocytes from mice with progressive EAE and RR-EAE were further cultured in a 24-well plate in the presence of 30 μg/mL MOG38–49 and PLP139–151 respectively for 3 days. The cells were then stimulated with cell activation cocktail (Biolegend) containing phorbol myristate acetage (PMA), ionomyocin and brefeldin A for 5–6 h. Cells were harvested and subjected to anti-CD11c, anti-MHC II, anti-CD86, anti-CD49 (α4 integrin), anti-CLEC12A, anti-CD4, anti-IL-17A, anti-CD25 and anti-FOXP3 antibodies (Biolegend) staining using BD Cytofix/CytoPerm Fixation/Permeabilization Solution Kit for staining of intracellular markers. Cells were then acquired using the FACS Calibur (BD Biosciences, San Jose, CA) for functional analysis of DCs and T cells.
Brains from mice with progressive EAE were isolated after perfusing the mice with 10 mL heparinized PBS. The brain tissue was mechanically dissociated by passing through a 40 μm cell strainer (Falcon™). The single-cell suspension was centrifuged (10 min at 400 g at room temperature), and the pellet was resuspended in 4 mL of 30 % Percoll (MP Biomedicals). This mixture was overlaid on top of 3.5 ml of 37 % and 3.5 mL of 70 % Percoll solution and centrifuged for 20 min at 1000 g at room temperature in the absence of brake. The mononuclear cells were then collected from the 37 % to 70 % interface and washed once with Hanks’ balanced salt solution (HBSS) (GIBCO) containing 10 % fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA). Cells were then stained with anti-CD45, anti-CD11c, anti-CD68, anti-CD4 and anti-CD8 antibodies for quantification.
Results
Apigenin Treatment Delays the Course and Attenuates EAE Through Reduction in Disease Severity and Body Weight Restoration Accompanied by Retention of Immune Cells in the Periphery
Flavonoids like Apigenin have traditionally been associated with tissue protection through their anti-oxidative and anti-inflammatory properties. However, not much is known of the in vivo effects of Apigenin in neuroinflammatory diseases. Hence, we wished to observe the anti-inflammatory effects of Apigenin in the EAE mouse model for autoimmune demyelinating MS. Upon immunization with MOG peptide in the absence of Apigenin treatment, C57BL/6 mice displayed disease onset on Day 13 that showed progression until disease peak on Day 23. To assess the therapeutic effect of Apigenin on EAE severity and progression, treatment was administered on Day 17 after disease onset and resulted in a significant attenuation of disease score (Fig. 1a, left panel). A significant decrease in disease score was particularly seen at the peak of disease on day 23. Further, an increase in body weight was observed in the Apigenin treated mice when compared to the weight loss observed in the untreated EAE mice (Fig. 1a, right panel). Even upon disease remission between Days 24 and 28, the Apigenin treated group showed significant disease tapering compared to EAE mice.
Fig. 1.
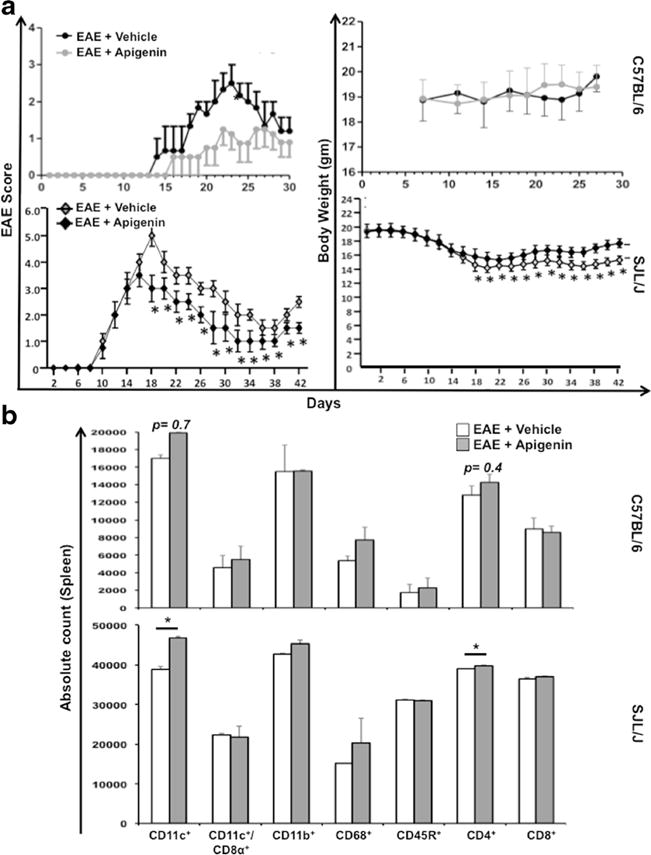
Impact of Apigenin treatment on EAE severity and relapse, restoration in body weight and myeloid cell characterization. a C57BL/6 mice were inoculated with MOG38–49 peptide for progressive EAE induction followed by treatment with Apigenin dissolved in PBS after disease onset for five consecutive days. Control animals with EAE were given PBS alone. The clinical disease scores and body weight of the control and treated animals were recorded every other day for 30 days. Data is indicative of mean scores and body weight from 3 animals per group (upper panel). For the relapsing remitting EAE (RR-EAE) model, SJL/J/J mice were inoculated with PLP323–339 peptide for EAE induction followed by treatment with Apigenin dissolved in distilled water containing 5 % sodium carboxyl methyl cellulose (CMC-Na) at disease onset till day 15. Control animals with EAE were given distilled water containing 5 % CMC-Na. The clinical disease scores and body weight of the control and treated animals were recorded every other day for 42 days. Data is indicative of mean scores and body weight from 5 animals per group (lower panel). b Cells isolated from the spleens of each of the C57BL/6 mice from the EAE + vehicle and EAE + Apigenin groups (n=2) were quantified. The cells were stained for CD11c, CD11c/CD8α, CD11b, CD68, CD45R, CD4, and CD8 immune cell markers. Plots represent absolute cell counts of all splenocytes from animals in each group (upper panel). Splenocytes isolated from EAE + vehicle and EAE + Apigenin groups in SJL/J/J mice were pooled in each group (n=5) and run in triplicates for cell quantification. The cells were stained as previously for C57BL/6 mice. Plots represent absolute cell counts of all splenocytes from animals in each group (upper panel). *p<0.05
Since more than 85 % of patients have relapse remitting-MS (RR-MS), we established the mouse model of RR-EAE by immunizing SJL/J mice with proteolipid protein (PLP) and determined the efficacy of Apigenin treatment after disease onset (Fig. 1a, left panel). The first appearance of clinical signs of RR-EAE resulting from the PLP immunizations occurred on Day 8 and peaked on Day 18 in the vehicle-treated mice. Clinical EAE scores for mice treated with Apigenin were significantly reduced at the disease peak on Day 18 along with significant alleviation in severity of disease progression. Additionally, Apigenin treated mice remained in more prolonged remission as compared to untreated mice. Concomitantly, disease relapse in the treated mice was significantly milder with limp tail phenotype at its most severe form. Figure 1a (right panel) illustrates an increase in body weight beginning on Day 18 as Apigenin treatment alleviates the EAE disease symptoms.
Upon quantification of immune cells in the spleen of the SJL/J mice, significantly higher numbers of CD11c+ DCs and CD4+ T cells were observed in the mice exposed to Apigenin treatment compared to the vehicle-treated EAE mice indicating the accumulation of immune cells in the spleens of treated mice (Fig. 1b). A similar accumulation was observed in the C57BL/6 mice although the differences were not significant. Further quantification of immune cells isolated from the blood and lymph nodes of RR-EAE SJL/J mice revealed accumulation of immune cells in the periphery of the Apigenin treated animals. A similar increase in the number of CD11c+ DCs was observed in the Apigenin treated SJL/J mouse lymphocytes in addition to increased numbers of CD8+ T cells and CD45R+ B cells. Furthermore, the blood of SJL/J mice showed retention of CD11c+ DCs, CD68+ macrophages and CD4+ T cells. CD11b+ cells were also significantly reduced in number in the Apigenin treated SJL/J lymphocytes (Supplementary Figure 1).
Apigenin Treatment Results in Down-Modulation of DC Associated Activation and Co-stimulatory Markers as Well as Adhesion and Antigen Uptake Molecules in EAE Mice
DCs seemed to be retained in all three compartments in the periphery of the Apigenin treated mice. Hence, we decided to further investigate the effects of Apigenin treatment on surface expression of activation and co-stimulatory molecules MHC II and CD86 on DCs, splenocytes from both C57BL/6 and SJL mice were subjected to FACS staining after peptide restimulation for 3 days. Down-modulation of MHC II and CD86 levels was observed in both the Apigenin treated C57BL/6 and SJL/J mice; however, this decrease was only significant in the SJL/J mice post-stimulation with PLP peptide (Fig. 2). Significant reduction in MHC II and CD86 expression levels was additionally seen in lymphocytes isolated from the SJL/J mice (Supplementary Figure 2). We also observed the down-modulation of levels of α4 integrin and CLEC12A found on splenocyte derived DCs in both EAE and RR-EAE models upon Apigenin treatment after peptide re-stimulation (Fig. 3a). The downmodulation was observed at a significant level specifically in the RR-EAE mouse model showing evidence of reduced antigen uptake and adhesion capacity of DCs to the BBB when treated with Apigenin (Fig. 3b). In lymphocytes isolated from SJL/J mice, significant down-modulation of α4 integrin was seen while significant up-regulation of CLEC12A was observed on Apigenin treatment (Supplementary Figure 2).
Fig. 2.
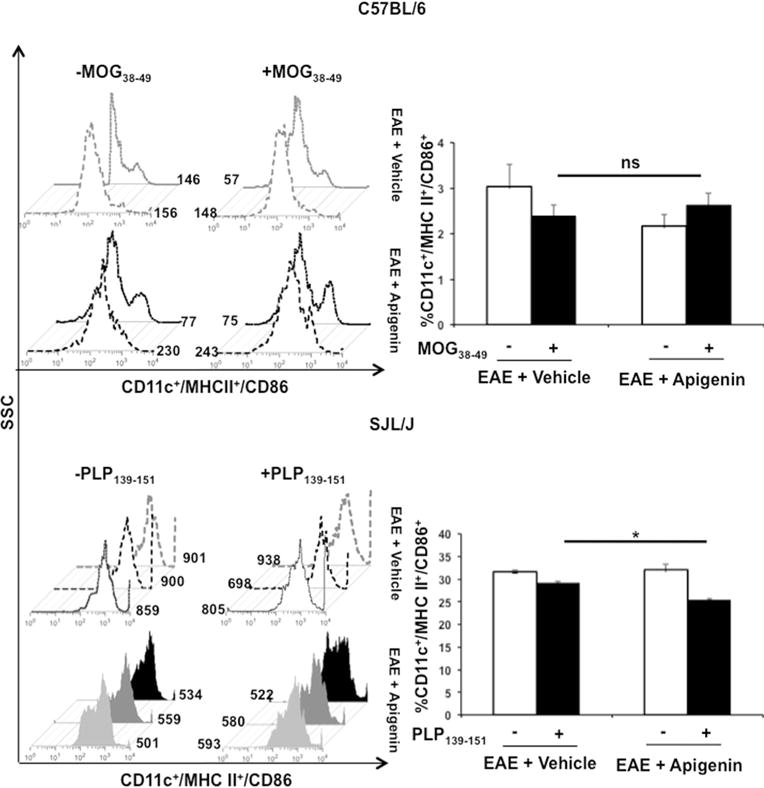
Effect of Apigenin on DC activation and antigen presentation. Splenocytes from both C57BL/6 and SJL/J mice (EAE + vehicle and EAE + Apigenin groups) were stimulated with MOG38–49 and PLP323–339 peptides respectively for 3 days followed by activation with PMA, ionomycin and brefeldin A for 5 h. These cells were subsequently stained with antibodies against CD11c, CD8α, MHC II, and CD86. Data represents CD11c+ dendritic cells from mice expressing MHCI I+CD86+ upon stimulation with MOG38–49 (top) and PLP323–339 (bottom). Each bar is representative of the mean percentage for every marker per group for both C57BL/6 (n=2/group) and SJL/J mice (n=5/group). Histogram plots are representative of one animal per group. *p<0.05
Fig. 3.
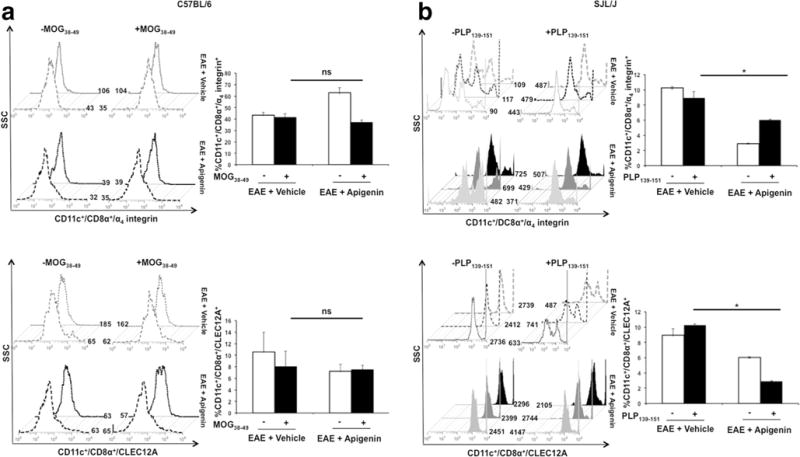
Impact of Apigenin on antigen uptake and immune cell adhesion. Splenocytes from both the EAE + Vehicle and EAE + Apigenin groups of C57BL/6 (n=2/group) and SJL/J mice (n=5/group) were stimulated with MOG38–49 and PLP323–339 peptides respectively for 3 days followed by activation with PMA, ionomycin and brefeldin A for 5 h. These cells were subsequently stained with antibodies against CD11c, CD8α, α4, and CLEC12A. Data represents dendritic cells from C57BL/6 (a) and SJL/J (b) mice expressing α4 integrin (top) and CLEC12A (bottom) upon stimulation with MOG38–49 and PLP323–339 respectively. Each bar is representative of the mean percentage for every marker per group. Histogram plots are representative of one animal per group. *p<0.05
Decreased Th17 and Increased Regulatory T Cell Response Upon Restimulation with MOG and PLP Peptides in Apigenin Treated EAE Mice
To evaluate the effect of Apigenin on T cell differentiation and the resultant immune response, we restimulated splenocytes from both the progressive and RR-EAE mice with MOG38–49 and PLP139–151 peptides respectively for 3 days. Investigation into the Th17 and T regulatory cell phenotypes on splenocytes showed increased IL17A+ and CD25+/FOXP3+ expression amongst CD4+ T cells in Apigenin treated C57BL/6 EAE mice in response to MOG peptide stimulation (Fig. 4a). Similarly, splenocytes isolated from SJL/J mice immunized with PLP showed a decrease in the percentage of IL17A+ cells and increase in CD25+/FOXP3+ in Apigenin treated mice indicating a decreased Th17 response upon restimulation and a heightened T regulatory response upon treatment (Fig. 4b). Also, significantly reduced expression of IL-17A and increased expression of FOXP3 was seen in Apigenin treated lymphocytes isolated from RR-EAE mice (Supplementary Figure 3).
Fig. 4.
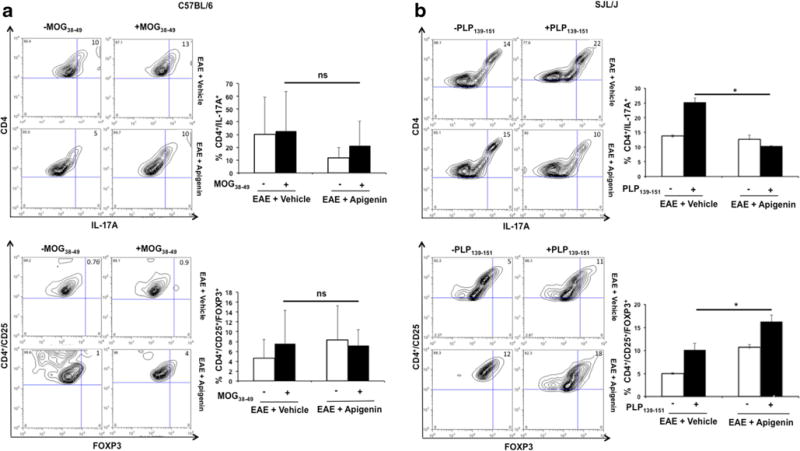
Effect of Apigenin on Treg and Th17 balance in neuroinflammation. Flow cytometry analysis representing CD4+ cells from C57BL/6 mice (a) and SJL/J mice (b) expressing IL-17A (top) and CD25+FOXP3 (bottom) upon stimulation with MOG38–49 and PLP323–339 peptides respectively for 3 days followed by activation with PMA, ionomycin and brefeldin A for 5 h. Each bar is representative of the mean percentage for every marker per group. Contour plots representative of one animal per group are shown on the left. *p<0.05
Treatment with Apigenin Promotes Restoration of T Cell Function
We further investigated the effects of Apigenin on T cell functionality in splenocytes from both the progressive and RR-EAE mice after restimulation with MOG and PLP peptides. Antigen-induced IFNγ production is an important indication of effector Tcell function. Especially in EAE, IFNγ production by auto-antigen-specific T cells has been implicated in disease development (Renno et al. 1994; Swanborg 2001). CD4+ T cells from C57BL/6 EAE mice showed a reduction in intracellular IFNγ levels in treated mice. Additionally, cell enumeration from SJL/J mice immunized with PLP showed a significant decrease in IFNγ expression indicating the restoration of regulatory functions of T cells (Fig. 5). A moderate but non-significant decrease in expression of IFNγ was additionally observed in the lymphocytes of RR-EAE mice treated with Apigenin (Supplementary Figure 4).
Fig. 5.
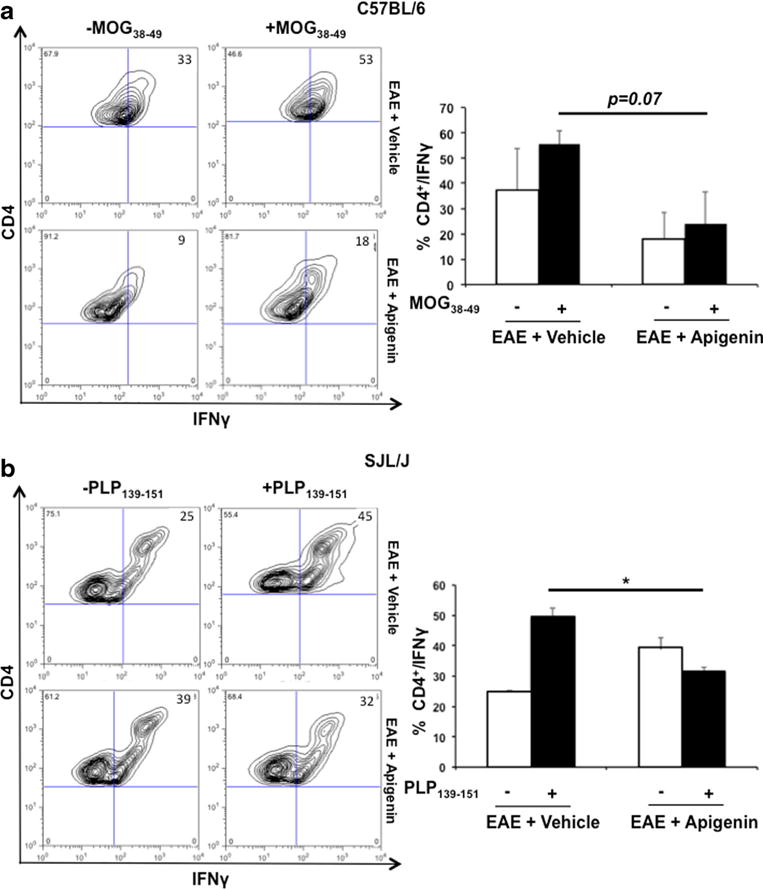
T cell functionality in the presence of Apigenin. Flow cytometry analysis representing CD4+ cells expressing IFN-γ in C57BL/6 mice upon stimulation with MOG38–49 a and SJL/J mice upon stimulation with PLP323–339 b. Splenocytes were isolated from mice in each group of C57BL/6 (n=2) and SJL/J (n=5) mice for quantifying and stimulating procedures. Each bar is representative of the mean percentage for every marker per group. Contour plots representative of one animal per group are shown on the left. *p<0.05
Apigenin Treatment Reduces Immune Cell Infiltration Within CNS of EAE Mice
On evaluation of spinal cord histopathology from untreated C57BL/6 mice irregular myelin staining was observed in both cervical and lumbar sections indicating severe demyelination. Apigenin treatment resulted in significantly less amounts of demyelination indicating a reduction in immune cell infiltration and neuroinflammation in the treated mice (Fig. 6a). H&E staining on spinal cord sections from C57BL/6 EAE mice indicated significantly lesser amount of cellular infiltration when treated with Apigenin indicating its therapeutic potential against inflammatory influx into the CNS. An analysis performed on brain cells isolated from C57BL/6 EAE mice showing a significant reduction in the number of CD45+/CD68+ cells when treated with Apigenin supports the immunohistochemistry findings (Fig. 6b). Similar myelin preservation and decreased cellular infiltration was observed in brain tissue from SJL/J mice upon Apigenin treatment (Supplementary Figure 5).
Fig. 6.
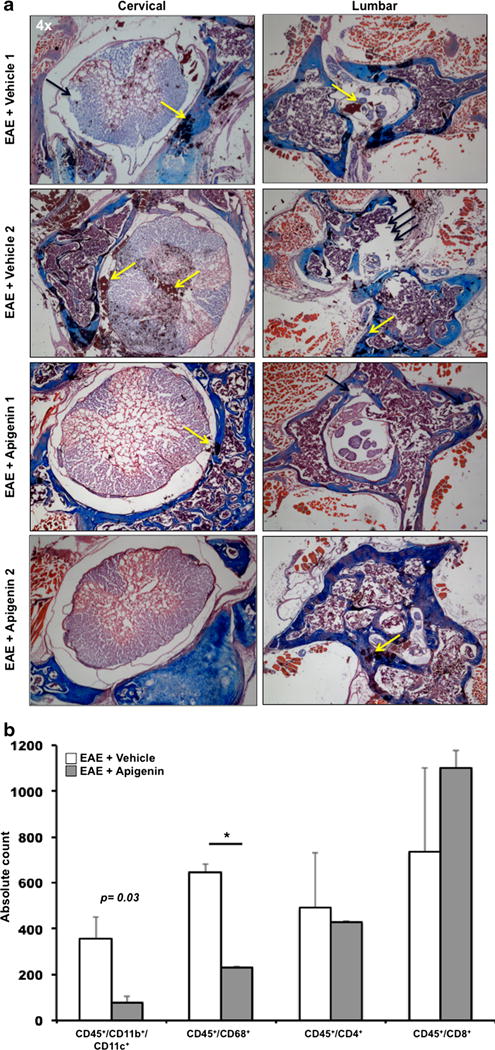
Apigenin treatment reduces immune cell migration into the CNS. Spinal cord tissues from progressive (C57BL/6) mice were sectioned. a Spinal cord tissue from C57BL/6 mice was subjected to LFB and H&E staining depicting areas of myelination (blue) and cellular infiltration (reddish brown). Some of the areas of demyelination have been pointed out by black arrows whereas those showing cellular infiltration have been indicated by yellow arrows. b Cells isolated from the brains of the EAE + vehicle and EAE + Apigenin groups in C57BL/6 mice were quantified and stained for CD45, CD11b, CD11c, CD68, CD4, and CD8 immune cell markers. Plots represent absolute brain cell counts from animals in each group. *p<0.05
Discussion
Natural plant-based flavonoids known for their anti-inflammatory properties have not been explored for use as therapeutic choices for neurodegenerative diseases. The use of Apigenin containing plants for years as a medicinal approach for asthma, Parkinson’s etc. are indicative of the role of the flavonoid in the regulation of inflammation (Jager and Saaby 2011; Patel et al. 2007). Currently, there is an unsubstantial amount of information regarding the effects of Apigenin on immune cells responsible for playing an important role in directing inflammatory responses within the CNS. Amongst them, MS is a neuroinflammatory autoimmune disease of the CNS characterized by a relapsing demyelinating phenomenon with a yet unknown etiology. There is currently no cure for MS and treatment is focused on accelerating recovery from relapses, delaying the progression of disease, and managing pain and symptoms. Natalizumab and dimethyl fumarate (DMF) are two of the drug therapies that have shown potential in controlling MS symptoms and relapse (Ali et al. 2013). The severe side effects from Natalizumab treatment including progressive multifocal leukoencephalopathy (PML) and viral infections have restricted its use to patients with severe MS or those patients who do not respond to other therapies (Oh and O’Connor 2015). Therefore, there remains an urgent need to develop a therapy targeting the inflammatory responses within the CNS during MS. Since, Apigenin is a naturally occurring ubiquitous plant compound it will serve as a non-toxic (Kang et al. 2009) alternative therapy with no severe side effects.
As a result of their vital role in immune surveillance, we wished to explore the effects of Apigenin on DC functionality. DCs are bestowed with the responsibility of regulating the immune response either through their stimulatory or inhibitory effects on various other immune cell populations. Besides their crucial role in peripheral immune responses, DCs are now emerging in the forefront of autoimmune and neuroinflammatory diseases (summarized in (Steinman and Cohn 1973)). These diseases are characterized by the dysregulation of DC function that results from several possible reasons, which include but are not limited to T cell anergy in response to persistent antigens displayed by long-lived lymphoid DCs and functional abnormality of DCs. In the present study, cell surface expression of MHC class II and CD86 molecules was significantly down modulated in mice with EAE upon Apigenin treatment thereby affecting DC capability to induce anti-inflammatory T cell response (Fig. 2). This was in accordance to the findings published earlier wherein a dose-dependent decrease in the expression of CD80, CD86, CD40, MHC class I, and MHC class II molecules on DCs exposed to lipopolysaccharide (LPS) was seen upon Apigenin treatment (Yoon et al. 2006).
Although DCs are the most potent antigen presenting cells contributing towards primary and secondary immune responses, their phenotype and function as they are recruited across the BBB are the least explored of amongst all leukocytes (Steinman and Hemmi 2006). The rise in immune cell infiltration across the BBB seen in neuroinflammation along with uncontrolled activation and antigen presentation are influenced by chemokines. The release of CCL2, CCL3 and CCL5 from cerebral endothelium and astrocytes (Ni et al. 2001) serve as key factors in regulating the trafficking of immature DCs across the BBB in CNS inflammation. In order for rolling and tethering to take place, DCs must be chemoattracted to be captured at the endothelium through the expression of their respective receptors (CCR2/CCR4 for MCP-1, CCR1/CCR5 for MIP-1α and CCR1/CCR3/CCR5 for RANTES). Lectins and integrins are the major group of receptors involved in this cascade. Low-avidity auto-reactive T cells that escape immune surveillance and cause neuroinflammation need to be reactivated in the CNS by APCs. Thus migration of DCs and other immune cells through the BBB must be regulated in order to inhibit the neuroinflammatory responses in MS and other CNS inflammatory diseases. The current disease modifying therapy used by MS patients, Natalizumab (Tysabri, Biogen Idec and Elan Pharmaceuticals), is a humanized IgG4 monoclonal antibody that targets the α4 chain of very late activation antigen-4 (VLA-4) adhesion molecule (α4β1) and α4β7 integrin (Gross et al. 2012; Oh and O’Connor 2015; Raiotach-Regue et al. 2012; Steinman 2012; Yednock et al. 1992). The α4β1 integrins are expressed on all leukocytes except neutrophils, while the α4β7 integrins are expressed on a more restricted set of leukocytes. The blocking of the α4-chain by Natalizumab restricts the ability of such leukocytes to bind to their cognate ligands - vascular cell adhesion molecule-1 (VCAM-1) or mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressed on the endothelial cell layer thereby preventing the transmigration of such leukocytes across the BBB. We observed a decrease in the cell surface expression of the α4-chain on peripheral DCs and CD4+ T cells on Apigenin treatment thus reducing the ability of these cells to cross the BBB (Fig. 3). Additionally, monocyte adherence to vascular endothelium has been shown to be affected through the down-regulation of VCAM-1, intracellular adhesion molecule-1 (ICAM-1), and E-selectin in the presence of Apigenin (Lee et al. 2007). Thus, Apigenin may serve as a chemopreventive measure against neuroinflammation by restricting DC and T cell trafficking to-and-fro between the CNS and periphery. An overall increase in the number of DCs and other immune cells in the periphery was observed upon Apigenin treatment as a possible result of the inability of these cells to cross the BBB (Fig. 1). A concomitant decrease in immune cell infiltration into the CNS and resultant decrease in demyelination was seen upon Apigenin treatment underlining the fact that Apigenin may have an effect on immune cell trafficking during inflammation (Fig. 6).
Further, a study performed in 2004 demonstrated that Apigenin impaired PLP antigen specific T cell proliferation and IFN-γ production from SJL/J mice with EAE (Verbeek et al. 2004). Also, FOXP3+ Treg cells play an important role in restraining Th17 cells and their down-modulation in MS leads to an inability to regulate IL-17 mediated autoimmune neuro-inflammation (Fletcher et al. 2009; Rouse et al. 2013; Singh et al. 2007). Our present study indicated a restoration in the T cell functional response in MS upon Apigenin treatment through an increase in FOXP3+ cells and decrease in IL-17 expression (Fig. 4). Additionally, reduced IFNγ production by CD4+ T cells was also seen upon Apigenin treatment (Fig. 5). This could also be a result of a reduced ability of DCs to uptake antigen in the presence of Apigenin as evidenced by the down-modulation of antigen uptake molecules such as CLEC12A on their surface (Fig. 3).
Finally, a significant decrease in EAE disease severity and relapse was seen upon administration of Apigenin with an accompanying restoration in body weight (Fig. 1). Despite the obvious anti-inflammatory properties of flavonoids, these molecules have not been thoroughly tested as therapeutic agents against neuroinflammatory diseases. In contrast, a study published in 2005 demonstrated that oral flavonoids Apigenin, luteolin, quercetin and hesperitin did not exert any beneficial effects on murine EAE (Verbeek et al. 2005). However, in our present study we observe a significant reduction in disease severity and several anti-inflammatory responses within the treated animals to warrant further study into the mechanism of Apigenin action under neuroinflammatory conditions. Apigenin could possibly function not only as a disease modifying agent but also exert neuroprotective effects. Recent publications have shown that reactive oxygen species and the accompanying oxidative damage are some of the contributing factors to the initial chronic stage of MS (Lassmann and van Horssen 2011). Hence, antioxidant properties of Apigenin (Romanova et al. 2001) might be a promising approach to reduce MS disease progression. Additionally, Apigenin has been shown to have an inhibitory effect on neurodegenerative factors like NO, iNOS and cyclooxygenase-2 (COX-2) (Jeong et al. 2009). Since, these molecules correlate with disease progression independently from ongoing inflammation, a therapeutic modality that targets them would serve a long way towards attenuating progressive disease. Also, the role of Apigenin in recovery from MS in terms of reparatory mechanisms such as restoration of myelin and reduction in neuro-cytotoxicity that results from ongoing inflammation also needs to be elucidated. In comparison to the existing treatment options available for MS, Apigenin could prove to be an extremely safe alternative helping not only patients with the relapse-remitting form but also progressive form of the disease.
Supplementary Material
Acknowledgments
The authors wish to acknowledge US Public Health Service/National Institutes of Health grants: R01CA054559 and R56AI077414 to PJ.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11481-015-9617-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no competing interests.
Contributor Information
Pooja Jain, Email: pjain@drexelmed.edu.
Zafar K. Khan, Email: zkhan@drexelmed.edu.
References
- Ali R, Nicholas RS, Muraro PA. Drugs in development for relapsing multiple sclerosis. Drugs. 2013;73:625–650. doi: 10.1007/s40265-013-0030-6. [DOI] [PubMed] [Google Scholar]
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: critical role for CD5 in experimental autoimmune encephalomyelitis: inhibition of engagement reverses disease in mice. J Immunol. 2004;173:2928–2932. doi: 10.4049/jimmunol.173.5.2928. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- Gross CC, Jonuleit H, Wiendl H. Fulfilling the dream: tolerogenic dendritic cells to treat multiple sclerosis. Eur J Immunol. 2012;42:569–572. doi: 10.1002/eji.201242402. [DOI] [PubMed] [Google Scholar]
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong GS, Lee SH, Jeong SN, Kim YC, Kim EC. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int Immunopharmacol. 2009;9:1374–1380. doi: 10.1016/j.intimp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Kang HK, Ecklund D, Liu M, Datta SK. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009;11:R59. doi: 10.1186/ar2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011;585:3715–3723. doi: 10.1016/j.febslet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res. 2007;30:1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- Lefort EC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. 2013;57:126–144. doi: 10.1002/mnfr.201200424. [DOI] [PubMed] [Google Scholar]
- Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Manuel SL, Rahman S, Wigdahl B, Khan ZK, Jain P. Dendritic cells in autoimmune diseases and neuroinflammatory disorders. Front Biosci. 2007;12:4315–4335. doi: 10.2741/2390. [DOI] [PubMed] [Google Scholar]
- Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Naves R, Singh SP, Cashman KS, Rowse AL, Axtell RC, Steinman L, Mountz JD, Steele C, De Sarno P, Raman C. The interdependent, overlapping, and differential roles of type I and II IFNs in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2013;191:2967–2977. doi: 10.4049/jimmunol.1300419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HT, Spellman SR, Jean WC, Hall WA, Low WC. Immunization with dendritic cells pulsed with tumor extract increases survival of mice bearing intracranial gliomas. J Neurooncol. 2001;51:1–9. doi: 10.1023/a:1006452726391. [DOI] [PubMed] [Google Scholar]
- Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- Nico B, Quondamatteo F, Ribatti D, Bertossi M, Russo G, Herken R, Roncali L. Ultrastructural localization of lectin binding sites in the developing brain microvasculature. Anat Embryol (Berl) 1998;197:305–315. doi: 10.1007/s004290050140. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Oh J, O’Connor PW. Established disease-modifying treatments in relapsing-remitting multiple sclerosis. Curr Opin Neurol. 2015;28:220–229. doi: 10.1097/WCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- Plattner VE, Germann B, Neuhaus W, Noe CR, Gabor F, Wirth M. Characterization of two blood-brain barrier mimicking cell lines: distribution of lectin-binding sites and perspectives for drug delivery. Int J Pharm. 2010;387:34–41. doi: 10.1016/j.ijpharm.2009.11.030. [DOI] [PubMed] [Google Scholar]
- Raiotach-Regue D, Grau-Lopez L, Naranjo-Gomez M, Ramo-Tello C, Pujol-Borrell R, Martinez-Caceres E, Borras FE. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol. 2012;42:771–782. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- Renno T, Lin JY, Piccirillo C, Antel J, Owens T. Cytokine production by cells in cerebrospinal fluid during experimental allergic encephalomyelitis in SJL/J mice. J Neuroimmunol. 1994;49:1–7. doi: 10.1016/0165-5728(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Romanova D, Vachalkova A, Cipak L, Ovesna Z, Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48:104–107. [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol. 2013;169:1305–1321. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2012a;7:74–94. doi: 10.1007/s11481-011-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation. 2012b;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. The discovery of natalizumab, a potent therapeutic for multiple sclerosis. J Cell Biol. 2012;199:413–416. doi: 10.1083/jcb.201207175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- Swanborg RH. Experimental autoimmune encephalomyelitis in the rat: lessons in T-cell immunology and autoreactivity. Immunol Rev. 2001;184:129–135. doi: 10.1034/j.1600-065x.2001.1840112.x. [DOI] [PubMed] [Google Scholar]
- Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Verbeek R, van Tol EA, van Noort JM. Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem Pharmacol. 2005;70:220–228. doi: 10.1016/j.bcp.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Wu GF, Laufer TM. The role of dendritic cells in multiple sclerosis. Curr Neurol Neurosci Rep. 2007;7:245–252. doi: 10.1007/s11910-007-0037-z. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yoon MS, Lee JS, Choi BM, Jeong YI, Lee CM, Park JH, Moon Y, Sung SC, Lee SK, Chang YH, Chung HY, Park YM. Apigenin inhibits immunostimulatory function of dendritic cells: implication of immunotherapeutic adjuvant. Mol Pharmacol. 2006;70:1033–1044. doi: 10.1124/mol.106.024547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


