Abstract
Background
Hepatocyte growth factor (HGF) is a pleotropic factor posited to have metabolic homeostatic properties. The purpose of this study is to examine whether level of HGF is associated with the development of type 2 diabetes.
Methods
Data from the Multi-Ethnic Study of Atherosclerosis (MESA) were used to examine the prospective association between serum level of HGF and incident diabetes. Fasting HGF was measured at Exam 1 (2000–2002) in 5395 participants free from diabetes (61.5 ± 10.2 years old) and incidence of diabetes was determined at four subsequent follow-up exams over 12 years. Hazard ratios (HR) for incident diabetes were estimated according to 1 standard deviation (SD) unit increment of HGF (1 SD =26 μg/l), before and after adjustment for age, sex, race/ethnicity, education, study center, smoking status, alcohol consumption, body mass index, waist circumference, fasting glucose and insulin, C-reactive protein, and interleukin-6 levels.
Results
A 1 SD increment of baseline HGF was associated with a 46% (95% CI =1.37, 1.56) increased risk of diabetes before adjustment. After adjustment, diabetes risk per 1 SD increment of HGF was attenuated but remained significantly increased (HR=1.21; 95% CI=1.12, 1.32). Men had a significantly greater HR compared to women per equivalent increase of HGF (p-value for sex interaction=0.04). There was no evidence of effect modification by race/ethnicity.
Conclusions
This study advances understanding from cross-sectional studies and investigation of incident insulin resistance, demonstrating higher level of HGF is associated with incident diabetes and may reflect a unique type of impaired metabolism.
Keywords: Hepatocyte growth factor, Incidence, Diabetes mellitus, Longitudinal, Ethnicity
1. Introduction
Hepatocyte growth factor (HGF) is a pleotropic factor synthesized and secreted by mesenchymal cells, involved in cell motility, growth, and morphogenesis of manifold cell types [1]. Levels of HGF differ slightly by sex and age and are altered during gestation, however compared to healthy individuals significant differences in HGF levels have been found in individuals with various disease states and by disease stage [2]. Level of circulating HGF is predictive of mortality in cancer and cardiovascular patients [3, 4]. Increased levels of HGF are also associated with incident cardiovascular events [5].
Animal and in vitro models suggest HGF may have a metabolic homeostatic role with functional properties similar to insulin for glucose uptake and utilization [6, 7]. In contrast, epidemiologic studies show HGF levels are increased in conditions of unfavorable metabolic health [8–11]. Specifically, levels of HGF are significantly increased in type 1 diabetes and positively associated with the prevalence of insulin resistance, type 2 diabetes, and metabolic syndrome [8–10, 12]. Levels of HGF are increased in obesity, with a strong positive association found between HGF and the spectrum of body mass index (BMI) and levels of HGF are significantly reduced following weight loss [11, 13, 14].
A recent study found elevated levels of HGF were associated with the development of insulin resistance [15]. Whether elevated levels of HGF or changes in HGF over time are associated with the development of type 2 diabetes is unknown. The objective of this study was two-fold: to assess the association between serum level of HGF and incident type 2 diabetes and identify any race/ethnicity heterogeneity; and to assess the association between change in HGF from Exam 1 to Exam 2 and incidence of type 2 diabetes in a sub-sample that had HGF measured at both Exam 1 and Exam 2.
2. Materials and methods
2.1. Study population
The general study design and objectives of the Multi-Ethnic Study of Atherosclerosis (MESA) have been described previously [16]. Briefly, it is a prospective cohort study of U.S. adult men and women who were free of clinical cardiovascular disease at the time of the initial exam. From July 2000 to August 2002, 6814 participants aged 45–84 were recruited and examined at six field centers located in Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and Saint Paul, Minnesota. Participants were from four racial ethnic groups including non-Hispanic white (38%), African American (28%), Hispanic (22%), and Chinese American (12%). All participants gave written informed consent at the baseline examination (Exam 1) and MESA study protocol and ancillary studies were approved by the Institutional Review Board at each site. Participants were contacted by telephone annually and invited to participate in 4 follow-up in-person clinic examinations, each roughly 2 years apart.
2.2. Measurement of HGF
Venous blood was obtained from fasting participants and serum separation was performed within 30 minutes of phlebotomy with aliquots subsequently stored at −70°C. Levels of serum HGF protein were measured with a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using the Human HGF Immunoassay kit (R&D Systems, Minneapolis, MN) on 6769 individuals at Exam 1 and on a sub-sample of 2403 individuals at Exam 2 as part of the Multi-scale Biology of Atherosclerosis in the Cellular Adhesion Pathway (HL98077, MESA Adhesion Ancillary Study). The method was validated by R&D Systems as specified in the package insert; validation was verified by the University of Minnesota laboratory. The lower limit of detection was 4 μg/l and the inter-assay laboratory coefficient of variations (CVs) for the HGF method were 12.0%, 8.0%, and 7.4% at respective mean concentrations of 69, 204, and 408 μg/l for lyophilized manufacturer’s controls; and 10.4% at a mean concentration of 69 μg/l for an in-house pooled serum control.
2.3. Determination of Diabetes
All participants with diabetes at Exam 1, determined by use of insulin or oral hypoglycemic medication or fasting glucose ≥ 7.0 mmol/L, were excluded from these analyses. Incident type 2 diabetes was ascertained over 4 follow-up examinations (from 2000–2011) defined by new use of insulin or oral hypoglycemic medication or fasting glucose ≥ 7.0 mmol/L at any exam. Serum glucose was measured by the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics).
2.4. Ascertainment of Other Characteristics
Self-administered and interviewer-administered questionnaires were used to collect information on demographics, education, current tobacco use and regular alcohol consumption, physical activity, and medication use was assessed using a validated medication inventory [17]. Intentional exercise was quantified as the sum of walking for exercise, sports/dancing, and conditioning MET hours/week [16]. Body-mass index (BMI: weight in kilograms divided by the square of height in meters) was calculated from weight measured to the nearest 0.45 kilogram and height measured to the nearest centimeter. Waist circumference (WC) was measured at the umbilicus (to the nearest 0.1 cm) using a steel measuring tape of a standard 4-oz tension. Resting seated blood pressure was measured three times using a Dinamap automated oscillometric sphygmomanometer (model Pro 100, Critikon, Tampa, Fl) with the average of the last two measurements used for this analysis. Total cholesterol and high-density lipoprotein cholesterol (HDL cholesterol) were measured using a cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN); HDL-cholesterol was measured after precipitation of non-HDL cholesterol with magnesium/dextran. Triglyceride concentrations were measured using Triglyceride Glycerol Blanked reagent (Roche Diagnostics). Low-density lipoprotein (LDL) cholesterol was calculated with the Friedewald equation [18]. Insulin was determined by a chemiluminescent immunoenzymatic sandwich assay using Access® Ultrasensitive Insulin Reagent Packs on the Access® Immunoassay system (Beckman Instruments, Inc.). High sensitivity C-reactive protein (CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring) and interleukin-6 (IL-6) was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems).
2.5. Statistical Analysis
When evaluating the association between Exam 1 HGF and incident diabetes, individuals were excluded from analysis if they had prevalent diabetes (n=859) or unknown diabetes status at Exam 1 (n=6), were missing covariate information (n=218), presented with fasting time <8 hours (n=3), or did not return for any follow-up exams (n=333), resulting in an analytic sample of 5395. A total of 2393 individuals had repeated measures of HGF. When evaluating the association between change in HGF from Exam 1 to Exam 2 and incident diabetes, we excluded those with prevalent diabetes at exam 2 (n=387), presenting with fasting time <8 hours at exam 2 (n=2), missing covariate information at exam 2 (n=10), or who did not return for any follow-up exams (n=79), leaving an analytic sample of 1915. Summary characteristics were calculated according to quintile of HGF. Categorical characteristics included race/ethnicity, study site, smoking status (current use), alcohol consumption (zero; any up to 1 drink daily; and greater than 1 drink daily), and educational attainment (less than high school degree; some college; college degree). All other characteristics were quantitative in nature and subsequently modeled continuously. Cox proportional hazards were used to estimate risk of incident diabetes (hazard ratios (HR) and 95% confidence limits) over the course of follow-up with each individual’s contributed time to the analysis concluding with the date of exam at which diabetes was ascertained or administrative censoring on the date of their last exam. For analyses of HGF at Exam 1, diabetes HRs were calculated by HGF quintile and per 1 standard deviation unit increment, before and after adjustment for age, sex, race/ethnicity, study site, smoking status, alcohol consumption, educational attainment, regular physical activity, body composition (BMI and WC), fasting glucose and insulin, and levels of IL-6 and CRP (restricted cubic splines, centering the spline at the median HGF value 88 μg/l, were used to describe the association). Given the strength of association between BMI and diabetes and to reduce residual confounding, all adjusted models containing BMI also included a BMI2 term to account for a possible nonlinear relationship. For analyses of change in HGF, HRs for diabetes were estimated according to continuous (HGFExam 2 − HGFExam 1) and percent change ((HGFExam 2 − HGFExam 1)/HGF Exam 1*100) in HGF between Exam 1 and Exam 2. We assumed age, sex, race/ethnicity, study site, smoking status, alcohol consumption, educational attainment, and regular physical activity were not on the pathway between change in HGF level from Exam 1 to Exam 2 and incident diabetes. However, the influence of HGF on subsequent BMI, WC, fasting glucose and insulin, and levels of IL-6 and CRP is less clear, and adjustment for Exam 2 values of these covariates may induce bias by conditioning on an intermediary [19]. Therefore, we conducted separate adjusted analyses using the data for these covariates at Exam 1 and compared them to analyses using the updated data for these covariates at Exam 2 (insulin, IL-6, and CRP were not available at Exam 2 on the entire sub-sample and left out of the fully adjusted model using updated data). A variable for the squared level of HGF was created for the assessment of quadratic relationship between HGF and risk of diabetes incidence. We tested the sensitivity of our results to measures of insulin resistance and unfavorable liver health (i.e. measures of HOMA-IR, liver attenuation, and gamma-glutamyl transferase (GGT)). To investigate if risk of diabetes according to level of HGF differed by sex or race/ethnicity, separate multiplicative interactions were tested by adding product terms to the proportional hazards model. Poisson regression was used to estimate incidence rates and assess potential effect modification on the additive scale. The proportional hazards assumption was assessed by inclusion of a product term between HGF level and natural log of contributed person-time. Cochran-Mantel-Haenszel Chi-square was used to test for linear trend of incident cases across HGF category. Tests of linear trend of HRs across categories of HGF were performed by assigning participants the median of their HGF category and entering this new variable into a separate Cox proportional-hazards regression model. Similarly this median HGF value was entered into separate linear regression models to test for linear trend of follow-up time and incidence rates across HGF category. Improvement-of-fit tests were performed using a likelihood-ratio Chi-squate test by subtracting the likelihood ratio value from the fully adjusted model with HGF included as a covariate from the likelihood ratio value from the fully adjusted model without HGF. Lastly, to fully evaluate diabetes status by ADA criteria, data on percent glycated hemoglobin (HbA1c) and oral glucose tolerance testing (OGTT) should be incorporated. In this study we used data only on fasting glucose and diabetes medications to identify diabetes cases. To address potential misclassification of diabetes cases at baseline and over follow-up, we conducted several sensitivity analyses incorporating data on percentage HbA1c measured at Exam 2. To address the potential of misclassification of baseline diabetes, we conducted sensitivity analyses in the subset of individuals with data on HGF at Exam 2, assessing the association of level of HGF and incident diabetes, with follow-up commencing after Exam 2, comparing the results before and after individuals with HbA1c ≥ 6.5% were excluded. To address potential misclassification of incident cases, we quantified additional cases of incident diabetes withHbA1c ≥ 6.5% at Exam 2 who were previously undetected with fasting glucose and medication use data. A combination of SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC) and STATA 13.0 software (College Station, Texas) was used for statistical analysis.
3. Results
3.1. Characteristics of the study population
Levels of HGF were approximately normally distributed at Exams 1 and 2 around mean values of 92 μg/l (standard deviation (SD)=26 μg/l) and 98 μg/l (SD=25 μg/l), respectively. Of the 1915 individuals included in the evaluation of change in HGF, change in HGF between Exam 1 and Exam 2 was approximately normally distributed around 7 μg/l (SD=19 μg/l with range −82 to 89 μg/l). Change in HGF between Exam 1 and Exam 2 was greatest in those with lower HGF at Exam 1. Participant characteristics at Exam 1 are presented in Table 1 according to quintile of HGF. At Exam 1, older age, female sex, current smoking and pack-years smoking, and higher BMI, WC, fasting glucose and insulin, CRP, and IL-6 levels were all associated with higher levels of HGF, while greater education and physical activity were associated with lower levels of HGF. Those included had lower mean levels of HGF, were younger, more likely to be female, had more formal education, were less likely to smoke, had lower mean BMI and WC, were less likely to be taking blood pressure or cholesterol lowering medications, and reported being more physically active at Exam 1 than those excluded.
Table 1.
Participant characteristics according to quintile of hepatocyte growth factor at Exam 1, The Multi-Ethnic Study of Atherosclerosis
| Quintile of Hepatocyte Growth Factor (HGF pg/mL) | |||||
|---|---|---|---|---|---|
| Quintile 1 (n=1079) | Quintile 2 (n=1079) | Quintile 3 (n=1079) | Quintile 4 (n=1079) | Quintile 5 (n=1079) | |
|
| |||||
| HGF (μg/L) | 61 ± 8 | 77 ± 4 | 89 ± 3 | 101 ± 5 | 130 ± 21 |
| Range | 29 – 71 | 71 – 83 | 83 – 95 | 95 – 111 | 111 – 275 |
| Age (years) | 58 ± 9 | 60 ± 10 | 62 ± 10 | 63 ± 10 | 66 ± 11 |
| Female (%) | 49 | 52 | 54 | 56 | 57 |
| Race (%) | |||||
| Non-Hispanic White | 49 | 43 | 40 | 41 | 41 |
| Chinese | 18 | 16 | 13 | 8 | 5 |
| African-American | 23 | 27 | 26 | 27 | 23 |
| Hispanic | 10 | 15 | 22 | 25 | 32 |
| Education (%) | |||||
| < High school (HS) | 9 | 12 | 17 | 19 | 21 |
| HS, some college | 41 | 44 | 45 | 48 | 53 |
| ≥ College degree | 50 | 44 | 38 | 32 | 25 |
| Smoking (%) | |||||
| Current use | 9 | 10 | 12 | 15 | 18 |
| Pack-years smoking | 9 ± 20 | 8 ± 16 | 10 ± 18 | 13 ± 24 | 14 ± 22 |
| Alcohol Use (%) | |||||
| Never | 18 | 20 | 19 | 20 | 19 |
| Former | 19 | 20 | 21 | 23 | 26 |
| Current; ≤ 1 drink daily | 51 | 49 | 50 | 46 | 47 |
| Current; > 1 drink daily | 12 | 11 | 9 | 11 | 8 |
| Physical activity (Mets) | 6300 ± 6212 | 6281 ± 5897 | 5763 ± 5709 | 5922 ± 6660 | 5168 ± 4960 |
| Systolic BP (mmHG) | 120 ± 19 | 123 ± 20 | 126 ± 21 | 128 ± 22 | 129 ± 21 |
| Diastolic BP (mmHG) | 72 ± 10 | 72 ± 10 | 72 ± 10 | 72 ± 10 | 71 ± 10 |
| BP-Lowering Medications (%) | 21 | 24 | 28 | 31 | 40 |
| BMI (kg/m2) | 26 ± 5 | 27 ± 5 | 28 ± 5 | 29 ± 5 | 30 ± 6 |
| Waist Circumference (cm) | 92 ± 13 | 95 ± 13 | 97 ± 14 | 99 ± 14 | 102 ± 14 |
| Fasting Glucose (mmol/L) | 4.8 ± 0.5 | 4.9 ± 0.6 | 5.0 ± 0.6 | 5.0 ± 0.6 | 5.1 ± 0.7 |
| Fasting Insulin (pmol/L) | 55.6 ± 27.8 | 62.5 ± 34.7 | 62.5 ± 34.7 | 69.5 ± 34.7 | 83.3 ± 48.6 |
| Total Cholesterol (mmol/L) | 5.0 ± 0.9 | 5.1 ± 0.9 | 5.1 ± 0.9 | 5.1 ± 0.9 | 5.0 ± 0.9 |
| LDL (mmol/L) | 3.0 ± 0.8 | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.0 ± 0.8 |
| HDL (mmol/L) | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 |
| Triglycerides (mmol/L) | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.5 ± 0.9 | 1.5 ± 0.8 | 1.5 ± 0.9 |
| Lipid-Lowering Medications (%) | 11 | 14 | 15 | 16 | 18 |
| C-Reactive Protein (nmol/L ) | 21.0 ± 30.5 | 26.7 ± 40.0 | 31.4 ± 40.0 | 37.1 ± 45.7 | 46.7 ± 61.0 |
| IL-6 (IU/mL) | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 |
Continuous variables are means ± standard deviations
Those excluded had higher levels of HGF, were older, more likely to be male, had less formal education, were more likely to smoke, had higher BMI and WC, were more likely to be taking blood pressure or cholesterol lowering medications, and reported being less physically active at Exam 1 than those included.
3.2. Exam 1 HGF and incident diabetes
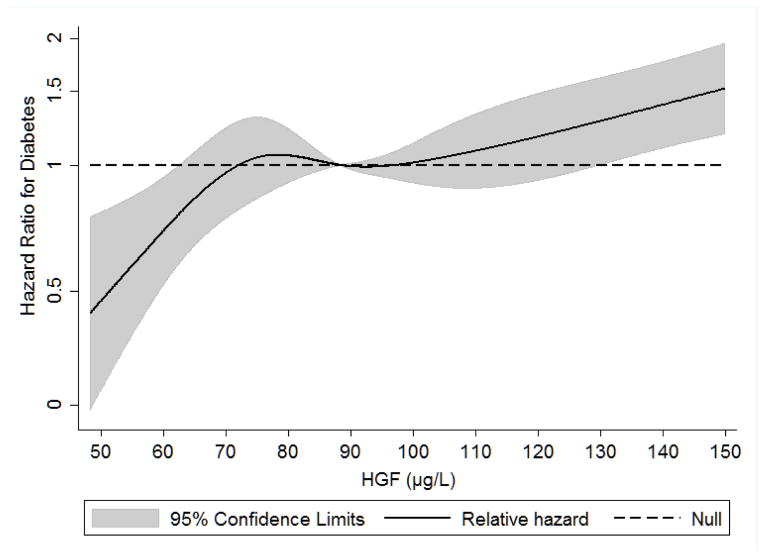
From 2000 to 2011, there were 670 incident cases of diabetes in this analytic sample of 5395 individuals (12%). The average follow-up time was 7.6 years. HRs with 95% confidence intervals from the unadjusted and adjusted analyses are presented in Table 2. No violations to the proportional hazards assumption were detected. The test for a quadratic association between Exam 1 level of HGF and incident diabetes was not statistically significant (p=0.16). Figure 1 shows the restricted cubic spline of continuous HGF and adjusted hazard for diabetes. Level of HGF at Exam 1 was positively and linearly associated with incidence of diabetes over follow-up (P for linear HGF < 0.0001). Specifically, a 1 SD unit increment in Exam 1 HGF level was associated with a 1.46 fold higher risk for diabetes (95% CI=1.37, 1.56), before adjustment (Table 2). The strongest attenuation of HR was observed with the adjustment for metabolic health characteristics (Model 3 in Table 2), primarily by BMI and fasting glucose. After adjustment, the risk for diabetes was attenuated but remained significantly increased (per 1 SD unit increment: HR=1.21; 95% CI=1.12, 1.32). Improvement-of-fit statistics were significantly lower for the adjusted model with HGF compared to a similarly adjusted model without the inclusion of HGF (−2 Log Likelihood with HGF=9619.97; −2 Log Likelihood without HGF=9639.22; LR Chi-square, 1 DF = 19.25, p < 0.001). A positive monotonic association between quintile of HGF and risk for diabetes was observed in unadjusted analysis (HR range 1.62 – 3.36). After adjustment for BMI and fasting glucose all HRs were attenuated (remaining significantly increased) and the monotonic association was no longer present; adjusted associations for HGF in were similar in quintiles 2–4 (HR=1.26–1.38) and highest in quintile 5 (HR=1.67). A formal test of interaction was not significant for race/ethnicity (p=0.23); however there was evidence for effect modification by sex (p=0.04). In sex stratified analyses, males had a greater increase in risk for diabetes per 1 SD unit increment in Exam 1 level of HGF compared to the same 1 SD unit increment in women (HRmales=1.31, HRfemales=1.14).
Table 2.
Hazard ratios (95% confidence intervals) for incident type 2 diabetes according to quintile of hepatocyte growth factor (μg/L) at Exam 1, and stratified by sex and race/ethnicity, The Multi-Ethnic Study of Atherosclerosis (2000–2002 to 2010–2011)
| Quintile of Hepatocyte Growth Factor (HGF μg/L) | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (n=1079) | Quintile 2 (n=1079) | Quintile 3 (n=1079) | Quintile 4 (n=1079) | Quintile 5 (n=1079) | p-for trend | |
| Incident cases (% total) | 82 (12%) | 120 (18%) | 136 (20%) | 129 (19%) | 203 (30%) | <0.001 |
| Years follow-up (average) | 9005 (8.3) | 8477 (7.9) | 8317 (7.7) | 7936 (7.4) | 7366 (6.8) | <0.001 |
| Incidence Rate | 911 | 1416 | 1635 | 1625 | 2756 | <0.001 |
| Unadjusted hazard model | 1.00 | 1.62 (1.23, 2.15) | 1.96 (1.49, 2.58) | 1.94 (1.47, 2.56) | 3.36 (2.59, 4.34) | <0.001 |
| Final adjusted model | 1.00 | 1.32 (0.99, 1.76) | 1.38 (1.03, 1.83) | 1.26 (0.94, 1.69) | 1.67 (1.25, 2.24) | <0.005 |
|
| ||||||
| Sex-stratified | n cases/N total | Adjusted HR per 1 SDa HGF | P for interaction | Hazard Ratios (95% CL) per 1 SD HGFa | ||
| Females | 349/2884 | 1.14 (1.02, 1.27) | 0.04 | Unadjusted | 1.46 (1.37, 1.56) | |
| Males | 321/2511 | 1.31 (1.15, 1.48) | Model 1 | 1.45 (1.36, 1.55) | ||
| Model 2 | 1.46 (1.36, 1.56) | |||||
| Race/ethnicity | Model 3 | 1.23 (1.14, 1.33) | ||||
| Non-Hispanic White | 202/2296 | 1.28 (1.10, 1.50) | P for interaction | Model 4 | 1.21 (1.12, 1.31) | |
| Chinese | 82/631 | 1.20 (0.90, 1.59) | 0.23 | |||
| African-American | 206/1357 | 1.16 (0.98, 1.37) | ||||
| Hispanic | 180/1111 | 1.19 (1.03, 1.36) | ||||
Incidence rate: per 100,000 person years
Final adjusted model: age, sex, race/ethnicity, study site, smoking status, alcohol consumption, educational attainment, regular physical activity, body mass index, waist circumference, fasting glucose and insulin, and levels of IL-6 and CRP
For HGF: 1 standard deviation (SD) = 26 μg/L
Model 1: Adjustment for age, sex, race/ethnicity, study site, and education
Model 2: Adjustment for M1 and smoking status, alcohol consumption, and regular physical activity
Model 3: Adjustment for M2 and body mass index, BMI2, waist circumference, and fasting glucose and insulin
Model 4: Adjustment for M3 and levels of IL-6 and CRP
Figure 1.
Restricted cubic spline of the association between hepatocyte growth factor (HGF μg/L) and incident diabetes, The Multi-Ethnic Study of Atherosclerosis (2000–2002 to 2010–2011)
The hazard ratio is per each absolute increase of 1 μg/L in the level of hepatocyte growth factor at MESA Exam 1 (P for quadratic HGF = 0.16; P for linear HGF < 0.0001). The shaded area is the 95% confidence interval from the restricted-cubic-spline model. The model is centered at the median (88 μg/L) and the plot was truncated at the 1.5th and 98.5th percentiles of hepatocyte growth factor (48 and 150 μg/L, respectively). The hazard ratio was adjusted for age, sex, race/ethnicity, education, study site, body mass index, BMI2, and waist circumference, smoking, alcohol, physical activity, fasting glucose and insulin, and levels of interleukin-6 and C-reactive protein.
3.3. Change in HGF and incidence of diabetes in sub-sample
In the sub-sample of individuals with measurements of HGF at both Exam 1 and 2, there were 200 incident cases of diabetes from 2002–2011 (excluding cases occurring between Exam 1 and Exam 2). Basic descriptive characteristics and results from change in HGF between Exam 1 and Exam 2 (continuous change; percent change not presented in tabular form) and incident diabetes analyses are presented in Table 3. There was no association between change in HGF and incidence of diabetes before adjustment; however after accounting for Exam 1 level of HGF, increase in HGF between exams was associated with increased risk for diabetes. This association remained significantly increased after adjustment for age, sex, race/ethnicity, study site, smoking status, alcohol consumption, educational attainment, and regular physical activity (per 1 SD unit increment: HR=1.31; 95% CI=1.13, 1.53). In the model using updated Exam 2 data, adjustment for BMI, WC, and fasting glucose resulted in attenuation of the HR, no longer significantly increased (per 1 SD unit increment: HR=1.12; 95% CI=0.95, 1.32). In the adjusted model using Exam 1 data for these covariates in addition to Exam 1 data for insulin, CRP, and IL-6, a 1 SD unit increment for change in HGF was associated with a 21% increased risk for developing diabetes (95% CI= 1.03, 1.43). A comparison of the adjusted models is presented in Table 3. When change in HGF was modeled as percent change (not shown), change in HGF was associated for increased risk for diabetes after adjustment for Exam 1 level of HGF, age, sex, race/ethnicity, study site, smoking status, alcohol consumption, educational attainment, and regular physical activity. After further adjustment for BMI, WC, fasting glucose and insulin, and IL-6 and CRP, the HRs were attenuated and no longer significantly increased. There was no evidence of effect modification by race/ethnicity or sex in any analyses for the association between change in HGF and incidence of diabetes. There was no evidence for effect modification on the additive scale for any associations. Likewise, we did not find evidence for violation of the proportional hazards assumption for any of the analyses.
Table 3.
Change in level of hepatocyte growth factor between Exam 1 and Exam 2 and hazard ratios (95% confidence intervals) for incident type 2 diabetes according to change in HGF, The Multi-Ethnic Study of Atherosclerosis (2000–2002 to 2010–2011)
| Quintile of Hepatocyte Growth Factor Exam 1 | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (n=383) | Quintile 2 (n=383) | Quintile 3 (n=383) | Quintile 4 (n=383) | Quintile 5 (n=383) | p-for trend | |
| Mean change in HGF(μg/L) Exam 1 to Exam 2 (95% Confidence Limits) | 17 (16, 19) | 12 (10, 14) | 7 (6, 9) | 3 (0.8, 4) | −6 (−8, −3) | <0.001 |
|
| ||||||
| Quintile of Change in Hepatocyte Growth Factor from Exam 1 to Exam 2 (HGF μg/L) | ||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
|
|
||||||
| Cases/Person years follow-up | 39/2384 | 42/2529 | 35/2554 | 43/2420 | 41/2556 | |
| Incidence Rate/100,000 person years | 1636 | 1661 | 1370 | 1777 | 1604 | NS |
|
| ||||||
| Hazard Ratios (95% Confidence Limits) per 1 SD increment in Change in Hepatocyte Growth Factor from Exam 1 to Exam 2 | ||||||
| Unadjusted hazard model | 1.02 (0.89, 1.18) | |||||
| Model 1 | 1.22 (1.06, 1.41) | |||||
| Model 2 | 1.28 (1.10, 1.49) | |||||
| Model 3 | 1.31 (1.13, 1.53) | Using Exam 1 clinical values* | ||||
| Model 4 | 1.12 (0.95, 1.32) | 1.21 (1.03, 1.45) | ||||
| Model 5 | NE | 1.21 (1.03, 1.43) | ||||
1 SD increment change in HGF = 19 μg/L
Change in HGF is presented as continuous (Exam 2 − Exam 1); percent change results similar and not shown
Model 1: Adjustment for HGF exam 1 value
Model 2: Adjustment for M1, and Exam 2 age, sex, race/ethnicity, study site, and education
Model 3: Adjustment for M2, and Exam 2 smoking status, alcohol consumption, and regular physical activity
Model 4: Adjustment for M3, and Exam 2 body mass index, waist circumference, and fasting glucose and insulin (unless noted*)
Model 5: Adjustment for M4, and Exam 2 levels of IL-6 and CRP (unless noted*)
Clinical values: body mass index, BMI2, waist circumference, fasting glucose and insulin and levels of IL-6 and CRP
NS: Not significant
NE: Not estimable due to large number for which IL-6 and CRP unmeasured at Exam 2
3.4. Incorporating data on HbA1c to identify diabetes case misclassification
In the subset of individuals with HGF measured at Exam 2, 30 individuals with HbA1c ≥ 6.5% at Exam 2 were identified who did not have prevalent diabetes detected based on fasting glucose and medication data. We did not observe any meaningful difference in the strength of association between level of HGF at Exam 2 and incident diabetes identified at Exam 3 through Exam 5 in crude or adjusted models when excluding these 30 individuals from prospective analaysis (Adjusted model including those with HbA1c ≥ 6.5%: HR=1.28 (1.11, 1.49); adjusted model excluding those with HbA1c ≥ 6.5%: HR=1.28 (1.10, 1.50)).
In analyses of HGF measured at Exam 1, we identified 84 additional cases of incident diabetes with HbA1c ≥ 6.5% at Exam 2 who were previously undetected with fasting glucose and medication use data. The total (detected and previously undetected) number of cases of diabetes in this sample was 1015, resulting in underreporting of 8% of cases. There was no discernable association between the proportion of cases missed and quintile of HGF at Exam 1 (results not shown).
4. Discussion
In this ethnically diverse population of U.S. adult men and women, higher level of circulating HGF was significantly associated with the development of type 2 diabetes over an average of 7.6 years of follow-up. By contrast, 2-year increase in HGF level was marginally associated with increased incidence of diabetes over follow-up. The association of HGF and incident diabetes was modified by sex, with an equivalent increase in level of HGF having a larger increase in hazard for diabetes in men compared to women. We did not identify any racial/ethnic difference in risk for diabetes according to level of HGF, despite the substantial disparities in the burden of diabetes among the various racial/ethnic groups in the US [20, 21].
While this is the first study to investigate the prospective effect of HGF levels on risk for diabetes, our results support and extend previous studies evaluating HGF and general metabolic health [8–11, 15]. Level of HGF was positively associated with levels of glucose and BMI at Exam 1 in our cohort, in agreement with previous cross-sectional studies reporting increased prevalence of diabetes and metabolic syndrome with higher levels of HGF and higher levels of HGF among individuals with obesity and type 1 diabetes [8–11]. A recent study of Japanese adults reported elevated levels of HGF were associated with increased incidence of insulin resistance over 10 years of follow-up [15]. Higher level of HGF was associated with higher prevalence of insulin resistnace in two large cohorts of elderly individuals without diabetes; however instrumental variable analysis using genetic markers did not find evidence to support a causal association [12]. Researchers were unable to account for physical activity level in their analyses and these Japanese individuals had lower BMI compared to MESA participants. However given the development of insulin resistance is generally a precursor to type 2 diabetes these results are largely complementary.
Work by Fafalios et al. may help explain the mechanism underlying HGF and metabolic dysfunction [7]. In vitro study of human hepatocytes found treatment with HGF stimulated the formation of an HGF receptor (Met) and insulin receptor (ISNR) complex [7]. In mouse models, reduced Met protein abundance was associated with a simultaneous reduction in glucose clearance while administration of HGF to mice genetically predisposed to hyperglycemia suppressed blood glucose levels compared to levels of those administered saline and treatment with HGF in combination with insulin did not induce overt hypoglycemia as did treatment with insulin alone [7]. Taken together, researchers concluded a potential metabolic homeostatic role of the HGF with therapeutic potential in type 2 diabetes [7]. As HGF has similar properties to that of insulin, it may be possible that the association we are seeing is due to “HGF resistance”, akin to insulin resistance, resulting in impaired glucose uptake to particular tissues [9, 15]. The addition of HGF significantly improved the fit of the model given our data compared to a model without HGF, suggesting HGF to have informative value in estimating the development of diabetes beyond traditional diabetes risk factors. However, it may be that the association we are observing is due to insulin resistance absent of diabetes or the result of poor metabolic health, higher levels of HGF induced by less favorable levels of diabetes risk factors. Adjustment at baseline for HOMA-IR or the restriction of the analytic sample to individuals with HOMA-IR values below established isulin resistance thresholds did not substantively alter the results. We observed modest attenuation of the HRs when adjusting for measures of metabolic health, but we included a comprehensive list of diabetes risk factors reducing the influence of residual confounding. Additionally, our estimates were robust to models including measures of liver attenuation and enzymes of liver dysfunction (GGT); signifying unfavorable liver health did not explain our results.
An advantage of the MESA study is the ability to make comparisons across racial/ethnic groups. At Exam 1 we observed differences in mean levels of HGF according to race/ethnicity; compared to non-Hispanic white, African Americans had similar levels of HGF while individuals with Chinese ancestry had significantly lower levels and Hispanics had higher levels of HGF. However, there was no evidence for differences in the association between HGF and incidence of diabetes by race/ethnicity group. Although statistically significant, the magnitude of effect modification by sex was small and it is unclear whether it is of clinical or biological significance.
We did not find evidence that a change in HGF level, over roughly 2 years, was associated with incidence in diabetes when evaluating the association using Exam 2 data for BMI, WC, fasting glucose and insulin, and levels of IL-6 and CRP. However, the placement of these values in relation to the mechanistic pathway between change in HGF over time and incidence of diabetes is unclear. If change in HGF from Exam 1 to Exam 2 affects BMI, WC, fasting glucose and insulin, and levels of IL-6 and CRP at Exam 2, adjustment for these characteristics using Exam 2 data may not be prudent [19]. When we adjusted for these characteristics using data specific to a time preceding change in HGF (Exam 1), we observed greater change in HGF between Exam 1 and Exam 2 was significantly associated with the development of type 2 diabetes. Given the discrepancy between these two results, these findings should be interpreted with caution. Additionally, levels of HGF increased most in those with the lowest Exam 1 levels and marginally in individuals with the highest Exam 1 levels. It is unclear if this signifies an irregular physiological occurence or normal individual variation. The range of average change is narrow and may reflect regression to the mean; levels of HGF measured over a one-year period in healthy individuals indicated low within person variability (ICC=0.83) [22].
Limitations of our study should be recognized when synthesizing our results with previous studies of HGF and metabolic health. First, as with any observational epidemiological study, confounding by unmeasured or unknown characteristics is possible. Second, of the individuals determined to not have diabetes at Exam 1, 9% were excluded from this analysis due to reasons of missing data and loss to follow-up. The analytic sample may be healthier, on average, but this will not necessarily lead to bias in estimates of the association between HGF and diabetes. Third, HGF was measured in singleton; repeated measures on HGF at baseline would improve classification of the exposure. However, the ELISA procedure method was validated by the manufacturer and the MESA central laboratory. Fourth, diabetes was ascertained by data on diabetes medication use and fasting glucose values and the addition of data from OGTT and HbA1c would likely identify additional diabetes cases at baseline and over follow-up. Sensitivity analyses in a subset of individuals with data on HbA1c showed the exclusion of individuals with HbA1c ≥ 6.5% at baseline from prospective analysis did not materially alter the estimates. We would be concerned with bias to our results if the underreporting of incident cases over follow-up was associated with level of HGF, but we found no evidence to indicate such a pattern. We have no reason to think that the proportion of cases detected solely by OGTT would differ systematically by level of HGF, and thereby introduce bias. Fifth, the association between 1 pg/mL unit (0.1 μg/l) of HGF and incidence of diabetes, while statistically significant, was small in magnitude and we presented results for the association according to 1 SD in HGF. This 1 SD unit increment is similar to the difference in average HGF between the first quintile and third quintile or also between the third quintile and fifth quintile, but the clinical application of this value is unclear. Finally, the association between change in HGF between Exams 1 and 2 was of marginal significance and careful interpretation should be given to these results. Strengths of this study include the prospective evaluation of this association in four distinct race/ethnic groups from six geographic locations, multiple measurements of HGF levels, objective determination of diabetes (not only self-report), and highly standardized laboratory, anthropometric, and behavioral characteristic measurements.
In conclusion, over 12 years of follow-up, higher levels of HGF were positively associated with incidence of diabetes. This association was consistent across four ethnically diverse groups. An equivalent increase in level of HGF corresponded to a larger increase in hazard for diabetes in men compared to women. Two-year change in levels of HGF was marginally associated with incidence of diabetes in a sub-sample. Future studies should look to confirm these significant associations in other diverse populations.
Acknowledgments
The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
M.P.B. was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL007779 to conduct the current work. The Multi-Ethnic Study of Atherosclerosis is conducted and supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from National Center for Research Resources. Adhesion protein measurements supported by Multi-scale Biology of Atherosclerosis in the Cellular Adhesion Pathway (HL98077). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CRP
C-reactive protein
- HGF
Hepatocyte growth factor
- ISNR
Insulin receptor complex
- MESA
Multi-Ethnic Study of Atherosclerosis
- Met
HGF receptor
- WC
Waist circumference
Footnotes
Author disclosures: None
Disclosure statement
The authors have no conflicts of interest.
Author contributions
Mr. Michael Bancks is the guarantor of this work, had full access to the data in this analysis, and takes responsibility for the integrity of the data and the accuracy of the data analysis. MB, SB, and JP developed the idea for this paper. MB wrote the manuscript and researched the data. SB and JP contributed input for analysis methods, the interpretation of results, discussion, and edited/reviewed the manuscript. PD, NL, HS, and CW contributed to the discussion and edited/reviewed the manuscript. NH oversaw laboratory analysis, contributed to the methods, and edited/reviewed the manuscript. All authors critically reviewed and contributed to the scientific content and approved the final version to be published.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael P. Bancks, Email: banck001@umn.edu.
Suzette J. Bielinski, Email: Bielinski.Suzette@mayo.edu.
Paul A. Decker, Email: Decker.Paul@mayo.edu.
Naomi Q. Hanson, Email: hanso047@umn.edu.
Nicholas B. Larson, Email: Larson.Nicholas@mayo.edu.
Hugues Sicotte, Email: Sicotte.Hugues@mayo.edu.
Christina L. Wassel, Email: cwassel@med.uvm.edu.
James S. Pankow, Email: panko001@umn.edu.
References
- 1.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funakoshi H, Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta. 2003;327:1–23. doi: 10.1016/s0009-8981(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka M, Adachi H, Jacobs DR, Jr, et al. Serum hepatocyte growth factor and cancer mortality in an apparently healthy Japanese population. J Epidemiol. 2012;22:395–401. doi: 10.2188/jea.JE20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamblin N, Susen S, Dagorn J, et al. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am Heart J. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Rajpathak SN, Wang T, Wassertheil-Smoller S, et al. Hepatocyte growth factor and the risk of ischemic stroke developing among postmenopausal women: results from the Women’s Health Initiative. Stroke. 2010;41:857–862. doi: 10.1161/STROKEAHA.109.567719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perdomo G, Martinez-Brocca MA, Bhatt BA, Brown NF, O’Doherty RM, Garcia-Ocana A. Hepatocyte growth factor is a novel stimulator of glucose uptake and metabolism in skeletal muscle cells. J Biol Chem. 2008;283:13700–13706. doi: 10.1074/jbc.M707551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fafalios A, Ma J, Tan X, et al. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat Med. 2011;17:1577–1584. doi: 10.1038/nm.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiratsuka A, Adachi H, Fujiura Y, et al. Strong association between serum hepatocyte growth factor and metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2927–2931. doi: 10.1210/jc.2004-1588. [DOI] [PubMed] [Google Scholar]
- 9.Rajpathak SN, Wassertheil-Smoller S, Crandall J, Liu S, Ho GY. Hepatocyte growth factor and clinical diabetes in postmenopausal women. Diabetes Care. 2010;33:2013–2015. doi: 10.2337/dc10-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulseng B, Borset M, Espevik T, Sundan A. Elevated hepatocyte growth factor in sera from patients with insulin-dependent diabetes mellitus. Acta Diabetol. 1998;35:77–80. doi: 10.1007/s005920050107. [DOI] [PubMed] [Google Scholar]
- 11.Rehman J, Considine RV, Bovenkerk JE, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/s0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 12.Nowak C, Sundstrom J, Gustafsson S, et al. Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes. 2015 doi: 10.2337/db15-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell LN, Ward JL, Degawa-Yamauchi M, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 14.Swierczynski J, Korczynska J, Goyke E, Adrych K, Raczynska S, Sledzinski Z. Serum hepatocyte growth factor concentration in obese women decreases after vertical banded gastroplasty. Obes Surg. 2005;15:803–808. doi: 10.1381/0960892054222678. [DOI] [PubMed] [Google Scholar]
- 15.Tsukagawa E, Adachi H, Hirai Y, et al. Independent association of elevated serum hepatocyte growth factor levels with development of insulin resistance in a 10-year prospective study. Clin Endocrinol (Oxf) 2013;79:43–48. doi: 10.1111/j.1365-2265.2012.04496.x. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Psaty B, Lee M, Savage P, Rutan G, German P, Lyles M. Assessing the use of medications in the elderly: Methods and initial experience in the cardiovascular health study. Journal of Clinical Epidemiology. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 20.Hertz RP, Unger AN, Ferrario CM. Diabetes, hypertension, and dyslipidemia in Mexican Americans and non-Hispanic whites. Am J Prev Med. 2006;30:103–110. doi: 10.1016/j.amepre.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34:353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S-A, Kallianpur A, Xiang Y-B, et al. Intra-individual Variation of Plasma Adipokine Levels and Utility of Single Measurement of These Biomarkers in Population-Based Studies. Cancer Epidemiology Biomarkers & Prevention. 2007;16:2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]