Summary
There is growing interest in the role that life-history traits of hosts, such as their ‘pace-of-life’, play in the evolution of resistance and tolerance to parasites.
Theory suggests that, relative to host species that have high syntopy (local spatial and temporal overlap) with parasites, host species with low syntopy should have lower selection pressures for more constitutive (always present) and costly defences, such as tolerance, and greater reliance on more inducible and cheaper defences, such as behaviour. Consequently, we postulated that the degree of host–parasite syntopy, which is negatively correlated with host pace-of-life (an axis reflecting the developmental rate of tadpoles and the inverse of their size at metamorphosis) in our tadpole–parasitic cercarial (trematode) system, would be a negative and positive predictor of behavioural resistance and tolerance, respectively.
To test these hypotheses, we exposed seven tadpole species to a range of parasite (cercarial) doses crossed with anaesthesia treatments that controlled for anti-parasite behaviour. We quantified host behaviour, successful and unsuccessful infections, and each species’ reaction norm for behavioural resistance and tolerance, defined as the slope between cercarial exposure (or attempted infections) and anti-cercarial behaviours and mass change, respectively. Hence, tolerance is capturing any cost of parasite exposure.
As hypothesized, tadpole pace-of-life was a significant positive predictor of behavioural resistance and negative predictor of tolerance, a result that is consistent with a trade-off between behavioural resistance and tolerance across species that warrants further investigation. Moreover, these results were robust to considerations of phylogeny, all possible re-orderings of the three fastest or slowest paced species, and various measurements of tolerance.
These results suggest that host pace-of-life and host–parasite syntopy are powerful drivers of both the strength and type of host defence strategies against parasites. Future research should evaluate how often and how strongly host pace-of-life and host–parasite syntopy are correlated and which is the better predictor of the strength and type of host investments in anti-parasite defences.
Keywords: amphibian, cercariae, cost of exposure, disease, helminth, life history, resistance, snail, tolerance, trematode
Introduction
There is growing interest in understanding how host traits, such as life history and ‘pace-of-life’ (suite of traits typically associated with the correlation between development and life span or duration of a life stage; Ricklefs & Wikelski 2002), affect the evolution of defence against parasites (Zuk & Stoehr 2002; Martin, Weil & Nelson 2006, 2007; Miller, White & Boots 2007; Cronin et al. 2010; Rohr et al. 2011; Johnson et al. 2012). Pace-of-life, for instance, can affect exposure to parasites because hosts generally accumulate parasites throughout their life (i.e. age–intensity relationship; Raffel et al. 2011). Hence, ‘fast-paced’ species with rapid growth and short life spans should have few per capita exposures to parasites relative to ‘slow-paced’ species and thus should have less selection pressure for constitutive (always present) defences against parasites. This lower investment in constitutive defences should allow fast-paced species to invest more in growth and reproduction, consistent with trade-offs emphasized by life-history theory (Promislow & Harvey 1990; Schmid-Hempel & Ebert 2003; Lee 2006; Martin, Weil & Nelson 2006; Lee et al. 2008; Hawley & Altizer 2011; Boots, Donnelly & White 2013).
Although support is building for this trait-based approach for understanding absolute investment in defences against parasites (Haydon et al. 2002; LoGiudice et al. 2003; Martin, Weil & Nelson 2007; Cronin et al. 2010; Johnson et al. 2012; Previtali et al. 2012), predictions become more challenging when attempting to understand relative investment in different types of host defences, such as resistance and tolerance. Resistance reduces intensity of infections, whereas tolerance mitigates the deleterious effects of a given parasite exposure and infection intensity (Miller, White & Boots 2007). Resistance and tolerance can be achieved through multiple mechanisms, such as through immunity (Raberg, Graham & Read 2009; Best et al. 2012; Boots, Donnelly & White 2013) and behaviour (Miller, White & Boots 2007; Sears, Snyder & Rohr 2013b). Although tolerance mechanisms are likely induced upon exposure to parasites, the primary mechanisms of tolerance are thought to be resource-intensive processes, such as wound healing (Allen & Wynn 2011) and regulation of self-damaging inflammation (Raberg, Graham & Read 2009; Sears et al. 2011), which likely require permanent investment in some immunological and physiological architecture. Thus, tolerance must be more on the constitutive and costly end of the spectrum than behavioural resistance. Additionally, these two defence strategies for managing infection might not be independent as several researchers have suggested a trade-off between resistance and tolerance (Raberg, Graham & Read 2009; Sears et al. 2011). The strength of this trade-off should dictate the relative investment in each defence type potentially posing further challenges for predicting defence investments based on host life history (Miller, White & Boots 2007; Boots et al. 2009; Boots, Donnelly & White 2013).
Even in the absence of infections, mere exposure to parasites can induce costly behavioural and immunological defences (Lochmiller & Deerenberg 2000). Here, we define costs of parasite exposure as any cost of being exposed to parasites that is independent of the costs of actual infections. The cost of parasite exposure can be quantified several ways, such as by comparing the ‘fitness’ (or proxy thereof) of hosts not exposed to parasites to hosts exposed to parasites but not infected or to hosts exposed to only chemical and visual cues of parasites. As an example, in studies of skin-infecting fungi and trematodes, tadpoles not exposed to these parasites had greater survival than those that were exposed to these pathogens but never got infected (Rohr, Raffel & Hall 2010; Rohr et al. 2013). This was presumably because the groups exposed to these pathogens paid the costs of preventing the infections, battling secondary infections associated with the wounds caused to the skin and repairing these wounds, all of which likely impose considerable selection pressures on hosts. Traditional tolerance estimates combine, and thus confound, these costs of parasite exposure with costs of actual infections (Rohr, Raffel & Hall 2010). Different mechanisms could be used to tolerate parasite exposure, which does not entail an intimate symbiotic interaction between a host and a parasite, and actual infection, which does. Consequently, costs of parasite exposure and costs of actual infections might be very different in magnitude and could be differentially affected by life-history strategies, emphasizing the value of identifying predictors of tolerance of parasite exposure and tolerance of actual infections in an unconfounded manner.
Here, we studied skin-penetrating cercariae (larval trematodes) released from planorbid snails inhabiting Tampa, Florida, USA, which infect tadpoles. This tadpole–cercarial system is ideal for addressing relationships between pace-of-life and resistance and tolerance for several reasons. Given that the source of infections to tadpoles are planorbid snails, the level of syntopy or local spatial and temporal overlap between tadpole species and planorbid snails should generally reflect the level of amphibian exposure to these cercariae and thus the extent of selection pressures on amphibians for constitutive anti-cercarial defences. Most rapidly developing tadpole species in Florida breed as soon as the summer rains begin in late May and early June and metamorphose in 2–6 weeks (Lannoo et al. 2005); thus, they have little temporal overlap with the peak of planorbid snail and cercarial populations, which occur in late July and August (Table A1 in Appendix S1, Supporting information). In contrast, slower developing tadpole species tend to breed later and can take as long as 2 years to metamorphose (Lannoo et al. 2005); thus, they have considerable temporal overlap with peak snail and cercarial populations (Table A1 in Appendix S1, Supporting information). Moreover, the degree of spatial overlap is also generally predictable by the pace-of-life gradient. Rapidly developing tadpole species tend to occur in ephemeral or semi-permanent waterbodies devoid of aquatic snails, whereas tadpole species with long larval periods and planorbid snails are often found in more permanent waterbodies (Table A1 in Appendix S1, Supporting information). Hence, the host–parasite syntopy and host pace-of-life gradients are correlated in this system, which reflects the negative correlation between parasite exposure and pace-of-life seen in dynamic, theoretical models of defence investment with host life span (Miller, White & Boots 2007).
Tadpole species also differ in their use of behavioural resistance against cercariae, a defence that is probably less constitutive and costly than tolerance. In the presence of cercariae, tadpoles increase their activity to avoid cercariae (Rohr et al. 2009) and, upon an infection attempt, swim rapidly with many directional changes (Taylor, Oseen & Wassersug 2004), behaviours that successfully reduce infections (Koprivnikar, Forbes & Baker 2006; Daly & Johnson 2011). Hence, the primary host response to cercariae is to increase swimming activity to reduce contact rates (i.e. create a moving target) and the secondary response is a series of violent body contortions that help to dislodge cercariae once they have made contact with the tadpole.
To test whether cercarial-coping strategies were predictable based on pace-of-life and host–parasite syntopy, we exposed tadpoles of seven anuran species that differed considerably in their life histories (Table A1 in Appendix S1, Supporting information; listed from fast to slow development) to a range of cercarial doses crossed with anaesthesia treatments (presence or absence to control for anti-parasite behaviour) and quantified tadpole behavioural responses, attempted infections, infection intensities and mass change. Tadpoles were exposed to cercariae for only 10 min, which was long enough for many cercariae to begin penetrating host skin but was typically not long enough for infections to establish, allowing us, for the most part, to experimentally isolate tolerance of parasite exposure from tolerance of actual infections.
Given that rapidly developing or fast-paced tadpole species rarely encounter cercariae in the environment, they should, on average, have little need to develop constitutive defences, such as strong immunological resistance or tolerance of cercarial exposures. Hence, we hypothesized that when these species do encounter cercariae, they should exhibit intense behavioural avoidance (i.e. strong behavioural resistance) because they would likely die from the infection otherwise (i.e. low tolerance or low immunological resistance). If one considers the opposite end of the spectrum, a constant barrage of parasites, host behavioural resistance alone would not be a viable strategy because it would require constant anti-cercarial behaviours that would minimize host fitness by reducing other vital behaviours, such as foraging, mating and anti-predator responses. Hence, as host–parasite syntopy increases or pace-of-life slows, we expected defences on the constitutive end of the spectrum, such as tolerance (and perhaps immunological resistance), to increase. In summary, we hypothesized that host–parasite syntopy (or the inverse of host pace-of-life) would be a significant negative and positive predictor of behavioural resistance and tolerance of cercarial exposures, respectively, and that behavioural resistance and tolerance would be negatively correlated across species.
Materials and methods
NATURAL HISTORY
The cercariae used in this experiment are shed from freshwater snails (Planorbella trivolvis), swim through the water in search for the second intermediate host, amphibians, attach to amphibian skin, and use a pointed stylet and proteolytic enzymes to encyst subcutaneously as metacercariae (Schell 1970). Because of this traumatic infection process, cercariae can wound the host even in the absence of successful encystment (Rohr, Raffel & Hall 2010). Once an infected tadpole is consumed by the appropriate definitive host (the host in which sexual reproduction occurs), the metacercariae excyst and develop into adult trematodes, which pass eggs into the host’s faeces. Ciliated miracidia hatch from the eggs, which in turn infect molluscs (Koprivnikar et al. 2012).
For this experiment, cercariae were obtained from five P. trivolvis collected from a wetland in Tampa, Florida, USA (28.164581, −82.31202). Snails were inspected under a dissecting microscope for the presence or absence of free-swimming armatae cercariae (Schell 1970). These cercariae have been identified as belonging to the family Plagiorchiidae, subfamily Reniferinae (Sears & Rohr 2013); although they have not been identified to species, reniferin trematodes are highly conserved in their host use, infecting freshwater snails, tadpoles and water snakes as their first intermediate, second intermediate and definitive hosts, respectively. Infected snails were housed in artificial spring water (ASW; Cohen, Neimark & Eveland 1980) and fed frozen spinach ad libitum.
Individual tadpoles from seven different species [from fastest to slowest paced: Scaphiopus holbrookii (Eastern spadefoot toad), Osteopilus septentrionalis (Cuban treefrog), Gastrophryne carolinensis (narrowmouth toad), Hyla femoralis (pinewoods treefrog), Pseudacris ocularis (little grass frog), Hyla gratiosa (barking treefrog) and Rana catesbeiana (bullfrog); see Table A1 in Appendix S1, Supporting information] were collected from wetlands in Tampa, Florida, USA, and all collection sites were sampled for snails to prevent prior trematode infections. Tadpoles exposed to zero cercariae (n = 84) were verified to be trematode-free after the experiment, indicating that they were not naturally infected prior to collection. Although these particular collection sites are believed to be free of shedding snails just before the time of tadpole collection, this does not mean that these species and populations have not been exposed to, or experienced selection pressures from, cercariae in their evolutionary past. Given likely gene flow throughout evolutionary time to these populations from sites with snails, these species would be expected to display variation in innate responses to these parasites that reflects their average level of exposure to, and thus their past selection pressures from, cercariae.
The syntopy rankings for the seven tadpoles species were determined by averaging the rankings based on (i) time to metamorphosis, (ii) hydroperiod of the natal habitat and (iii) the degree of phonological overlap with P. trivolvis (see Table A1 in Appendix S1, Supporting information). Despite pace-of-life rankings being based on time to metamorphosis only, they were the exact inverse of the syntopy rankings (Table A1 in Appendix S1, Supporting information). Before experimentation, tadpoles were housed in 37-L aquaria filled with ASW, which was constantly cycled through a carbon filter, and fed frozen spinach ad libitum. To prevent confounding developmental stage with species identity, almost all the tadpoles (93%) were Gosner stages 24–27 when exposed to the treatments. All the treatments were administered in a laboratory maintaining 23 °C and a 12:12 photoperiod.
BEHAVIOURAL RESISTANCE
Before parasite exposure, tadpoles were weighed and placed individually in plastic specimen cups with 30 mL of either 0.001% benzocaine solution or ASW (control) for 10 min (modified from Koprivnikar, Forbes & Baker 2006; Fig. A1 in Appendix S1, Supporting information). The concentration of benzocaine used here has been demonstrated to have no side effects on white blood cell counts and does not affect encystment success by armatae cercariae (Sears, Snyder & Rohr 2013a). This exposure to benzocaine is sufficient to induce a minimum of 10 min of immobility. After anaesthetic or ASW exposure, tadpoles were collected in a small net, rinsed with ASW to remove any residual anaesthetic and placed individually in a plastic cup with 30 mL of ASW and 0–30 cercariae (0, 10, 15, 20 or 30 cercariae; n = 6 tadpoles per cercarial dose for O. septentrionalis, G. carolinensis, H. femoralis, H. gratiosa and R. catesbeiana; 0, 15 or 30 cercariae; n = 3 tadpoles per dose for P. ocularis and S. holbrookii because we had too few tadpoles to have all doses represented). One- to six-hour-old (this age maximizes infectivity) cercariae were collected directly from a specimen cup containing infected snails in ASW using a micropipette and dissecting microscope. Each tadpole was exposed to the assigned cercarial dosage for 10 min, which can allow for skin penetration (especially for anaesthetized tadpoles) but minimizes actual infections. Twenty-four-hour exposure of each tadpole species to these cercariae resulted in successful infections demonstrating that these cercariae are capable of infecting each host species. During the 10-min exposure to cercariae, all tadpoles were videotaped from above using a fixed-mount camera.
After cercarial exposure, tadpoles were transferred to individual 1-L containers. For all tadpole species except O. septentrionalis, G. carolinensis and P. ocularis (because data for these species were unfortunately collected before quantification of attempted infections was implemented), the cercarial exposure water received several drops of Lugol’s iodine, which killed and stained any remaining cercariae. The number of cercariae remaining was subtracted from the total cercariae administered to calculate the number of ‘attempted infections’. Hence, attempted infections are any cercariae that successfully penetrated the skin causing a wound. Many attempted infections are killed by the host immune system and thus only a fraction ever become established metacercarial infections.
After parasite exposure, tadpoles were maintained in 1 L of ASW, fed frozen spinach ad libitum and monitored for mortality daily. Water was changed 4 days after cercarial exposure. Seven days after cercarial exposure, tadpoles were weighed and euthanized in a 0.1% benzocaine solution. Tadpoles not surviving through this 7-day period were weighed and preserved immediately after death. For O. septentrionalis, G. carolinensis, H. femoralis, H. gratiosa and R. catesbeiana, three of the six tadpoles from each cercarial dosage were preserved in 70% ethanol, whereas the other three were stored in RNAlater (Ambion, Inc., Austin, TX, USA) for utilization in a separate study. For P. ocularis and S. holbrookii, all tadpoles were preserved in 70% ethanol. RNAlater-preserved tadpoles thus contributed behaviour and attempted infection data, but not infection intensity data. To make encysted metacercariae visible, ethanol-preserved tadpoles were cleared according to Hanken & Wassersug (1981) and metacercariae were counted at 100× magnification under a compound microscope.
STATISTICAL ANALYSES
We conducted two tests for a phylogenetic signature in our resistance and tolerance traits using the fitContinuous function in the geiger package of R statistical software (see Appendix S4 for methodological details and Fig. D1, Supporting information, for phylogeny). Regardless of the test, there was no indication of a phylogenetic signature or even any trends for a signal (Appendix S4 Results, Table D1 in Supporting information). For all tested traits, sample-size-corrected Akaike’s information criteria were lowest for the white-noise or non-phylogenetic model of evolution (Table D1 in Appendix S4, Supporting information). These findings suggest that a statistical correction for phylogeny was unnecessary for this data set. Johnson et al. (2012) also found no effect of phylogeny with 13 different species of tadpoles exposed to cercariae.
All behavioural analyses were performed in JWatcher (Blumstein, Evans & Daniel 2006) by a single observer. Swimming behaviour was scored as either normal (slow pace, no directional changes; see Appendix S2, Supporting information, for video), angled (swimming faster than normal and at an angle to the container with few directional changes; see Appendix S2, Supporting information, for video), evasive (explosive swimming with many, rapid directional changes; see Appendix S3, Supporting information, for video) or none/resting. Normal, angled and no swimming were scored as continuous states within JWatcher, whereas evasive manoeuvres were scored as discrete events because of their very short duration.
We tested for effects of treatments on four different measures of resistance: (i) the slope of the relationship between cercarial dose and anti-cercarial behaviours (a reaction norm or species-level estimate of behavioural resistance), (ii) the difference between the number of attempted infections per tadpole in the control and anaesthetized groups, (iii) the overall number of attempted infections and (iv) the number of successful metacercarial infections (Table 1). For behavioural resistance, we used a MANOVA to test for the effects of species and cercarial dose (a continuous predictor) on the proportion of time spent normal and angled swimming (arcsine square-root-transformed), and the number of evasive events per minute (log-transformed). Fisher’s post hoc tests were used to detect treatment- and species-level differences. For number of attempted infections, we conducted a negative binomial regression analysis controlling for cercarial dose and testing for the main and interactive effects of species and anaesthesia treatment.
Table 1.
Description of the response variables quantified in this study and whether they are estimates of behavioural resistance, resistance or tolerance of parasite exposure
| Responses | Estimates | No. of species for which response could be calculated |
|---|---|---|
| Slope of the relationship between cercarial dose and anti-cercarial behaviours | Behavioural resistance | 7 |
| Difference between number of attempted infectionsa when anaesthetized or not | Behavioural resistance | 7 |
| Number of attempted infectionsa | Behavioural resistance | 4 |
| Number of successful metacercarial infections | Resistance | 3 |
| Slope of the relationship between cercarial dose and mass change | Tolerance of infections plus tolerance of parasite exposure | 7 |
| Slope of the relationship between cercarial dose and mass change excluding the few tadpoles that were infected | Tolerance of parasite exposure | 7 |
| Slope of the relationship between cercarial dose or number of attempted infectionsa (when available) and mass change excluding the few tadpoles that were infected | Tolerance of parasite exposure | 4 |
| Slope of the relationship between cercarial dose and mass change statistically controlling for the number of actual infections | Tolerance of parasite exposure | 7 |
| Slope of the relationship between cercarial dose or number of attempted infectionsa (when available) and mass change statistically controlling for the number of actual infections | Tolerance of parasite exposure | 4 |
An attempted infection is defined as any cercaria that penetrated a tadpole, thus causing a wound, regardless of whether it successfully infected the tadpole or was killed by the tadpole’s immune response.
For all tolerance analyses, we used an estimate of cercarial exposure as the predictor (see below) and daily percentage mass change as the response variable (to control for size differences between species and minor differences in the durations of tadpole survival). Additionally, all tolerance analyses included both the anaesthetized and non-anaesthetized treatments, the zero cercarial dose level to control for differences in vigour among species (see Stowe et al. 2000; Tiffin & Inouye 2000) and all tadpoles that lived for at least 4 days after cercarial exposure (to ensure enough time for mass to change). Hence, tolerance of parasite exposure is always presented as a reaction norm defined as the slope for the relationship between an estimate of cercarial exposure and host mass change across anaesthesia treatments. Finally, we conducted our tolerance analyses in five different ways (Tables 1, A2 and A3 in Appendix S1, Supporting information). First, we simply regressed cercarial dose against mass change for each species. This analysis, however, confounds tolerance of actual infections with tolerance of exposure to parasites (Table 1). To address this, we controlled for actual infections in our tolerance analyses in two different ways; we excluded the handful of tadpoles with actual infections from the analysis, and we controlled for actual infections in each tadpole statistically by including the number of metacercariae as a covariate (Tables 1, 2, A2 and A3 in Appendix S1, Supporting information). We also suspected that attempted infections were the primary cause of any cost of exposure. So, for the final two tolerance analyses, we repeated the previous two analyses that controlled for actual infections, but we replaced cercarial dose with the number of attempted infections when this information was available (Tables 1, A2 and A3 in Appendix S1, Supporting information).
Table 2.
Statistical results for the effects of anaesthesia, tadpole species and cercarial dose on tadpole mass change per day (log-transformed). The results did not change when we controlled for actual infections in two different ways
| Effects | df 1 | All tadpoles not controlling for actual infections
|
Controlling for actual infections by excluding infected tadpoles
|
Controlling for actual infections statistically
|
|||
|---|---|---|---|---|---|---|---|
| F | Pa | F | Pa | F | Pa | ||
| Intercept | 1 | 3205.23 | <0.001 | 2957.67 | <0.001 | 2427.93 | <0.001 |
| No. of metacercariae | 1 | – | – | – | – | 0.78 | 0.380 |
| Anaesthesia treatment | 1 | 0.11 | 0.743 | 0.04 | 0.842 | 0.20 | 0.656 |
| Species | 6 | 26.96 | <0.001 | 24.98 | <0.001 | 17.16 | <0.001 |
| Cercarial dose | 1 | 5.43 | 0.020 | 4.23 | 0.041 | 6.68 | 0.011 |
| Anaesthesia × species | 6 | 0.93 | 0.473 | 0.89 | 0.503 | 1.06 | 0.388 |
| Anaesthesia × dose | 1 | 7.65 | 0.006 | 6.40 | 0.012 | 4.61 | 0.033 |
| Species × dose | 6 | 3.74 | 0.001 | 3.36 | 0.003 | 2.83 | 0.012 |
| Anaesthesia × species × dose | 6 | 0.70 | 0.650 | 0.49 | 0.818 | 0.54 | 0.775 |
Bolded probability values are below the alpha threshold of 0.05.
Tolerance of parasite exposure can be broken down into mitigating the costs of attempted infections, which entail substantial wound repair, and any costs of exposure independent of attempted infections, such as general up-regulation of immunity or stress from being in a risky environment. To partition these two components of tolerance of exposure, we conducted a general linear model with percentage mass change per day as the response variable, number of attempted infections, syntopy/pace-of-life ranking and anaesthesia treatment as crossed predictors, and cercarial dose as a covariate. If both cercarial dose and number of attempted infections are significant predictors of mass change, then it would suggest that parasite exposure has costs above and beyond the cost of attempted infections.
Given that we did not have attempted infection data for every species and not all tadpoles were free of infections, we could not evaluate our syntopy/pace-of-life hypotheses by testing for an interaction between pace-of-life and ‘cercarial exposure’. Hence, to test our hypothesis that pace-of-life is a negative predictor of tolerance, we regressed the rank order of species’ syntopy/pace-of-life (Table A1 in Appendix S1, Supporting information) against each of the five estimates of tolerance (Table A3 in Appendix S1, Supporting information), weighting by the inverse of the variance of the tolerance slope parameter; therefore, more weight was given to slopes with less error. Hence, these analyses use the species as the replicate and incorporate the error of the tolerance slope parameter. For consistency purposes, we tested for syntopy/pace-of-life relationships with our measures of behavioural resistance using a similar approach. That is, the slope coefficient was for the relationship between cercarial dose and each behavioural response, the species was the replicate, and the inverse of the variance estimate was used as a weight in the regression analysis.
It is possible that our pace-of-life ranking has error; that is, the true ranking might be slightly different than the ranking we used. To determine how robust our results were to any potential error, we performed all re-orderings of the presumed three fastest paced and three slowest paced species and reconducted the regression analyses for the relationships between pace-of-life and behavioural resistance and pace-of-life and tolerance (1234567 vs. 3214567, 1234765, 2134567, etc.; see Tables A4 and A5 in Appendix S1, Supporting information).
Although previous pace-of-life infection studies have used factor analyses conducted on various life-history traits to identify a primary, hypothetical life-history axis (Cronin et al. 2010; Johnson et al. 2012), we chose not to take this approach for several reasons. First, although traits can be correlated and there can be selection pressures on suites of traits across developmental stages of hosts, we had little a priori reason to believe that the traits of adult frogs, which often rarely encounter cercariae, must be coupled or correlated with the defences of aquatic larvae that often regularly encounter aquatic cercariae. So considering the role of life-history traits of adult frogs in defences against parasites mostly encountered by larval frogs seemed to have less of an a priori justification relative to host–parasite syntopy and thus seemed to run the risk of (i) adding noise that could increase the chances of a type II error or (ii) generating a spurious suggestion that some adult trait is most important simply because there are more adult life-history traits (e.g. size at maturity, age at maturity, clutch size, maximum age, maximum body size and egg size) than there are larval life-history traits (time and size at metamorphosis). Secondly, the most defensible approach we could come up with to assess the robustness of our findings was to consider reasonable re-orderings of the pace-of-life or host–parasite syntopy gradient. Hence, this requires taking a continuous life-history axis and turning into rank orders anyhow. Finally, resistance and tolerance are inherently outcomes of host–parasite interactions, but a host pace-of-life gradient completely ignores the parasite. In contrast, the host–parasite syntopy gradient has the benefit of considering both the host and parasite species.
To assess support for a trade-off between behavioural resistance and tolerance, we tested for a negative correlation between tolerance (using the tolerance estimate that was best predicted by our syntopy/pace-of-life gradient) and four estimates of behavioural resistance (again weighting by the inverse of the variance estimate): (i–iii) the reaction norm (i.e. slope) of each of the three measured behaviours as a function cercarial dose and (iv) the effectiveness of behaviour at reducing attempted infections (coefficient for the effect of anaesthesia treatment on attempted infections controlling for cercarial dose). Given that there was error in our estimate of both behavioural resistance and tolerance, we used major axis regression to minimize the sums of squares of the perpendicular distance between each point and the regression line. Statistical analyses were performed using a combination of R v3.0.2 (R Core Team 2014) and Statistica v12 (StatSoft 2009).
Results
RESISTANCE TO TREMATODE CERCARIAE
Only three of the seven species exposed to cercariae became infected: O. septentrionalis, H. gratiosa and H. femoralis. In each case, actual infections were low (Fig. A2 in Appendix S1, Supporting information); <2% of the cercariae infected O. septentrionalis and H. gratiosa and <8% infected H. femoralis (Fig. A2 in Appendix S1, Supporting information). Thus, no statistical analyses were conducted on actual infections and we were generally successful at keeping the cost of parasite exposure independent of costs of actual infections.
A MANOVA revealed that there was species variation in the use of the three anti-parasite behaviours – overall swimming, angled swimming and evasive behaviours – as a function of cercarial dose (Species × cercarial dose: Wilk’s F18, 436.06 = 2.64, P < 0.001; Fig. 1). Fast-paced species or species with low host–parasite syntopy generally exhibited a greater increase in anti-parasite behaviours as a function of cercarial dose than slow-paced species, such that pace-of-life ranking was a significant, positive predictor and host– parasite syntopy was a significant negative predictor of the relationship between cercarial dose and both total time spent swimming (F1,5 = 18.07 P = 0.008; Fig. 1a) and time spent angled swimming (F1,5 = 13.32 P = 0.014; Fig. 1b). These results were generally robust to re-orderings of the three fastest and three slowest paced species (P < 0.10; Tables A4 and A5 in Appendix S1, Supporting information). Pace-of-life or host–parasite syntopy were not significant predictors of the relationship between cercarial dose and evasive manoeuvres (F1,4 = 1.63 P = 0.271; Fig. 1c), but evasive manoeuvres only occur if the primary response, swimming activity, is ineffective at preventing longer-term contacts between the host and cercariae. Thus, evasive behaviours were rare relative to overall and angled swimming, providing less statistical power to detect an effect.
Fig. 1.
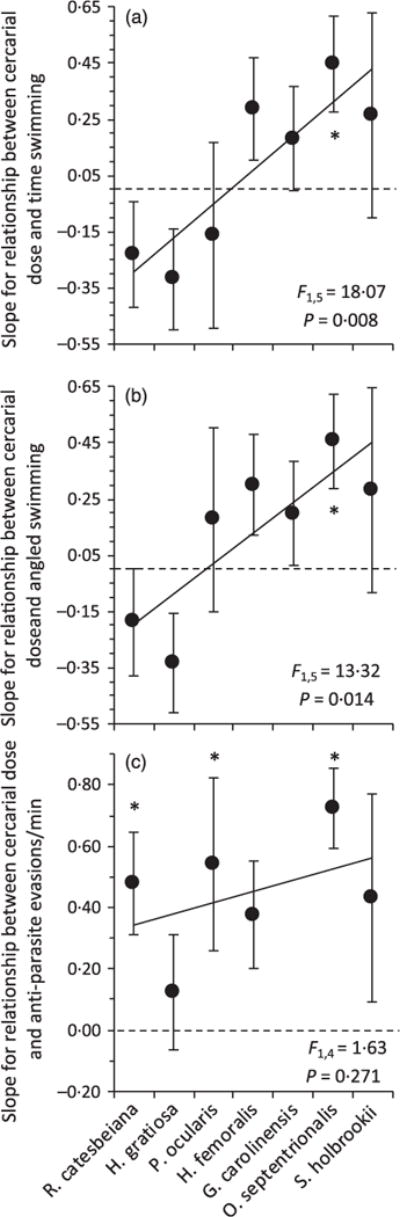
Relationship between cercarial dose and three swimming behaviours in seven tadpole species: (a) total time spent swimming, (b) angled swimming, (c) anti-parasite evasions per minute. Species are arranged from slow to fast pace-of-life, left to right. Each point is the standardized slope (±1 SE) of the behaviour as a function of exposure to 0–30 trematode cercariae (see Figs A4–7 in Appendix S1 for individual scatterplots associated with each slope parameter). Asterisks indicate slopes that are significantly different from zero. Statistics are for the regression between the rank order of pace-of-life and the slope of each behaviour as a function of cercarial dose, weighting by the inverse of the variance estimate associated with each slope parameter.
Only the fast-paced species (S. holbrookii, H. femoralis), which exhibited stronger behavioural responses to cercariae than the slow-paced species (H. gratiosa, R. catesbeiana; Fig. 1), had a significant difference in attempted infections between the anaesthetized and non-anaesthetized treatments (Fig. 2a), resulting in a significant interaction between anaesthesia treatment and host species ( , P = 0.024) and anaesthesia treatment and pace-of-life ( , P = 0.005). In the absence of behaviour (i.e. anaesthetized), the relationship between pace-of-life and attempted infections was positive but marginally non-significant ( , P = 0.069), whereas in the presence of behaviour, pace-of-life was a significant negative predictor of attempted infections ( , P = 0.022; Fig. 2a). When cercarial dose was added to this statistical model, both attempted infections and cercarial dose were significant predictors of mass change per day ( , P = 0.011; , P = 0.029, respectively), suggesting costs of parasite exposure independent of attempted infections. As expected, as cercarial dose increased so did attempted infections ( , P < 0.001).
Fig. 2.
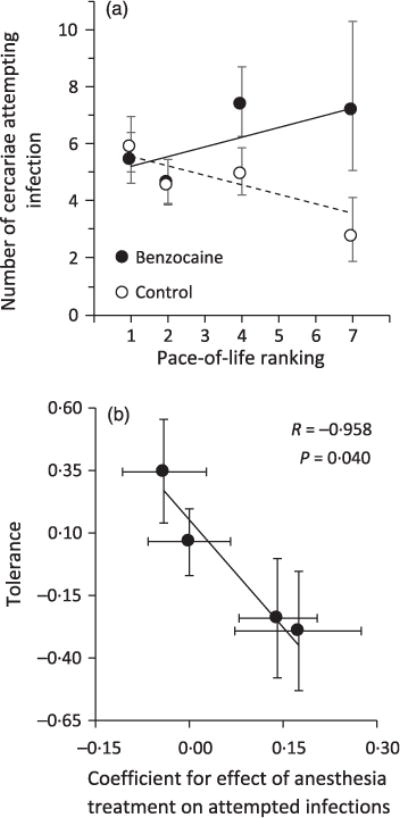
(a) Effect of pace-of-life on number of cercariae attempting to infect (i.e. not recovered after exposure to tadpole hosts) four anuran tadpoles species (R. catesbeiana, H. gratiosa, H. femoralis, S. holbrookii, ordered from slowest to fastest pace-of-life) exposed to benzocaine anaesthesia or not (averaged across cercarial doses) and (b) a negative relationship between tolerance of parasite exposure and the effectiveness of behaviour at reducing attempted infections, an estimate of behavioural resistance. In panel (a), shown are back-transformed mean predicted values (±1 SE) and regression fits from a negative binomial model controlling for cercarial dose and testing for the interaction between anaesthesia treatment and pace-of-life rank. In panel (b), tolerance is defined as the slope of the relationship between attempted infections and mass change controlling for the number of actual infections (see Fig. A6 in Appendix S1 for individual scatterplots associated with each slope parameter). Behavioural resistance (x-axis) is the coefficient for the effect of anaesthesia on the number of attempted infections controlling for cercarial dose. Each point in panel b is the standardized slope (±1 SE) of the tolerance and resistance estimate, and the fitted lines and statistics are from weighted (by the inverse of the variance) major axis regression.
TOLERANCE TO CERCARIAL EXPOSURE
In general, hosts suffered mass loss after cercarial exposure and the number of cercariae to which a host was exposed was inversely associated with mass change (Table 2). Furthermore, species identity was a significant predictor of mass change (F6,301 = 26.96, P < 0.001), as was the interaction between species and cercarial dose (F6,301 = 3.745, P = 0.001; Table 2). There was also a significant interaction between cercarial dose and anaesthesia (F1,301 = 7.65, P = 0.006); when tadpoles were not anaesthetized and thus could exhibit anti-cercarial behaviours, their mass remained relatively constant across cercarial doses, but when tadpoles were anaesthetized and thus could not exhibit anti-cercarial behaviours, mass declined as cercarial dose increased (Fig. 3a). These results did not change if we controlled for actual infections statistically or by excluding infected tadpoles from the analysis (Table 2), indicating that the few actual infections that established were inconsequential to the results.
Fig. 3.
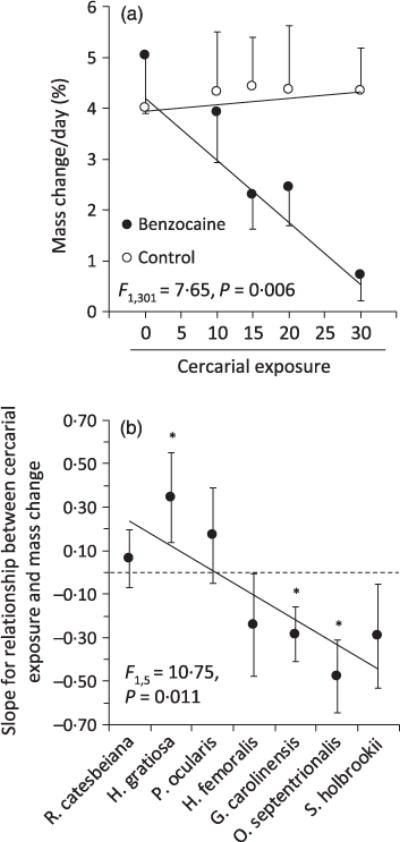
Tolerance of seven anuran species to cercarial exposure measured as the standardized slope of the relationship between cercarial exposure or attempted infections (when available) and mass change controlling for the number of actual infections. (a) Mean tolerance (±1 SE; averaging across the seven species) when tadpoles were or were not anaesthetized to control for anti-cercarial behaviours. Shown are back-transformed mean predicted values from a general linear model with log-positivized mass change as the response variable and species, anaesthesia treatment (continuous), and cercarial dose as predictors. (b) Mean tolerance (standardized slope ± 1 SE) of tadpole species arranged by tadpole pace-of-life, from slow (left) to fast (right). Asterisks indicate slopes that are significantly different from zero, and statistics listed are for the regression between the pace-of-life rank and the slope of mass change as a function of cercarial dose, weighting by the inverse of the variance estimate associated with each slope parameter (see Fig. A6 in Appendix S1 for individual scatterplots associated with each slope parameter).
The host pace-of-life and host–parasite syntopy rankings were significantly negative and positive predictors of tolerance, respectively, regardless of which of the five estimates of tolerance was used (Tables A2 and A3 in Appendix S1, Supporting information, Fig. 3b), emphasizing that the results were robust to the different tolerance estimates. Indeed, tolerance estimates based on cercarial dose and attempted infections were correlated positively (P = 0.038; Fig. A3 in Appendix S1, Supporting information). Because the results were independent of our tolerance estimate and for brevity, we choose to highlight tolerance estimate 4 (see Tables A2 and A3 in Appendix S1, Supporting information) in our figures and subsequent analyses because it used all the tadpoles that lived for at least 4 days after cercarial exposure (i.e. we statistically controlled for actual infections) and because it used all available information on both cercarial dose and attempted infections. Importantly, this significant negative association between pace-of-life and this measure of tolerance of parasite exposure was robust to re-orderings of the three fastest and slowest paced species, with pace-of-life remaining a significant positive predictor for all ten re-orderings (Table A4 and A5 in Appendix S1, Supporting information).
RELATIONSHIP BETWEEN RESISTANCE AND TOLERANCE
As predicted, the more intolerant species were of cercarial exposure, the greater their number and efficacy of anti-cercarial behaviours or, in other words, tolerance was inversely correlated with behavioural resistance. More specifically, tolerance was negatively correlated with the slope between cercarial dose and time spent swimming (R = −0.964, P < 0.001; Fig. 4a) and time spent angled swimming (R = −0.846, P = 0.012; Fig. 4b) and nearly negatively correlated with the rarely displayed evasive behaviours (R = −0.491, P = 0.126; Fig. 4c), three separate measures of behavioural resistance. Additionally, tolerance was negatively correlated with the effectiveness of behaviour at preventing attempted infections (R = −0.958, P = 0.040; Fig. 2b).
Fig. 4.
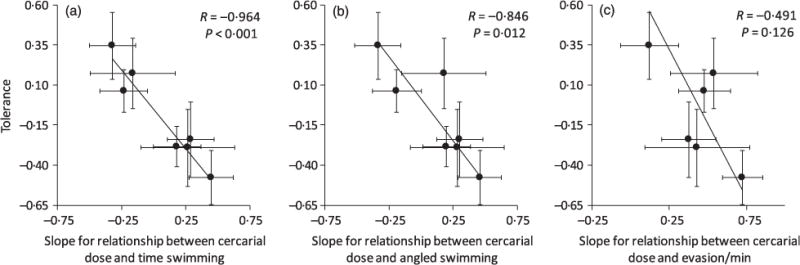
Negative relationships between tolerance of cercarial exposure in seven species of tadpoles and three measures of the strength of their behavioural resistance to cercariae: (a) total time spent swimming, (b) angled swimming, (c) anti-parasite evasion manoeuvres. Tolerance is defined as slope of the relationship between cercarial exposure or attempted infections (when available) and mass change controlling for the number of actual infections. Each point is the standardized slope (±1 SE) of the tolerance and resistance estimate, and the fitted lines and statistics are from weighted (by the inverse of the variance) major axis regression that minimizes the sums of squares of the perpendicular distance between each point and the regression line (to account for error in both the x and y variables). See Figs A3–7 in Appendix S1 for individual scatterplots associated with each slope parameter.
Discussion
The results of our comparative study across seven species of amphibian hosts revealed that tadpole anti-cercarial behaviours generally increased with cercarial dose (Fig. 1b,c) and more cercariae penetrated tadpoles that were anaesthetized than not anaesthetized (Fig. 2a). Additionally, tadpole mass loss was independent of cercarial dose when tadpoles could exhibit anti-cercarial behaviours but, when anaesthetized, mass loss increased with dose even when controlling for the few infections that established (Fig. 3a). These results indicate that behaviour generally reduced parasite exposure, parasite exposure was costly even in the absence of infections, and anti-cercarial behaviours increased sufficiently to keep mass gain similar across parasite doses. These results are consistent with several previous studies that emphasized the importance of behaviour for reducing exposure to cercariae (Koprivnikar, Forbes & Baker 2006; Rohr et al. 2009; Daly & Johnson 2011) and the costs of parasite exposure even in the absence of actual infections (Rohr, Raffel & Hall 2010; Rohr et al. 2013). The fact that mass loss across all species did not decline as cercarial dose increased when tadpoles could exhibit behaviours highlights that anti-cercarial behaviours were cheap in these trials. However, the opportunity costs of anti-cercarial behaviours would be expected to be greater in the field where they might preclude foraging and predator avoidance.
The overall costs of parasite exposure in this study could have included increased energy expenditures associated with (i) anti-parasite behaviours, (ii) immunity to fight potential trematode or secondary infections and (iii) wound repair. However, we suspect that most of the cost of parasite exposure was associated with immunity or wound repair for several reasons. First, exposure to parasites only occurred for 10 min and this duration of anti-cercarial behaviour is unlikely to have a substantial effect on tadpole mass. Indeed, anti-cercarial behaviours were not positively correlated with mass loss; rather, they seemed to prevent mass loss (Fig. 3a). In contrast, some of the chief mechanisms of tolerance are thought to be resource-intensive physiological processes such as wound healing (Allen & Wynn 2011) and regulation of self-damaging inflammation (Raberg, Graham & Read 2009; Sears et al. 2011), and thus, it is not surprising that wounds in the absence of infection should invoke costs for cercariae-exposed tadpoles. In support of this, mass loss was positively correlated with cercarial dose for anaesthetized tadpoles (Fig. 3a), consistent with greater investment in immunity or wound repair as parasite dose increased. In addition, even when controlling for the number of attempted infections, there was still a significant association between cercarial dose and mass loss. This result suggests that there are costs of parasite exposure beyond repairing wounds from attempted infections, which might be caused by an up-regulation of costly immunity. Future studies that quantify tadpole growth rates in the presence and absence of cercariae that cannot contact tadpoles would further elucidate whether cercarial exposure in the absence of attempted infections is costly, whereas studies with actual infections would be useful to elucidate costs of infection.
The results of our study also underscore the importance of host life-history traits or host–parasite syntopy in determining the evolution of both behavioural resistance to cercariae and tolerance of cercarial exposure. Experimental exposures to various doses of skin-penetrating cercariae in the presence and absence of host behaviours revealed profound species-level variation in (i) the types of behavioural responses to cercariae (Fig. 1), (ii) the strength of anti-cercarial responses as a function of cercarial dose (Fig. 1), (iii) the importance of behaviour in minimizing attempted infections (Fig. 2a) and (iv) the tolerance of cercarial exposure (Figs 2b and 4a–c). All of these responses were predicted by correlated host pace-of-life and host–parasite syntopy gradients. Fast-paced tadpole species exhibited strong behavioural resistance to cercariae, whereas slow-paced tadpole species favoured tolerance, supporting the hypothesis that pace-of-life and/or host–parasite syntopy are predictive of the selective pressures for both the amount and type of investments hosts make in parasite defences. These patterns were unlikely a product of phylogenetic inertia or geography because there was little evidence of a phylogenetic signal for any response variable or predictor and all the tadpoles were collected from nearby wetlands in Tampa, Florida, USA. Moreover, all possible re-orderings of the three fastest or slowest paced species and different measures of tolerance of parasite exposure did not change these results, emphasizing how robust these results were. That the results were robust to re-orderings of the fastest and slowest paced species could suggest a pace-of-life threshold for investment in defences as opposed to linear relationships, but tests of more species will be necessary to discriminate between threshold and linear responses and to further evaluate the generality of our results. Pace-of-life has been used previously to explain host reservoir potential and investment in immune defences (Haydon et al. 2002; LoGiudice et al. 2003; Lee 2006; Martin, Weil & Nelson 2007; Lee et al. 2008; Cronin et al. 2010; Previtali et al. 2012), but this is the first study to use a trait-based approach in an animal system to explain experimental patterns of behavioural resistance and tolerance in a manner that does not confound cost of parasite exposure with costs of actual infections.
Several researchers have suggested evolutionary trade-offs between resistance and tolerance within individuals (Raberg, Graham & Read 2009; Sears et al. 2011), but patterns across species have been less thoroughly explored. Across the seven species we studied, the four measures of behavioural resistance were generally negatively correlated with tolerance of parasite exposure (Figs 2b and 4a–c). Species with high parasite syntopy that were strongly tolerant of cercarial exposure exhibited the weakest behavioural resistance responses, whereas species with low parasite syntopy that were least tolerant of cercarial exposure exhibited the strongest behavioural resistance responses. These results suggest an evolutionary trade-off between tolerance and behavioural resistance across species that was predictable based on host pace-of-life and host–parasite syntopy gradients that should be explored in other systems. However, to verify this trade-off, it will be necessary to establish the genetic basis for the traits involved.
Although the perspectives presented here, and in other literature on functional traits and parasitism (Haydon et al. 2002; LoGiudice et al. 2003; Lee 2006; Martin, Weil & Nelson 2007; Lee et al. 2008; Cronin et al. 2010; Previtali et al. 2012), are generally ‘host-centric’, it undoubtedly will be important to more thoroughly consider the perspective of the parasite and the dynamical evolutionary interplay of host–parasite interactions in the future (Miller, White & Boots 2006; Boots et al. 2009). For example, in this study, when tadpoles were anaesthetized, more cercariae attempted to infect fast-paced than slow-paced species (Fig. 2), despite fast-paced species being smaller and thus releasing less chemical cues to attract cercariae. These differences among anaesthetized species can be interpreted as differences in their attractiveness to cercariae. Indeed, Sears, Schlunk & Rohr (2012) demonstrated that cercariae tend to be attracted to the least immunologically resistant tadpole species, which are generally the fastest paced (Johnson et al. 2012). Likewise, it has been proposed that parasites should evolve to infect the most common host species (Ostfeld & Keesing 2000; Keesing, Holt & Ostfeld 2006; Crossan, Paterson & Fenton 2007; Joseph et al. 2013), which generally are the fast-paced species that invest heavily in fecundity and dispersal. It remains unclear how evolutionary responses on the part of the parasite to host defences and host commonness alter predictions and patterns regarding the trait-, life-history-, and host–parasite syntopy-based frameworks for understanding host–parasite interactions.
We suspect that most host pace-of-life and host–parasite syntopy gradients are negatively correlated, at least in time (perhaps less so in space), offering a general mechanism by which these gradients might predict investment in different types of parasite defences. In the tadpole–cercarial system, fast-paced tadpole species have short aquatic larval periods and low spatial and temporal overlap with cercariae and thus have little selection pressure for constitutive defences against cercariae. Hence, rather than pay the upfront and permanent cost for the physiological architecture to resist and tolerate cercariae (Hawley & Altizer 2011), we hypothesize that fast-paced species should only pay the opportunity costs of parasite avoidance on the rare occasion when cercariae are encountered. However, these avoidance responses should be strong because of their low tolerance of attempted or actual infections. In contrast, slow-paced tadpole species have long aquatic larval periods and high spatial and temporal overlap with cercariae and thus strong selection pressures for constitutive defences against cercariae (Promislow & Harvey 1990; Schmid-Hempel & Ebert 2003; Lee 2006; Martin, Weil & Nelson 2006; Miller, White & Boots 2007; Lee et al. 2008). In fact, the greater the exposure to cercariae, the more futile the anti-cercarial behaviours should become because there would be fewer places where hosts could go to avoid cercariae and anti-cercarial behaviours would have to occur so often that they would regularly preclude foraging, mating and predator avoidance. Hence, we postulate that as parasite exposure increases or as hosts become more slow paced, behavioural responses should become less effective at maintaining fitness and more constitutive responses, such as tolerance, and perhaps immunological resistance should be favoured.
Interestingly, the pattern of species with low host–parasite syntopy using more behavioural resistance and species with high host–parasite syntopy using more constitutive defences, such as immunological resistance and tolerance, seems to be supported by several studies conducted on the tadpole–cercarial system, suggesting that host–parasite syntopy might generally be an important predictor of host defences in this host–parasite system. For example, toad tadpoles that have low syntopy with cercariae avoided cercariae (Rohr et al. 2009) and a ranid tadpole species with lower host–parasite syntopy exhibited more anti-cercarial behaviours than a ranid species with higher host–parasite syntopy (Thiemann & Wassersug 2000). Additionally, a recent study by Koprivnikar, Refern & Mazier (2014) discovered that pace-of-life did not seem to be as strong of a predictor of behavioural resistance to cercariae as host–parasite syntopy. Tadpoles of the American toad (Bufo americanus) and wood frog (Lithobates sylvaticus), species with low spatiotemporal overlap with cercariae, exhibited much stronger anti-cercarial responses than tadpoles of grey tree frogs (Hyla versicolor) and northern leopard frogs (Lithobates pipiens), species with high syntopy with cercariae (Koprivnikar, Refern & Mazier 2014). Wood frogs, however, have similar larval periods and size at metamorphosis and thus have a comparable pace-of-life as the grey tree frogs and northern leopard frogs. Moreover, we revealed a negative relationship between behavioural resistance and tolerance of tadpoles species, and Johnson et al. (2012) also showed that tadpole species that have high host–cercarial syntopy exhibited greater immunological resistance and less pathology (an indirect estimate of tolerance) than tadpole species with low host-cercarial syntopy.
An important question that remains, however, is which is more predictive of host defences against parasites, host pace-of-life or host–parasite syntopy. Although much of the emphasis in the scientific literature has been on pace-of-life, we suspect that host–parasite syntopy will be the more powerful predictor of host anti-parasite defences because it inherently considers both the host and parasite and explicitly considers the selection pressures for host defences against parasitism. The pace-of-life hypothesis does not emphasize selection pressures for anti-parasite defences; rather, it highlights energetic trade-offs that can happen with traits other than host defences and thus might not have effects on defence investments. Moreover, the pace-of-life hypothesis assumes correlations among suites of traits that drive syndromes or life-history strategies despite many traits becoming decoupled over evolutionary time. As an example, imagine a scenario where a slow-paced, long-lived organism has very little spatial or temporal overlap with a given parasite; it should experience little selection pressures for defences against this parasite despite being slow-paced. In this case, one might expect host–parasite syntopy to more accurately predict defence investments than pace-of-life. Ultimately, the degree of correlation between the host–parasite syntopy and pace-of-life gradients will determine how interchangeable they are as predictors of host defences.
Given that parasitism is a nearly universal phenomenon, most hosts likely balance the cost of coping with parasites against other life-history investments (Sheldon & Verhulst 1996). Although our results provide evidence that functional traits and the life history of hosts can predict the strength and type of host defence strategies against parasites, we have only recently begun to appreciate these relationships (Martin, Weil & Nelson 2007; Johnson et al. 2012). To better assess generality, we encourage further empirical studies on the relationships among functional traits, disease ecology, host immunology and life-history theory that span a broader range of host and parasite taxa, that consider evolutionary responses of both hosts and parasites and that compete life-history and host–parasite syntopy gradients as predictors of anti-parasite defences. This approach should improve scientific understanding of host–parasite interactions and the management of diseases of conservation concern.
Supplementary Material
Appendix S1. Supporting tables and figures.
Appendix S2. Video of normal and angled swimming of tadpoles.
Appendix S3. Video of tadpoles exhibiting an evasive manoeuver in the presence of cercariae.
Appendix S4. Methods and results for phylogenetic analyses.
Acknowledgments
We thank L.B. Martin, N.T. Halstead, M.H. Hersh and M.D. Venesky for comments on the manuscript and S. Reed for field and laboratory assistance. This work was supported by grants from the US Department of Agriculture (NRI 2008-00622 20, 2008-01785, 2013-04712), US Environmental Protection Agency (STAR R83-3835, CAREER 83518801) and National Science Foundation (EF-1241889) to J.R.R.
Footnotes
AUTHOR CONTRIBUTIONS
BFS and JRR developed the question and designed the experiments. BFS and PWS conducted the experiment and analysed all the behavioural videos. BFS conducted quality control and curated the data. JRR conducted the statistical analyses, developed the figures and tables, wrote the manuscript and handled the manuscript submission and revision. All authors edited the paper.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.k77ck (Sears, Snyder & Rohr 2014).
Handling Editor: Priyanga Amarasekare
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue-destructive pathogens. PLoS Pathogens. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A, Long G, White A, Boots M. The implications of immunopathology for parasite evolution. Proceedings of the Royal Society B-Biological Sciences. 2012;279:3234–3240. doi: 10.1098/rspb.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Evans CS, Daniel JC. JWATCHER v. 1.0. 2006 See www.jwatcher.ucla.edu.
- Boots M, Donnelly R, White A. Optimal immune defence in the light of variation in lifespan. Parasite Immunology. 2013;35:331–338. doi: 10.1111/pim.12055. [DOI] [PubMed] [Google Scholar]
- Boots M, Best A, Miller MR, White A. The role of ecological feedbacks in the evolution of host defence: what does theory tell us? Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:27–36. doi: 10.1098/rstb.2008.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LM, Neimark H, Eveland LK. Schistosoma mansoni: response of cercariae to a thermal gradient. Journal of Parasitology. 1980;66:362–364. [PubMed] [Google Scholar]
- Cronin JP, Welsh ME, Dekkers MG, Abercrombie ST, Mitchell CE. Host physiological phenotype explains pathogen reservoir potential. Ecology Letters. 2010;13:1221–1232. doi: 10.1111/j.1461-0248.2010.01513.x. [DOI] [PubMed] [Google Scholar]
- Crossan J, Paterson S, Fenton A. Host availability and the evolution of parasite life-history strategies. Evolution. 2007;61:675–684. doi: 10.1111/j.1558-5646.2007.00057.x. [DOI] [PubMed] [Google Scholar]
- Daly EW, Johnson PTJ. Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia. 2011;165:1043–1050. doi: 10.1007/s00442-010-1778-y. [DOI] [PubMed] [Google Scholar]
- Hanken J, Wassersug R. The visible skeleton. Functional Photography. 1981;16:22–26. [Google Scholar]
- Hawley DM, Altizer SM. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology. 2011;25:48–60. [Google Scholar]
- Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerging Infectious Diseases. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecology Letters. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Joseph MB, Mihaljevic JR, Orlofske SA, Paull SH. Does life history mediate changing disease risk when communities disassemble? Ecology Letters. 2013;16:1405–1412. doi: 10.1111/ele.12180. [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecology Letters. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Forbes MR, Baker RL. On the efficacy of anti-parasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2006;84:1623–1629. [Google Scholar]
- Koprivnikar J, Refern JC, Mazier HL. Variation in anti-parasite behaviour and infection among larval amphibian species. Oecologia. 2014;174:1179–1185. doi: 10.1007/s00442-013-2857-7. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Marcogliese DJ, Rohr JR, Orlofske SA, Raffel TR, Johnson PTJ. Macroparasite infections of amphibians: what can they tell us? EcoHealth. 2012;9:342–360. doi: 10.1007/s10393-012-0785-3. [DOI] [PubMed] [Google Scholar]
- Lannoo M, Gallant AL, Nanjappa P, Blackburn L, Hendricks R. Species accounts. In: Lannoo MJ, editor. Amphibian Declines: The Conservation Status of United States Species. University of California Press; Berkeley, CA: 2005. pp. 349–914. [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lee KA, Wikelski M, Robinson WD, Robinson TR, Klasing KC. Constitutive immune defences correlate with life-history variables in tropical birds. Journal of Animal Ecology. 2008;77:356–363. doi: 10.1111/j.1365-2656.2007.01347.x. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Refining approaches and diversifying directions in ecoimmunology. Integrative and Comparative Biology. 2006;46:1030–1039. doi: 10.1093/icb/icl039. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution. 2006;60:945–956. [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. Host life span and the evolution of resistance characteristics. Evolution. 2007;61:2–14. doi: 10.1111/j.1558-5646.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conservation Biology. 2000;14:722–728. [Google Scholar]
- Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB. Relationship between pace of life and immune responses in wild rodents. Oikos. 2012;121:1483–1492. [Google Scholar]
- Promislow DEL, Harvey PH. Living fast and dying young: a comparative analysis of life-history variation among mammals. Journal of Zoology. 1990;220:417–437. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel TR, Lloyd-Smith J, Sessions SK, Hudson PJ, Rohr JR. Does the early frog catch the worm? Disentangling potential drivers of a parasite age-intensity relationship in tadpoles. Oecologia. 2011;165:1031–1042. doi: 10.1007/s00442-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends in Ecology & Evolution. 2002;17:462–468. [Google Scholar]
- Rohr JR, Raffel TR, Hall CA. Developmental variation in resistance and tolerance in a multi-host-parasite system. Functional Ecology. 2010;24:1110–1121. [Google Scholar]
- Rohr JR, Swan A, Raffel TR, Hudson PJ. Parasites, info-disruption, and the ecology of fear. Oecologia. 2009;159:447–454. doi: 10.1007/s00442-008-1208-6. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, et al. Frontiers in climate change-disease research. Trends in Ecology & Evolution. 2011;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, et al. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell SC. How to Know the Trematodes. Wm. C. Brown Company Publishers; Dubuque, IA: 1970. [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends in Ecology & Evolution. 2003;18:27–32. [Google Scholar]
- Sears B, Rohr JR. Loss of trematode parthenitae in Planorbella trivolvis (Mollusca: Gastropoda) Journal of Parasitology. 2013;99:738–739. doi: 10.1645/12-111.1. [DOI] [PubMed] [Google Scholar]
- Sears BF, Schlunk A, Rohr J. Do parasitic trematode cercariae demonstrate a preference for susceptible host species? PLoS One. 2012;7:e51012. doi: 10.1371/journal.pone.0051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears B, Snyder PW, Rohr J. No effects of two anesthetic agents on circulating leukocyte counts or resistance to trematode infections in larval amphibians. Journal of Herpetology. 2013a;47:498–501. [Google Scholar]
- Sears BF, Snyder PW, Rohr JR. Infection deflection: hosts control parasite location with behaviour to improve tolerance. Proceedings of the Royal Society B Biological Sciences. 2013b;280:20130759. doi: 10.1098/rspb.2013.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears BF, Snyder PW, Rohr JR. Data from: Host life-history and host-parasite syntopy predict behavioral resistance and tolerance of parasites. Dryad Digital Repository. 2014 doi: 10.1111/1365-2656.12333. http://dx.doi.org/10.5061/dryad.k77ck. [DOI] [PMC free article] [PubMed]
- Sears BF, Rohr JR, Allen JE, Martin LB. The economy of inflammation: when is less more? Trends in Parasitology. 2011;27:382–387. doi: 10.1016/j.pt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology & Evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- StatSoft. Statistica. StatSoft; Tulsa, OK: 2009. [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annual Review of Ecology and Systematics. 2000;31:565–595. [Google Scholar]
- Taylor CN, Oseen KL, Wassersug RJ. On the behavioural response of Rana and Bufo tadpoles to echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Canadian Journal of Zoology. 2004;82:701–706. [Google Scholar]
- Thiemann GW, Wassersug RJ. Patterns and consequences of behavioural responses to predators and parasites in Rana tadpoles. Biological Journal of the Linnean Society. 2000;71:513–528. [Google Scholar]
- Tiffin P, Inouye BD. Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution. 2000;54:1024–1029. doi: 10.1111/j.0014-3820.2000.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr AM. Immune defense and host life history. American Naturalist. 2002;160:S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting tables and figures.
Appendix S2. Video of normal and angled swimming of tadpoles.
Appendix S3. Video of tadpoles exhibiting an evasive manoeuver in the presence of cercariae.
Appendix S4. Methods and results for phylogenetic analyses.


