Filamentous fungi exhibit rapid, polarized growth governed by precise segregation of endocytosis and exocytosis at the site of cell expansion. Endocytosis, in particular, is concentrated at a zone termed the endocytic collar, surrounding the cell tip, where secretory vesicle fusion occurs. How the separation of these processes is established and maintained is still unclear, but one possible purpose for this organization is the ability of endocytosis to remove and recycle proteins that diffuse away from the site of growth. The phospholipid flippases DnfA and DnfB localize to sites of growth and endocytosis in the fungus Aspergillus nidulans, and may be involved in secretion. DnfA is endocytosed in part through an NPFxD motif, but whether DnfB is endocytosed or recycled is unknown. Here, we demonstrate that DnfB is also endocytosed, and that recycling through the late Golgi maintains its organization in the growing tip.
Results
Endocytosis and endocytic recycling are conserved processes that provide cells the chance to interact with their environment and regulate plasma membrane dynamics, among other things. Inhibiting endocytosis has many effects on filamentous fungi, including disrupting their ability to maintain polarity, an almost complete abolishment of growth, and causing aberrant cell shape.1-6 The direct reason for these phenotypes has not been demonstrated. Most mutations or treatments that target endocytosis may also target the actin cytoskeleton, which has several ancillary roles in growth and cell shape including intracellular membrane trafficking.3,7 Some hypotheses for the purpose of the endocytosis in growth and shape have been proposed, including the apical recycling model, which suggests that endocytosis acts to counteract to the passive diffusion of proteins from the site of growth. In this system, proteins that are necessary for certain processes at the cell tip are internalized at the endocytic collar as they are misplaced during growth. Additionally, some of these proteins could potentially be recycled back to the site of growth through a variety of endomembrane trafficking pathways.1,5,8,9
To understand the function of endocytosis in fungi, as well as the results that come from mutations that block endocytosis, it is helpful to characterize proteins that are cargo of the endocytic collar. To this date, however, only a few proteins have been shown to be endocytosed in the tips of filamentous fungi,10,11 One of these proteins is the Aspergillus nidulans phospholipid flippase DnfA.12 Phospholipid flippases control lipid asymmetry, primarily in the plasma membrane and throughout the secretory pathway.13-15 DnfA has an NPFxD motif, which is known to associate with the endocytic adaptor Sla1p in yeast.16-18 Upon mutation of this motif, DnfA relocalized from the hyphal tip to being present throughout the plasma membrane in an apolar manner, suggesting it is endocytosed in part through this motif. DnfA-GFP was, moreover, diffuse throughout the cytoplasm in a vftD deletion mutant. VftD is part of the Golgi Associated Retrograde Protein (GARP) complex, a 4-subunit tethering complex required for fusion of vesicles traveling from early and late endosomes to the late Golgi, and thus for endocytic recycling of proteins through the late Golgi.12,19 A vftD deletion confers a strong growth phenotype, which suggests that GARP function is abolished in this mutant, and, further, that endocytic recycling through the late Golgi could act to polarize tip components in A. nidulans.
Another flippase, DnfB, is also primarily localized to the hyphal tip and the plasma membrane under normal conditions (Fig. 1A).12 Particularly, it localizes to the Spitzenkörper: a spherical, fungal organelle just behind the growing cell tip, composed primarily of secretory vesicles. When endocytosis is blocked by downregulating the endocytic gene fimA (fimbrin)6 with the nitrate-expressing, ammonium-repressible niiA promoter,3,20,21 DnfB-mCherry depolarizes and moves from the tip (Fig. 1C) to being present throughout the plasma membrane (Fig. 1D). Interestingly, although DnfB trails off of the plasma membrane before DnfA in wild type cells,12 it does not possess an NPFxD motif, and its mechanism for being recognized by the endocytic machinery is not yet known.
Figure 1.
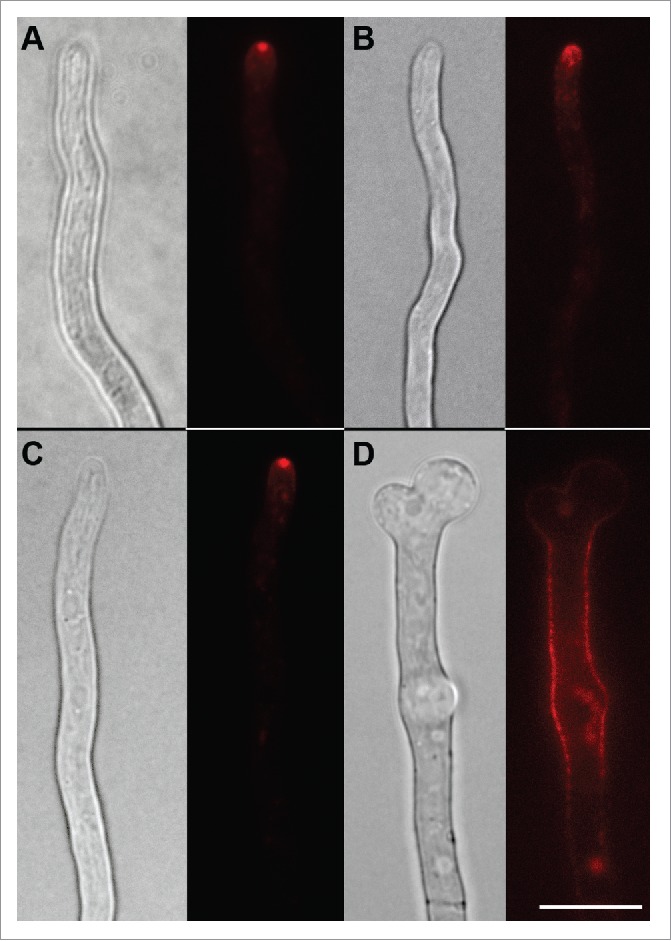
DnfB is endocytosed and possibly recycles through the late Golgi. DnfB-mCherry is present in the Spitzenkörper and the apical plasma membrane in wild type hyphae (A), while this organization is lost when vftD is deleted (B). When endocytosis is depleted through regulation of fimA by the ammonium-repressible niiA promoter, DnfB-mCherry relocalizes from the Spitzenkörper (C, grown with NO3) to the plasma membrane (D, grown with NH4). Scale bar = 5µm.
DnfB is also present at the late Golgi in wild type.12 To see if it also traffics to the late Golgi after being endocytosed, we viewed DnfB-mCherry in a vftD deletion mutant. As seen in Figure 1B, although DnfB-mCherry is still polarized, its apical accumulation is less organized, suggesting that the presence of DnfB-mcherry at the Spitzenkörper, and possibly Spitzenkörper structure, is dependent upon traffic between endosomes and the late Golgi. Whether DnfB can be polarized through a secondary recycling pathway is unknown. The homolog in yeast, Drs2p, has been shown to act in concert with Rcy1p to support endocytic recycling 22, but the importance of the A. nidulans Rcy1p homolog, RcyA, in recycling is unclear,23 and could be resolved in the future. What is clear is that one effect of abolished endocytosis is the likely loss of plasma membrane lipid asymmetry, the consequences of which is a currently unexplored topic in fungal cell biology.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Schultzhaus ZS, Shaw BD. Endocytosis and exocytosis in hyphal growth. Fungal Biol Rev 2015; 29(2):43-53. [Google Scholar]
- [2].Araujo‐Bazán L, Peñalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol 2008; 67:891-905; PMID:18179595; http://dx.doi.org/ 10.1111/j.1365-2958.2007.06102.x [DOI] [PubMed] [Google Scholar]
- [3].Hervás-Aguilar A, Peñalva MA. Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans. Eukaryot Cell 2010; 9:1504-18; PMID:20693304; http://dx.doi.org/ 10.1128/EC.00119-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peñalva MÁ. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr Opin Microbiol 2010; 13:684-92; PMID:20920884; http://dx.doi.org/ 10.1016/j.mib.2010.09.005 [DOI] [PubMed] [Google Scholar]
- [5].Shaw BD, Chung D-W, Wang C-L, Quintanilla LA, Upadhyay S. A role for endocytic recycling in hyphal growth. Fungal Biol 2011; 115:541-6; PMID:21640317; http://dx.doi.org/ 10.1016/j.funbio.2011.02.010 [DOI] [PubMed] [Google Scholar]
- [6].Upadhyay S, Shaw BD. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol Microbiol 2008; 68:690-705; PMID:18331474; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06178.x [DOI] [PubMed] [Google Scholar]
- [7].Pantazopoulou A, Pinar M, Xiang X, Peñalva MA. Maturation of late Golgi cisternae into RabE/RAB11 exocytic post-Golgi carriers visualized in vivo. Mol Biol Cell 2014; 25:2428-43; PMID:24943841; http://dx.doi.org/ 10.1091/mbc.E14-02-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee SC, Schmidtke SN, Dangott LJ, Shaw BD. Aspergillus nidulans ArfB plays a role in endocytosis and polarized growth. Eukaryot Cell 2008; 7:1278-88; PMID:18539885; http://dx.doi.org/ 10.1128/EC.00039-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caballero-Lima D, Kaneva IN, Watton SP, Sudbery PE, Craven CJ. The spatial distribution of the exocyst and actin cortical patches is sufficient to organize hyphal tip growth. Eukaryot Cell 2013; 12:998-1008; PMID:23666623; http://dx.doi.org/ 10.1128/EC.00085-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taheri-Talesh N, Horio T, Araujo-Bazán L, Dou X, Espeso EA, Peñalva MA, Osmani SA, Oakley BR. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell 2008; 19:1439-49; PMID:18216285; http://dx.doi.org/ 10.1091/mbc.E07-05-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pantazopoulou A, Peñalva MA. Characterization of Aspergillus nidulans RabC/Rab6. Traffic 2011; 12:386-406; PMID:21226815; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01164.x [DOI] [PubMed] [Google Scholar]
- [12].Schultzhaus Z, Yan H, Shaw B. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar, and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol Microbiol 2015; 97:18-32; PMID:25846564; http://dx.doi.org/ 10.1111/mmi.13019 [DOI] [PubMed] [Google Scholar]
- [13].Backer JM, Dawidowicz EA. Reconstitution of a phospholipid flippase from rat liver microsomes. Nature 1987; 327:341-3; PMID:3587349; http://dx.doi.org/ 10.1038/327341a0 [DOI] [PubMed] [Google Scholar]
- [14].Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci U S A 2012; 109:E290-E8; PMID:22308393; http://dx.doi.org/ 10.1073/pnas.1115725109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takeda M, Yamagami K, Tanaka K. Role of phosphatidylserine in phospholipid flippase-mediated vesicle transport in Saccharomyces cerevisiae. Eukaryot Cell 2014; 13:363-75; PMID:24390140; http://dx.doi.org/ 10.1128/EC.00279-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Howard JP, Hutton JL, Olson JM, Payne GS. Sla1p serves as the targeting signal recognition factor for NPFX (1, 2) D-mediated endocytosis. J Cell Biol 2002; 157:315-26; PMID:11940605; http://dx.doi.org/ 10.1083/jcb.200110027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu K, Hua Z, Nepute JA, Graham TR. Yeast P4-ATPases Drs2p and Dnf1p are essential cargos of the NPFXD/Sla1p endocytic pathway. Mol Biol Cell 2007; 18:487-500; PMID:17122361; http://dx.doi.org/ 10.1091/mbc.E06-07-0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Piao HL, Machado IM, Payne GS. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol Biol Cell 2007; 18:57-65; PMID:17065552; http://dx.doi.org/ 10.1091/mbc.E06-08-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Conibear E, Stevens TH. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol Biol Cell 2000; 11:305-23; PMID:10637310; http://dx.doi.org/ 10.1091/mbc.11.1.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cove D. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta 1966; 113:51-6; PMID:5940632; http://dx.doi.org/ 10.1016/S0926-6593(66)80120-0 [DOI] [PubMed] [Google Scholar]
- [21].Punt PJ, Strauss J, Smit R, Kinghorn JR, Van den Hondel C, Scazzocchio C. The intergenic region between the divergently transcribed niiA and niaD genes of Aspergillus nidulans contains multiple NirA binding sites which act bidirectionally. Mol Cell Biol 1995; 15:5688-99; PMID:7565720; http://dx.doi.org/ 10.1128/MCB.15.10.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hanamatsu H, Fujimura-Kamada K, Yamamoto T, Furuta N, Tanaka K. Interaction of the phospholipid flippase Drs2p with the F-box protein Rcy1p plays an important role in early endosome to trans-Golgi network vesicle transport in yeast. J Biochem 2014; 155:51-62; PMID:24272750; http://dx.doi.org/ 10.1093/jb/mvt094 [DOI] [PubMed] [Google Scholar]
- [23].Herrero S, Takeshita N, Fischer R. F-Box Protein RcyA Controls Turnover of the Kinesin-7 Motor KipA in Aspergillus nidulans. Eukaryot Cell 2014; 13:1085-94; PMID:24951440; http://dx.doi.org/ 10.1128/EC.00042-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


