Abstract
Background—
We sought to determine the overall impact of a nurse-led, multidisciplinary home-based intervention (HBI) adapted to hospitalized patients with chronic forms of heart disease of varying types.
Methods and Results—
Prospectively planned, combined, secondary analysis of 3 randomized trials (1226 patients) of HBI were compared with standard management. Hospitalized patients presenting with heart disease but not heart failure, atrial fibrillation but not heart failure, and heart failure, as well, were recruited. Overall, 612 and 614 patients, respectively, were allocated to a home visit 7 to 14 days postdischarge by a cardiac nurse with follow-up and multidisciplinary support according to clinical need or standard management. The primary outcome of days-alive and out-of-hospital was examined on an intention-to-treat basis. During 1371 days (interquartile range, 1112–1605) of follow-up, 218 patients died and 17 917 days of hospital stay were recorded. In comparison with standard management, HBI patients achieved significantly prolonged event-free survival (90.1% [95% confidence interval, 88.2–92.0] versus 87.2% [95% confidence interval, 85.1–89.3] days-alive and out-of-hospital; P=0.020). This reflected less all-cause mortality (adjusted hazard ratio, 0.67; 95% confidence interval, 0.50–0.88; P=0.005) and unplanned hospital stay (median, 0.22 [interquartile range, 0–1.3] versus 0.36 [0–2.1] days/100 days follow-up; P=0.011). Analyses of the differential impact of HBI on all-cause mortality showed significant interactions (characterized by U-shaped relationships) with age (P=0.005) and comorbidity (P=0.041); HBI was most effective for those aged 60 to 82 years (59%–65% of individual trial cohorts) and with a Charlson Comorbidity Index Score of 5 to 8 (36%–61%).
Conclusions—
These data provide further support for the application of postdischarge HBI across the full spectrum of patients being hospitalized for chronic forms of heart disease.
Clinical Trial Registration—
URL: http://www.anzctr.org.au. Unique identifiers: 12610000221055, 12608000022369, 12607000069459.
Keywords: case management, heart diseases, mortality, outcome assessment (health care), patient readmission, secondary prevention
There is an increasing pressure to develop cost-effective strategies to mitigate persistently high levels of rehospitalization and premature mortality associated with chronic heart disease. Beyond pharmacological agents and devices, disease management programs are integral to applying flexible, guideline-based management, coordinating care, and providing individualized support.1,2 However, the evidence to support their application across the full spectrum of heart disease remains incomplete and fragmented. For example, although the overall evidence supporting short-term cardiac rehabilitation3 and chronic heart failure (CHF) management4 programs is extensive and mostly favorable, there is a paucity of research to support programs designed to prevent progressive cardiac dysfunction in patients who survive an acute coronary syndrome5 or to optimize outcomes in those with atrial fibrillation (AF).6,7 Moreover, a diverse range of disease management techniques have been tested. Ideally, the same form of disease management program adapted to the individual patient profile would be tested across a diverse range of cardiac patients receiving high levels of standard care to determine its overall potential to improve health outcomes in the real-world setting.
Editorial, see p 1836
Clinical Perspective on p 1877
In the absence of a single definitive trial, we conducted 3 contiguous trials examining the benefits of the same model of care (nurse-led, multidisciplinary, home-based intervention [HBI]) to prevent secondary events in hospitalized patients spanning the continuum of heart disease. In each trial, we postulated that the benefits of HBI would transcend a patient’s specific cardiac needs and would be more responsive to their wider clinical and psychosocial needs with improvements in all-cause health outcomes. We now report on a prospectively planned, composite analysis of study outcomes from these trials. A priori, we postulated that, in the overall setting of chronic heart disease, HBI is superior to standard care in preventing recurrent hospitalization and premature mortality on an all-cause basis in patients with any form of chronic heart disease.
Methods
Study Design and Patients
Baseline and outcome data were combined from 3 trials targeting hospitalized patients with: (1) a range of chronic heart disease states (most presented with acute coronary syndrome) but not CHF (n=611) enrolled in the Nurse-led Intervention for Less Chronic Heart Failure (NIL-CHF) Study8; (2) chronic AF but not CHF (n=335) enrolled in the Standard versus Atrial Fibrillation Specific Management Strategy (SAFETY) Trial9; and (3) CHF (n=280) enrolled in the Which Heart Failure Intervention Is Most Cost-effective & Consumer Friendly in Reducing Heart Failure Hospital Care (WHICH?) Trial.10 All 3 trials were registered via www.anzctr.org.au (12608000022369,12610000221055, and 12607000069459, respectively) and conducted with appropriate ethics approval.
Detailed descriptions of each study design5,11,12 and subsequent outcomes8–10 (including extended follow-up of the WHICH? Cohort)13 were published between 2011 and 2015. Each adhered to CONSORT guidelines for conducting pragmatic health service interventions.14 Applying the same methods of recruitment, profiling, and follow-up, with only minor differences in clinical management specific to the target patient population, data were derived from 1226 patients recruited from 6 tertiary referral hospitals across 5 Australian states with a combined follow-up of 4517 person-years (see Table 1).
Table 1.
Major Design Features of the NIL-CHF Study, SAFETY Trial, and WHICH? Trial
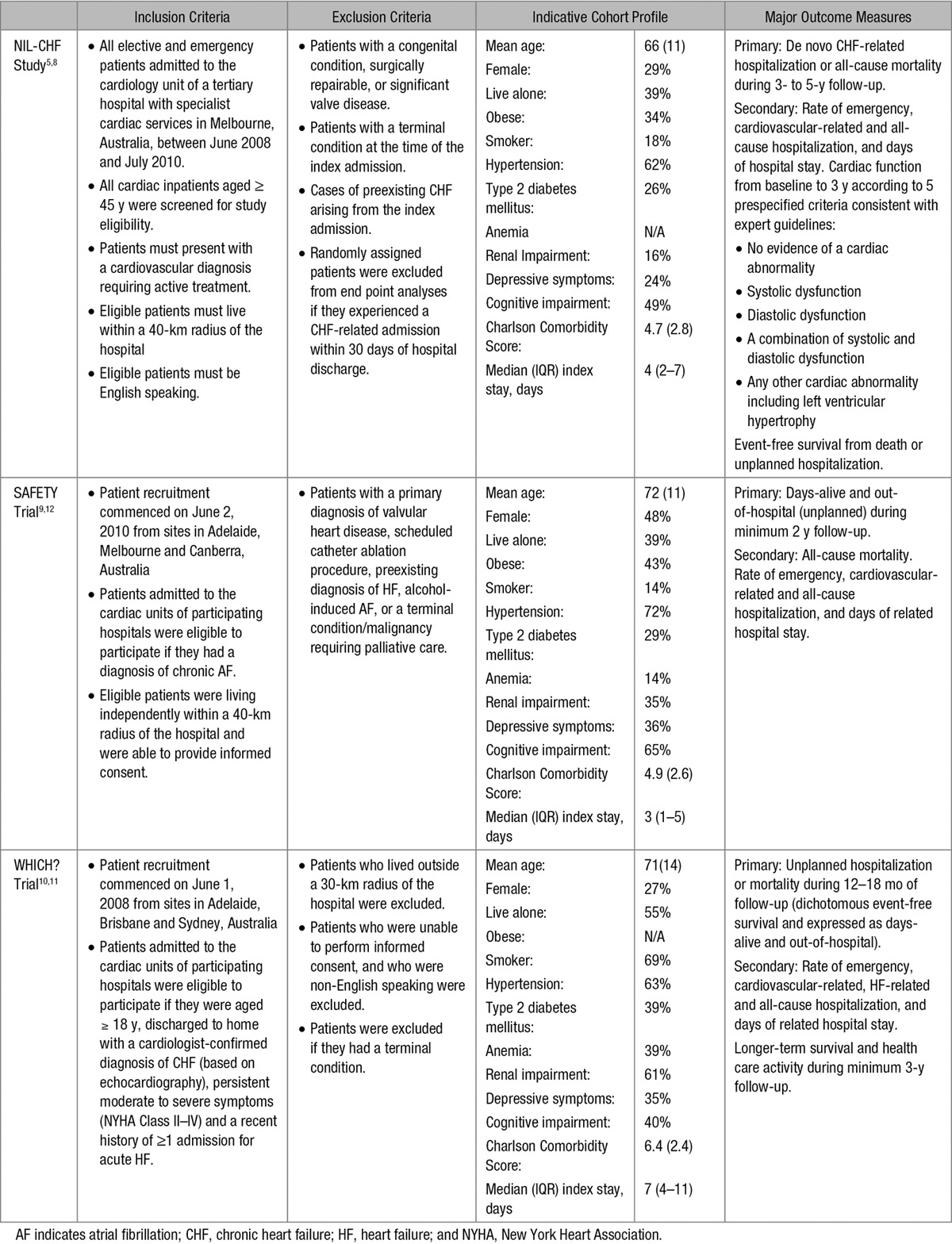
A blinded, computer-generated randomized protocol was implemented by an independent data management team via telephone for each study with a predetermined, block randomization sequence for individual study sites. Stratification for reduced versus preserved ejection fraction in the WHICH? Trial of patients with CHF and nominated rhythm versus rate control in the SAFETY Trial of patients with AF were applied. Overall, 612 and 614 patients were allocated to HBI and standard management, respectively.
Study Data
All patients were comprehensively profiled during their index admission by using the same framework of profiling and data entry. This included sociodemographic status, past medical history (including details specific to their primary reason for trial inclusion), clinical profile, in-hospital management, and planned postdischarge care.
Clinical Management
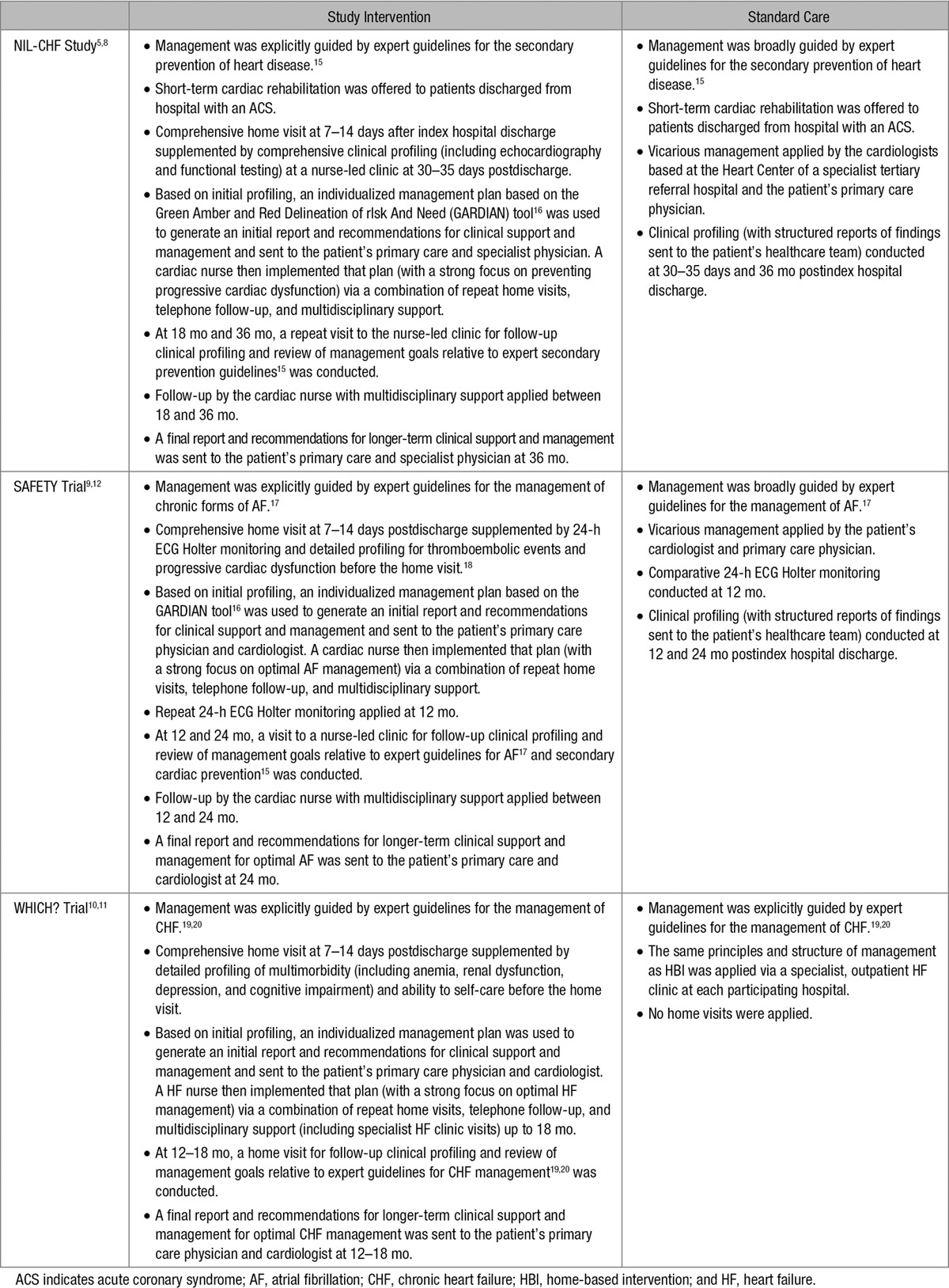
No restrictions on the level of standard management (other than no structured home visits) were applied. Treating physicians were routinely sent a copy of the hospital discharge summary and information about the trial and group allocation. Postdischarge, patients had unrestricted access to outpatient management via >50 specialist physicians (predominantly cardiologists), routine follow-up provided by 941 primary care physicians, subsidized pharmacological treatment, and referral to allied healthcare services when required. Common elements of the HBI were as follows: (1) a home visit 7 to 14 days posthospitalization conducted by a registered cardiac nurse with tertiary qualifications; (2) comprehensive automated reports derived from baseline and home profiling providing treating physicians with recommendations to address any treatment or management deficits; (3) coordination of multidisciplinary follow-up; (4) telephone follow-up by the cardiac nurse with both scheduled and patient-initiated contacts; (5) a strong focus on the 6 months postindex hospitalization to address residual risk with reapplication of home visits if a patient experienced an unplanned hospitalization; and (6) structured review and generation of a comprehensive report and recommendations for optimal long-term management once active study management was discontinued. Table 215–20 summarizes the specific features of postdischarge management for the 3 studies.
Table 2.
Characteristics of Clinical Management in the NIL-CHF Study, SAFETY Trial, and WHICH? Trial
End Points
Health outcomes were collected via electronic health records by personnel masked to group allocation. Subsequent adjudication of the type and nature of recurrent hospitalizations were determined by each study’s blinded end point committee. The primary end point for this composite analysis was event-free survival expressed as the proportion of actual versus maximal unplanned days-alive (a combined total of 1 648 560 days) and out-of-hospital (DAOH). For example, a patient who experienced 10 days of unplanned hospitalization and died on day 700 of 730 possible days follow-up achieved 690 of 730 DAOH (94.5% of maximal). The 2 components of this end point, all-cause deaths and days of unplanned hospitalization, were also examined separately. Hospitalization data (episodes and related overnight hospital stay) were also examined on an all-cause, unplanned, and cardiovascular-specific basis.
Statistical Analysis
Profiling and outcome data from the 3 studies were pooled and analyzed using SPSS v22.0. Discrete variables were summarized by frequencies and percentages and continuous variables by means (standard deviation) and medians (interquartile range) where appropriate. Between-group comparisons of health outcomes (including the primary end point) were assessed by Mann-Whitney U test. All efficacy analyses were undertaken on an intention-to-treat basis and blinded to group allocation. Multivariate analyses used the baseline variables listed in Table 3.21,22 Survival data were used to generate Kaplan–Meier survival curves and group comparisons analyzed with the log-rank test. A Cox proportional hazards model was constructed to derive adjusted hazard ratios (HRs) for independent correlates of all-cause mortality using a backward, stepwise approach. All predictors included in the Cox model were from baseline and were not time varying. We tested only for covariates of mortality, not management group, because covariates were from baseline, and random assignment to treatment group will ensure no systematic effects beyond chance. The assumption of proportional hazards was tested for the effect of treatment by using time-weighted score tests and the assumption was not rejected (P>0.05). Because of incomplete data for left ventricular ejection fraction (n=839) and cognitive function (n=1004), these 2 variables were initially excluded and then added to the final iteration to determine their impact on the explanatory variables. Patient hospital activity data (unplanned, all-cause, and cardiovascular related) were converted to the rate of hospitalizations and days of hospital stay per 100 days of follow-up. An exploration of the relative impact of HBI on being in the worst quintile of DAOH and experiencing a fatal event was conducted by including a condition×trial interaction in logistic regression models. We also explored the possibility that there was a differential intervention effect according to the age of the patient and their burden of disease (as measured by the Charlson Comorbidity Index Score23); both were treated as continuous variables and tested by including a condition×age and condition×age2 or condition×comorbidities and condition×comorbidities2 interaction terms in the regression models. Quadratic terms were included to allow the possibility that there was a U- or J-shaped pattern of interventional effect. If the quadratic term was not statistically significant, it was dropped.
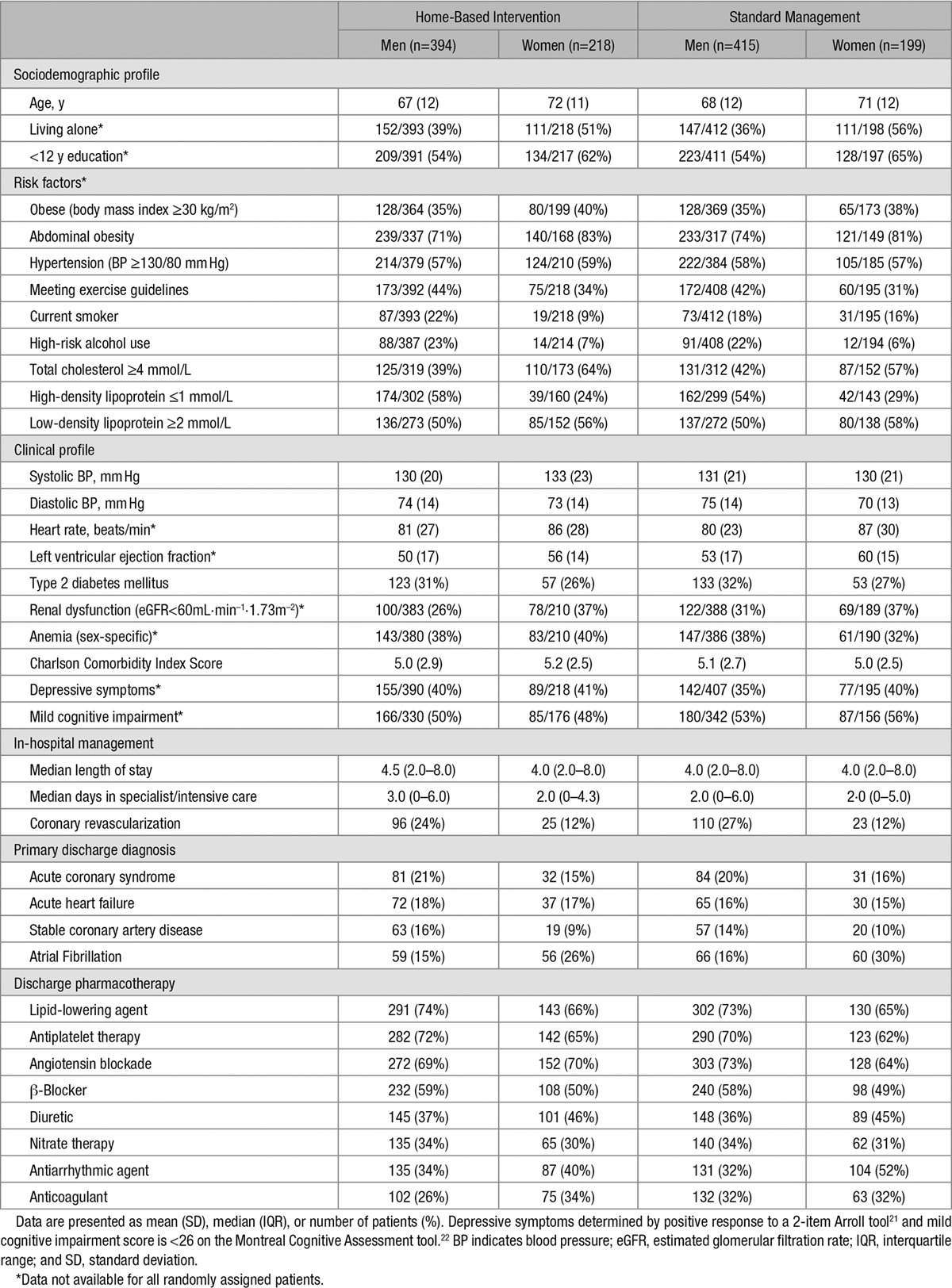
Table 3.
Baseline Characteristics According to Group Assignment and Sex (n=1226)
Results
Cohort Profile
Between June 1, 2008 and March 31, 2011, 1226 of 10 382 cardiac inpatients at participating hospitals were eligible for study entry, randomly assigned to a study group, and followed for study outcomes (Figure 1). Median follow-up of 1371 days (interquartile range, 1112–1605) across the 3 studies was completed by March 31, 2014. Reflective of the increasing severity of disease across this family of trials, multimorbidity, as measured by the mean (age-adjusted) Charlson Comorbidity Index Score23 was 4.7, 4.9, and 6.2 in the NIL-CHF, SAFETY, and WHICH? Trial cohorts. The HBI and standard management groups were well matched for sociodemographic characteristics and clinical profile according to sex (Table 3). The most common primary discharge diagnoses were AF (20%), acute coronary syndrome (19%), and acute HF (17%); the majority being admitted for a cardiovascular-related diagnosis (Figure 2). Women comprised 34% of patients and were on average 4 years older than men. Overall, patients had high levels of antecedent risk for secondary events and noncardiovascular multimorbidity complicating their clinical management. Pharmacological management included appropriately prescribed levels of antiplatelet/coagulant agents, lipid-lowering, angiotensin blockade, β-blockade, diuretics, and antiarrhythmic agents (Table 3).
Figure 1.
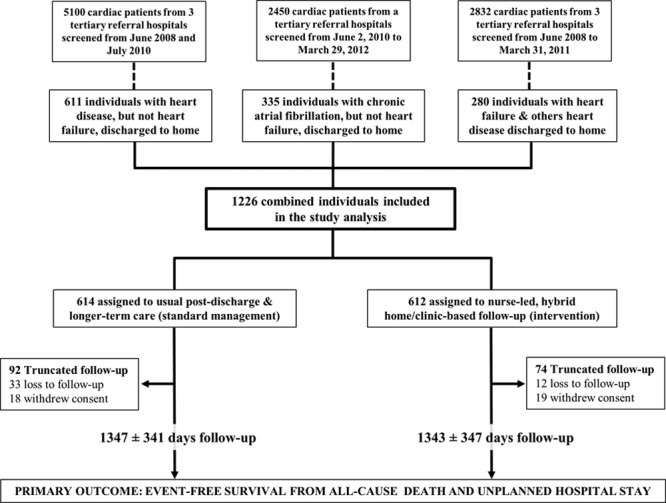
Combined study flow-chart.
Figure 2.
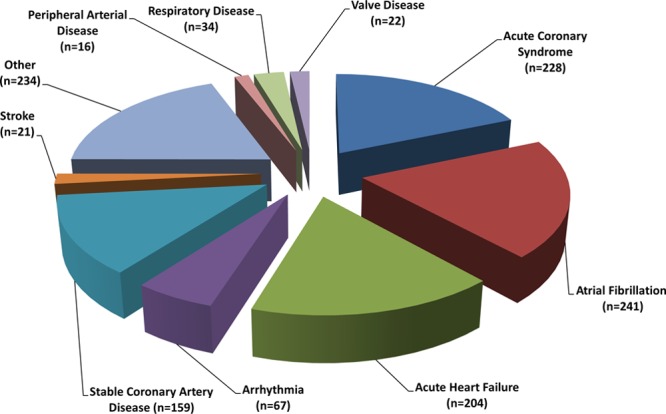
Primary cause of index hospitalization.
Primary End Point: Days Alive and Out of Hospital
Based on favorable outcome data with respect to both survival and hospital stay (see Survival Profile and Hospitalizations below), the HBI group accumulated more DAOH (737 852 of a maximal 821 707 days) than those allocated to standard management (723 527 of 826 853 days). Specifically, HBI patients experienced a mean of 1210 (standard deviation, 463) DAOH equating to 90.1% (95% confidence interval [CI], 88.2–92.0) days event free. In comparison, standard management patients experienced 1184 (standard deviation, 494) DAOH equating to 87.2% (95% CI, 85.1–89.3) days event free (P=0.02).
Survival Profile
Overall, 94 of 612 (15%) patients allocated to HBI died in comparison with 124 of 614 (20%) patients allocated to standard management. HBI was associated with significantly prolonged survival (unadjusted HR, 0.75; 95% CI, 0.57–0.98; P=0.032; Figure 3). The effect was even stronger (HR, 0.67; 95% CI, 0.50–0.88; P=0.005) after adjusting for age (HR, 1.04; 95% CI, 1.02–1.05 per year; P<0.001); age-adjusted Charlson Comorbidity Index Score (HR, 1.22; 95% CI, 1.16–1.28 per unit score; P<0.001), AF (HR, 2.02; 95% CI, 1.46–2.79; P=0.006), being treated for hypertension (HR, 0.67; 95% CI, 0.51–0.88; P=0.004), prescribed a diuretic (HR, 2.96; 95% CI, 2.12–4.13; P<0.001), and index length of stay (HR, 1.03; 95% CI, 1.03–1.04 per day; P<0.001). When added in a separate model, left ventricular ejection fraction (HR, 0.97; 95% CI, 0.96–0.98 per unit increment) was also correlated with survival without substantially altering the original model.
Figure 3.
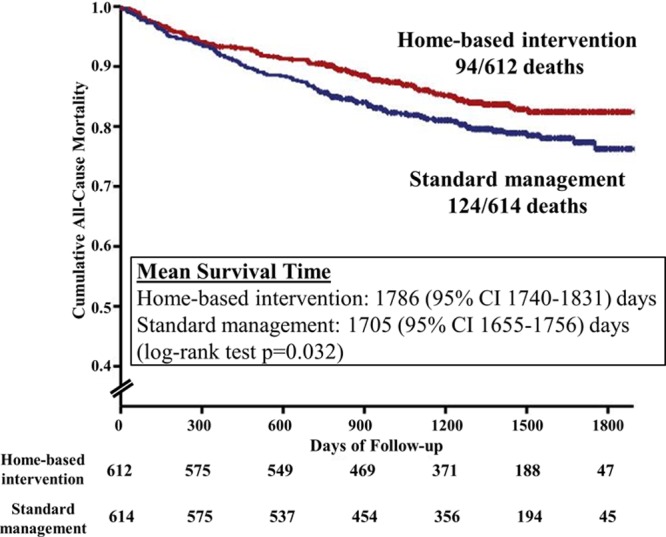
Kaplan–Meier (all-cause) survival plots. Nurse-led, multidisciplinary home-based intervention (518 censored observations) and standard management (490 censored observations). CI indicates confidence interval.
Hospitalizations
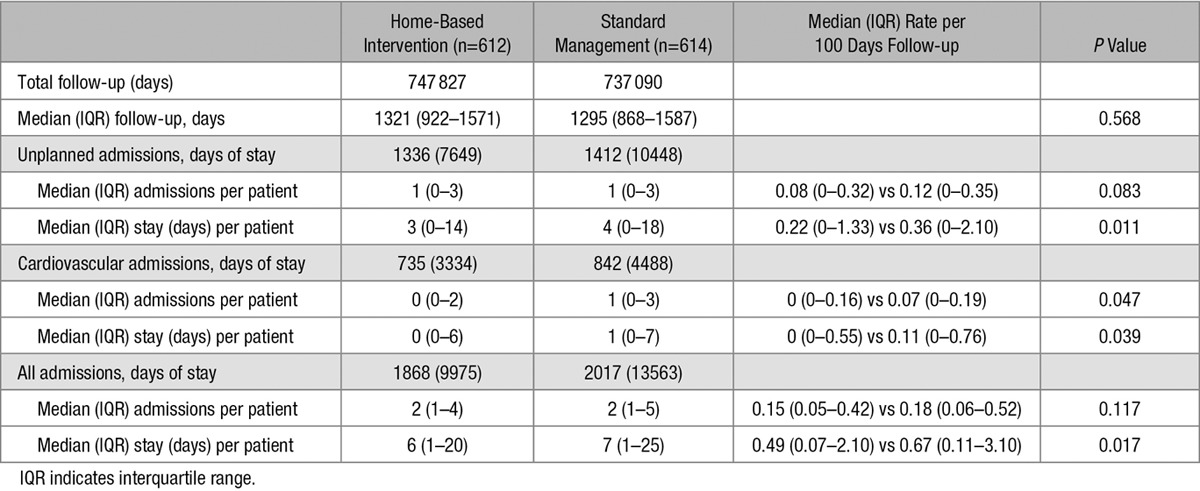
The HBI group accumulated 7469 days of hospital stay from 1336 unplanned hospitalizations in comparison with 10 448 days from 1412 unplanned hospitalizations in the standard management group. Adjusting for duration of follow-up, the HBI group accumulated significantly fewer cardiovascular admissions and accumulated significantly fewer days of hospital stay across all 3 categories of hospital activity (unplanned, cardiovascular related, and total all-cause; see Table 4).
Table 4.
Group Comparison of Hospital Outcomes
Differential Impact of HBI
For the worst quintile of DAOH (greatest number of days lost to hospital stay and death), the omnibus test of the trial×condition interaction did not reach statistical significance (P=0.055). There were no significant differences in the intervention effect between the WHICH? and SAFETY trials (P=0.928), and the intervention effects were comparable (odds ratio, 0.50; 95% CI, 0.28–0.88 in SAFETY and odds ratio, 0.49; 95% CI, 0.30–0.78 in WHICH?). However, there was a difference between the NIL-CHF Study and WHICH? (P=0.026) and the SAFETY Trial (P=0.049), and the intervention effect was smaller overall in NIL-CHF (odds ratio, 1.12; 95% CI, 0.64–1.97).
For all-cause mortality, the omnibus test of the trial×condition interaction was not statistically significant (P=0.208). Examining the specific contrasts between trials also revealed no statistically significant differences (WHICH? versus SAFETY, P=0.946; WHICH? versus NIL-CHF, P=0.085; SAFETY versus NIL-CHF, P=0.168). For lowest quintile of DAOH, the condition×age2 interaction was significant (P=0.045), revealing a U-shaped relationship (see Figure 4). HBI had a significant, beneficial effect for adults who were 59 to 81 years old; this age range was predominant in WHICH? (58%), SAFETY (62%), and NIL-CHF (64%). For extent of comorbidity, the direction of the effect was the same U-shaped relation as for age. However, the condition×comorbidities2 interaction did not reach statistical significance (P=0.083). For all-cause mortality, the condition×age2 interaction was significant (P=0.005), revealing a U-shaped relationship. HBI had a significant, beneficial effect for adults who were 60 to 82 years old, an age range also predominant in WHICH? (59%), SAFETY (63%), and NIL-CHF (65%). For extent of comorbidity, the condition×comorbidities2 interaction was also significant (P=0.041) and also displayed a U-shaped relationship. HBI had a significant, beneficial effect when the Charlson Comorbidity Index Score ranged from 5 to 8. This range was predominant in WHICH? (61%) and was common in SAFETY (45%) and NIL-CHF (36%).
Figure 4.
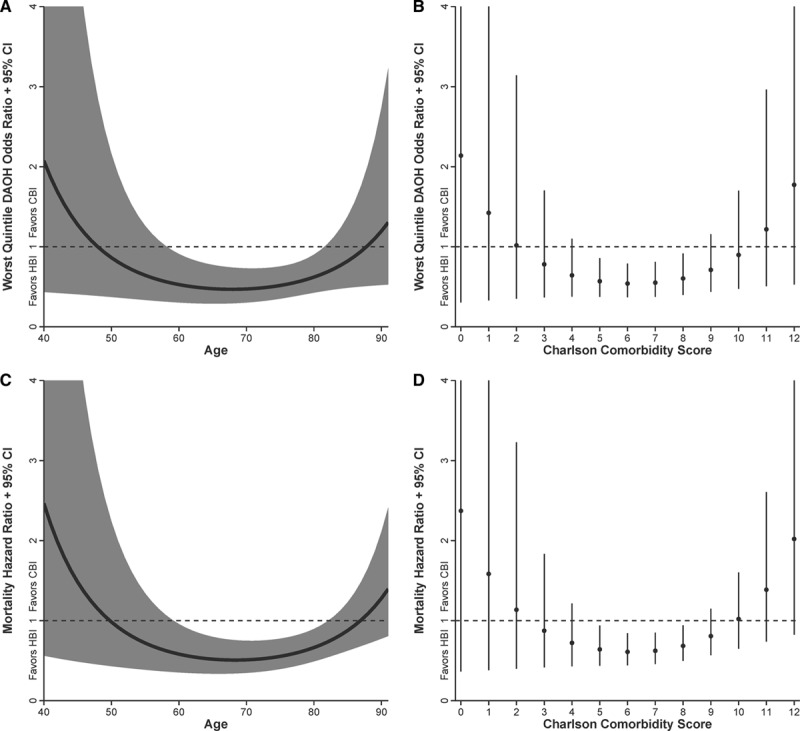
Interaction between age and Charlson Comorbidity Score and impact of HBI on the risk of being in the worst quintile of DAOH (A and B) or dying (C and D). CBI indicates clinic-based intervention (standard management); CI, confidence interval; DAOH, days-alive and out-of-hospital; and HBI, home-based intervention.
Discussion
We combined outcome data from 3 closely related trials to examine the impact of HBI delivered by cardiac nurses with multidisciplinary support across the spectrum of heart disease. Historically, this model of care is reserved for those with CHF. However, as postulated, our composite findings suggest that HBI consistently prolongs DAOH in comparison with standard management across the full spectrum of chronic heart disease. DAOH represents a realistic and patient-focused outcome because it does not rely on a single event, but rather reflects the entirety of the patient’s journey in terms of major outcomes.24 Moreover, it has particular relevance to interventions seeking to prolong life while avoiding recurrent and often costly rehospitalization. Accordingly, positive findings were derived from a significant reduction in unplanned hospital stay and prolonged survival during study follow-up. Absolute differences between groups were clinically compelling. These positive differences were monitored over 2 to 5 years follow-up in contrast to the frequent reporting of short-term outcomes in those exposed to a secondary prevention program.3,4 For all-cause mortality, the number needed to treat to avert 1 additional death via the application of HBI was 21 patients. At the same time, HBI was associated with close to 600 fewer days of hospital stay per 100 patients. Overall, these unique data provide a strong case for extending the application of this model of care more widely to those hospitalized with any form of chronic heart disease.
The benefits of home-based models of care, with their ability to improve the contextual profiling of affected patients to strengthen factors shown to improve health outcomes are now well described.8–10 Benefits include addressing often unknown issues such as cognitive impairment and clinical instability via an individualized, case management approach and improving self-care behaviors. Accordingly, across the 3 trials, there was evidence (albeit not definitive) of greater levels of healthcare engagement, more patient-initiated contacts, greater preservation of cardiac function through better blood pressure control, and more proactive uptitration of gold-standard therapies.8–10 The model of care applied in this instance was initially developed and successfully applied25 in patients with a broad range of chronic disease states before being specifically adapted for those with CHF; the subsequent trial report was the first to demonstrate both prolonged survival and reduced risk of recurrent hospitalization in this patient population.26 Since then, the literature has consistently demonstrated that an in-person, multidisciplinary approach is superior to other modes of management (including remote management strategies) to improve health outcomes in CHF, and this is likely to be the case for all forms of chronic heart disease.2,4,15,17,19 Based on the totality of evidence, expert guidelines have recommended the application of multidisciplinary, face-to-face programs of management for those hospitalized with CHF,20 resulting in a global network of such programs. However, there has been a paucity of research and evidence to demonstrate that such an approach is beneficial more widely across the spectrum of chronic heart disease. These data, therefore, address an important gap in the literature and evidence base.
Beyond questioning the relevance of data derived from Australia (characterized by high standards of subsidized health care), it is worth considering whether a blanket approach to applying HBI is truly warranted. For example, in the recent Young@Heart Study,27 applying HBI in a wealthier cohort of cardiac patients with greater access to a specialist Cardiologist, men but not women appeared to benefit from HBI; the latter potentially attributable to suboptimal application of HBI at 1 study site.27 Beyond health system factors, the relative impact of HBI will undoubtedly vary according to clinical acuity and risk of premature mortality and recurrent hospital stay. Exploratory analyses of the interaction between HBI and a patient’s age and comorbidity clearly showed a U-shaped response with respect to DAOH and survival alone, those at either end of the spectrum demonstrating an increased propensity to have more events when exposed to HBI. The potential to identify who benefits most from HBI and therefore to apply it on a more selective basis should not, however, detract from the overall benefits observed by this composite analysis; in particular, when one considers the unique advantage of HBI in being patient centered and detecting otherwise unknown clinical instability.4,28 The Green Amber Red Delineation of Risk and Need (GARDIAN) tool16 was designed to achieve the former and is being used to guide the intensity of disease management according to the level of need in current trials of HBI in the setting of CHF. It is certainly possible that high levels of standard management alone, including traditional outpatient care, deliver the best health outcomes in younger patients with less complex clinical needs. Alternatively, the danger of not transitioning to a potentially more effective HBI over time is tempered by the natural history of heart disease toward more progressive, multiorgan disease and poor health outcomes in the long term.8,29 With an increasingly ageing cardiac patient population in whom multimorbidity and rehospitalizations are becoming more common,30 our positive findings with respect to HBI, as a reflection of the overall benefits of applying successful programs involving a home-based31 or transitional care32 approach to chronic heart disease management, are likely to become more clinically relevant over time.
Beyond previously identified trial limitations, including nonblinding of patients and health workers, a number of specific issues require comment. First, despite the purposeful nature of our program of research and efforts to standardize trial methodology,14 there were potentially important differences in the application of clinical management in each trial. One trial (the NIL-CHF Study)8 was conducted in a single tertiary center. The applicability of our findings (both negative and positive) from studies conducted within the Australian healthcare system requires careful consideration in terms of their generalizability. Nevertheless, our analyses of who benefits most from HBI remain ongoing and we are yet to undertake economic analyses to determine whether there is a cost threshold at which HBI might be applied. Finally, our composite findings are also yet to be tested via a single appropriately powered, prospective study.
In conclusion, across 3 contiguous clinical trials applying the same form of nurse-led, multidisciplinary, HBI adapted to the target patient population, this model of care was associated with more DAOH than standard management. These data support the wider application of HBI to optimize the immediate and longer-term postdischarge management of patients with a broad spectrum of heart disease states.
Sources of Funding
This study (1055214), along with 1041796 (to Dr Stewart), 1112829 (to Dr Ball) and 1032934 (to Dr Carrington), was supported by National Health and Medical Research Council of Australia.
Disclosures
None.
CLINICAL PERSPECTIVE
These data have important clinical implications for the application of disease management across the full spectrum of heart disease to prolong survival without provoking the competing risk of recurrent hospitalization. In combining 3 contiguous trials of the same form of management adapted to clinical profile (from younger individuals surviving an acute coronary event to older individuals hospitalized with more advanced forms of atrial fibrillation and chronic heart failure), we were able to tackle an important gap in the evidence base. Overall, we demonstrated that, in comparison with high levels of standard management, a nurse-led, multidisciplinary, home-based intervention was associated with both prolonged survival and reduced hospital stay over the longer term. Given that in clinical practice disease management programs are typically applied according to a single cardiac diagnosis (most notably chronic heart failure), overall, these data support the wider application of this approach to any patient discharged home from the hospital with a form of chronic heart disease; particularly in older individuals with multimorbidity. On this basis, there is scope to extend preexisting health services applying this kind of approach to the full spectrum of heart disease on a cost-effective basis. This would involve modifications in screening and referral protocols (to identify eligible patients), more health resources to accommodate an increased case load, and critical adjustments to patient profiling and needs assessments to adapt this strategy on an individual basis.
References
- 1.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood) 2009;28:75–85. doi: 10.1377/hlthaff.28.1.75. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sochalski J, Jaarsma T, Krumholz HM, Laramee A, McMurray JJ, Naylor MD, Rich MW, Riegel B, Stewart S. What works in chronic care management: the case of heart failure. Health Aff (Millwood) 2009;28:179–189. doi: 10.1377/hlthaff.28.1.179. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 4.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Carrington MJ, Stewart S NIL-CHF Study Investigators. Bridging the gap in heart failure prevention: rationale and design of the Nurse-led Intervention for Less Chronic Heart Failure (NIL-CHF) Study. Eur J Heart Fail. 2010;12:82–88. doi: 10.1093/eurjhf/hfp161. doi: 10.1093/eurjhf/hfp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCabe PJ, DeVon HA. Home-based management of patients with atrial fibrillation. Lancet. 2015;385:752–753. doi: 10.1016/S0140-6736(14)62013-4. doi: 10.1016/S0140-6736(14)62013-4. [DOI] [PubMed] [Google Scholar]
- 7.Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R, Pison LA, Blaauw Y, Tieleman RG. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–2699. doi: 10.1093/eurheartj/ehs071. doi: 10.1093/eurheart/ehs071. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Chan YK, Wong C, Jennings G, Scuffham P, Esterman A, Carrington M NIL-CHF Investigators. Impact of a nurse-led home and clinic-based secondary prevention programme to prevent progressive cardiac dysfunction in high-risk individuals: the Nurse-led Intervention for Less Chronic Heart Failure (NIL-CHF) randomized controlled study. Eur J Heart Fail. 2015;17:620–630. doi: 10.1002/ejhf.272. doi: 10.1002/ejhf.272. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C, Abhayaratna WP, Chan YK, Esterman A, Thompson DR, Scuffham PA, Carrington MJ. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet. 2015;385:775–784. doi: 10.1016/S0140-6736(14)61992-9. doi: 10.1016/S0140-6736(14)61992-9. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Carrington MJ, Marwick TH, Davidson PM, Macdonald P, Horowitz JD, Krum H, Newton PJ, Reid C, Chan YK, Scuffham PA. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol. 2012;60:1239–1248. doi: 10.1016/j.jacc.2012.06.025. doi: 10.1016/j.jacc.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Carrington MJ, Marwick T, Davidson PM, Macdonald P, Horowitz J, Krum H, Newton PJ, Reid C, Scuffham PA Which Heart failure Intervention is most Cost-effective & consumer friendly in reducing Hospital care trial Investigators. The WHICH? trial: rationale and design of a pragmatic randomized, multicentre comparison of home- vs. clinic-based management of chronic heart failure patients. Eur J Heart Fail. 2011;13:909–916. doi: 10.1093/eurjhf/hfr048. doi: 10.1093/eurjhf/hfr048. [DOI] [PubMed] [Google Scholar]
- 12.Carrington MJ, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C, Abhayaratna WP, Haluska B, Thompson DR, Scuffham PA, Stewart S. Navigating the fine line between benefit and risk in chronic atrial fibrillation: rationale and design of the Standard versus Atrial Fibrillation spEcific managemenT studY (SAFETY). Int J Cardiol. 2013;166:359–365. doi: 10.1016/j.ijcard.2011.10.065. doi: 10.1016/j.ijcard.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 13.Stewart S, Carrington MJ, Horowitz JD, Marwick TH, Newton PJ, Davidson PM, Macdonald P, Thompson DR, Chan YK, Krum H, Reid C, Scuffham PA. Prolonged impact of home versus clinic-based management of chronic heart failure: extended follow-up of a pragmatic, multicentre randomized trial cohort. Int J Cardiol. 2014;174:600–610. doi: 10.1016/j.ijcard.2014.04.164. doi: 10.1016/j.ijcard.2014.04.164. [DOI] [PubMed] [Google Scholar]
- 14.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D CONSORT group; Pragmatic Trials in Healthcare (Practihc) group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 16.Carrington MJ, Kok S, Jansen K, Stewart S. The Green, Amber, Red Delineation of Risk and Need (GARDIAN) management system: a pragmatic approach to optimizing heart health from primary prevention to chronic disease management. Eur J Cardiovasc Nurs. 2013;12:337–345. doi: 10.1177/1474515112451702. doi: 10.1177/1474515112451702. [DOI] [PubMed] [Google Scholar]
- 17.European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 18.Ball J, Carrington MJ, Thompson DR, Horowitz JD, Stewart S Standard versus Atrial Fibrillation spEcific managemenT studY Investigators. Post-discharge electrocardiogram Holter monitoring in recently hospitalised individuals with chronic atrial fibrillation to enhance therapeutic monitoring and identify potentially predictive phenotypes. Eur J Cardiovasc Nurs. 2015;14:384–394. doi: 10.1177/1474515114547650. doi: 10.1177/1474515114547650. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ National Heart Foundation of Australia; Cardiac Society of Australia and New Zealand. 2011 update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust. 2011;194:405–409. doi: 10.5694/j.1326-5377.2011.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 21.Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144–1146. doi: 10.1136/bmj.327.7424.1144. doi: 10.1136/bmj.327.7424.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Ariti CA, Cleland JG, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, McMurray JJ, Michelson EL, Ostergren J, Yusuf S. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J. 2011;162:900–906. doi: 10.1016/j.ahj.2011.08.003. doi: 10.1016/j.ahj.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46:174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354:1077–1083. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 27.Carrington MJ, Chan YK, Calderone A, Scuffham PA, Esterman A, Goldstein S, Stewart S Young at Heart Investigators. A multicenter, randomized trial of a nurse-led, home-based intervention for optimal secondary cardiac prevention suggests some benefits for men but not for women: the Young at Heart study. Circ Cardiovasc Qual Outcomes. 2013;6:379–389. doi: 10.1161/CIRCOUTCOMES.111.000006. doi: 10.1161/CIRCOUTCOMES.111.000006. [DOI] [PubMed] [Google Scholar]
- 28.Tappenden P, Campbell F, Rawdin A, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess. 2012;16:1–72. doi: 10.3310/hta16200. doi: 10.3310/hta16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 30.Condelius A, Edberg AK, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr. 2008;46:41–55. doi: 10.1016/j.archger.2007.02.005. doi: 10.1016/j.archger.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Clark AM, Haykowsky M, Kryworuchko J, MacClure T, Scott J, DesMeules M, Luo W, Liang Y, McAlister FA. A meta-analysis of randomized control trials of home-based secondary prevention programs for coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2010;17:261–270. doi: 10.1097/HJR.0b013e32833090ef. [DOI] [PubMed] [Google Scholar]
- 32.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52:675–684. doi: 10.1111/j.1532-5415.2004.52202.x. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]


