Abstract
Background
Classic lichen planopilaris (LPP) is a patchy form of primary lymphocytic cicatricial alopecia localized on the vertex of the scalp. It is important, however, to be aware of other, less recognized presentations that may be missed without dermatoscopy and pathology.
Methods and Results
We report 26 patients with LPP presenting with subtle erythema and scaling colocalized in the area of patterned thinning (androgenetic alopecia, AGA). All patients had been treated for seborrheic dermatitis in the past. Dermatoscopy showed the presence of 2-4 hairs emerging as a tuft from the same ostium surrounded by erythema, peripilar casts and interfollicular scaling associated with hair miniaturization. Histopathology obtained from those areas corresponded to LPP with concomitant follicular miniaturization.
Conclusion
Subtle or focal cases of LPP may be missed for seborrheic dermatitis when overlapping with AGA. Dermatoscopy-guided biopsy from the affected scalp is the best approach to make a timely diagnosis. This is particularly important in patients with AGA evaluated to undergo hair transplantation, as active LPP is a contraindication for these patients.
Key Words: Lichen planopilaris, Androgenetic alopecia, Hair transplantation
Introduction
Androgenetic alopecia (AGA) is a common form of nonscarring hair thinning affecting both men and women, which is usually diagnosed by clinical examination. Many times it is associated with seborrheic dermatitis characterized by pruritus, interfollicular redness and scaling [1]. Lichen planopilaris (LPP) is a primary lymphocytic cicatricial alopecia, which starts as a sudden, irregular, patchy hair loss in the vertex area, and it is also associated with redness, scaling and pruritus. LPP has a worse prognosis than AGA, as it leads to follicular dropout and scarring [2,3].
We report 26 patients who presented with hair thinning, redness and scaling in the same area simulating seborrheic dermatitis associated with AGA. Most of them had already been diagnosed with AGA clinically but had progressed despite treatment. We show that such patients require scalp biopsy from the affected area, as otherwise the diagnosis of subtle LPP overlapping AGA can be missed for years.
Methods and Results
Nine females and 17 males experiencing hair thinning and scalp scaling were diagnosed with LPP at the Department of Dermatology, University of Miami, Miami, Fla., USA (4), at the Hair Transplant Institute of Miami, Coral Gables, Fla., USA (13), and at the Department of Dermatology, Santa Casa Hospital, Porto Alegre, Brazil (9). All patients were Caucasians aged between 22 and 65 years. They presented with asymptomatic progressive hair thinning of different grades: 1 female patient had Christmas tree pattern thinning (Ludwig type I and II; fig. 1a) and 1 male patient showed male pattern hair loss (MPHL; ranging from stage II to VI according to the Norwood-Hamilton classification; fig. 1b). All presented with a complaint of hair thinning, and 14 out of 17 males requested hair transplantation. When asked, all patients reported subtle scaling, and some of them had itching (12/26). None reported trichodynia. Table 1 summarizes the demographic, clinical, dermatoscopic and pathologic features.
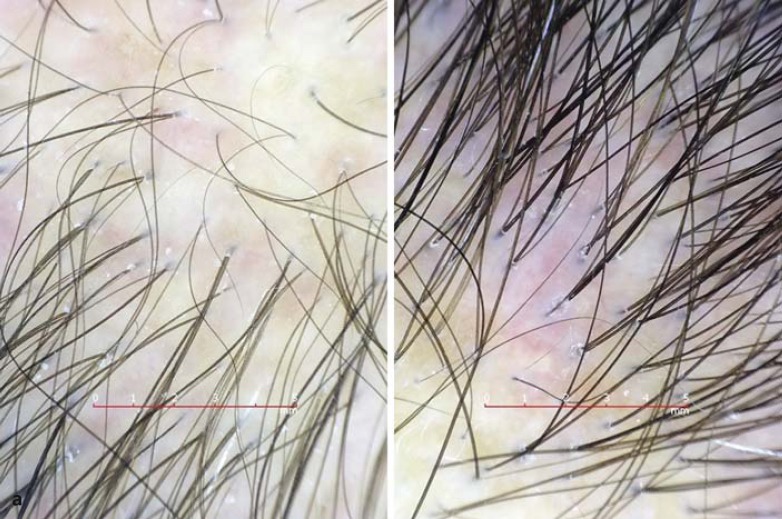
Fig. 1.
Clinical findings. a Diffuse hair thinning on the frontoparietal scalp of a female with AGA. Note the mild erythema. b Long-standing AGA (Norwood-Hamilton classification, stage IV) in a patient who requests hair transplantation. There are no apparent features for scarring alopecia, but mild redness and scaling are present at the periphery of the bald area.
Table 1.
Summary of the demographic, clinical, dermatoscopic and pathologic features of 26 patients with LPP
| Males | 17 (65%) |
| Females | 9 (35%) |
| Age range, years | 22–65 |
| Clinical findings | |
| Males | Asymptomatic or slightly pruritic scalp MPHL ranging from stage II to VI (Norwood-Hamilton classification) Variable scaling and redness |
| Females | Asymptomatic or slightly pruritic scalp Christmas tree pattern hair thinning (Ludwig type I or II) Variable scaling and redness |
| History of hair transplantation | None |
| Patients requesting hair transplantation | |
| Males | 14/17 |
| Females | 1/9 |
| Dermatoscopic findings | Hair shaft variability >20% on the frontal, parietal and vertex scalp |
| Erythema | |
| Groups of 2–4 hairs emerging as one stem from a single ostium in the same area of the variability | |
| Peripilar casts | |
| Interfollicular scaling | |
| Focal absence of follicular ostia | |
| Pathologic findings | Compound follicular structures |
| Perifollicular lichenoid infiltrate at the | |
| upper level | |
| Perifollicular fibrosis | |
| Partial loss of sebaceous glands | |
| Follicular miniaturization |
On dermatoscopy, hair shaft variability of more than 20% was present in all cases in the frontal, parietal and vertex area. Focal absence of follicular openings suggesting scarring alopecia was present in only 8 cases. The main finding was groups of hairs (2-4) emerging as one stem from a single ostium surrounded by perifollicular and interfollicular scaling and erythema (fig. 2). Scalp biopsies were obtained from these sites using the dermatoscope as a guiding tool.
Fig. 2.
Dermatoscopic findings. Dermatoscopy shows hair shaft variability and preserved follicular ostia. There are several groups of hairs emerging as a single stem from the same ostium. Note the peripilar casts and the erythema (Handyscope; Fotofinder Systems, Bad Birnbach, Germany; ×20).
The pathology was characterized by an altered follicular architecture due to focal areas of absent sebaceous glands and the presence of compound follicular structures surrounded by perifollicular fibrosis and a lichenoid infiltrate. The infiltrate was mild to moderate in 21 cases and dense in 5 cases, and it was most prominent at the level of the isthmus and infundibulum (fig. 3a, b). Marked follicular miniaturization with a terminal-to-vellus ratio of <2:1 was noted in all cases and was confirmed by concomitant AGA.
Fig. 3.
Histological findings. a The horizontal section trough the upper follicle level from the patient on fig. 1a shows an altered follicular architecture due to the loss of sebaceous glands and the presence of one compound follicular structure (black arrows) of a terminal and vellus follicle surrounded by peripilar fibrosis and lichenoid inflammation. Other affected follicles are highlighted with white arrows (scalp biopsy. HE. ×10). b The horizontal section through the upper follicular level from the patient on fig. 1b shows an altered follicular architecture due to the focal loss of sebaceous glands and the presence of compound follicular structures (black arrows) surrounded by peripilar fibrosis and lichenoid inflammation. A cleft is noted between the zone of fibrosis and the follicular epithelium. Note the preserved follicular architecture at the periphery (scalp biopsy. HE. ×10).
Discussion
We report 26 patients with an unusual presentation of subtle LPP associated with AGA who had a red and scaly scalp in the thinning area. Such cases may go unrecognized for years, as the hair loss may be attributed only to the AGA, with the erythema and scaling being attributed to concomitant seborrheic dermatitis. Cases may also accidentally be discovered as new onset of LPP after hair transplantation was performed without scalp biopsies prior to the procedure. Cases of LPP after hair transplant surgery have been reported recently [4,5,6], and although it has been suggested that LPP occurs due to the loss of immune privilege after surgery [6], it is possible that LPP in at least some of the reported patients did not occur de novo but rather had coexisted with the MPHL prior to the surgery. In fact, if our 14 male patients who requested hair transplantation for their MPHL had not been biopsied prior to the transplantation they could have developed classic LPP afterwards.
Dermatoscopy failed to show the loss of follicular ostia and white patches in 18 cases, but the presence of compound hairs emerging from the same ostium with peripilar casts was suspicious for scarring alopecia, as in AGA, most pilosebaceous units in the frontal and parietal scalp contain a single hair (65 and 55%, respectively) [7]. To the best of our knowledge, follicular counts in seborrheic dermatitis have not been reported, probably due to the lack of association with hair loss. Table 2 summarizes the percentage of single hair follicular units in scalp disorders, and table 3 summarizes the number of hair follicles, including terminal hairs per follicular unit [8,9]. Previous data from patients with established LPP showed that the hair count per follicular unit is lower than in AGA patients, probably due to the follicular dropout. [8] The counts per follicular unit in LPP may vary depending on the site of the biopsy, as we specifically used the dermatoscope to sample areas with groups of hairs emerging from the same ostium, which on pathology corresponds to compound follicular structures of two or three follicles surrounded by perifollicular fibrosis and lichenoid inflammation. In fact, it was previously suggested that two packs (compound follicular structures of 2 follicles) are a clue to the diagnosis of lymphocytic cicatricial alopecia on horizontal sections [10].
Table 2.
Percentage of single hair follicular units in scalp disorders
| Diagnosis | Frontal area | Temporal area | Occipital area |
|---|---|---|---|
| AGA | 65.2±19.9% | 55±15% | 36.8±18.6% |
| Normal scalp* | 27.3±13% | 32±15% | 22.6±12.6% |
Female patients [7].
Table 3.
Hair follicles and terminal hairs per follicular units in scalp disorders
The presence of peripilar casts is a known dermatoscopic clue of classic LPP [11], but the presence of compound hairs has only been described in one previous paper [11], although it can be observed in the images of several reports [6,12]. One possible explanation is that in classic LPP, the fibrosis and inflammation have already destroyed part of the compound follicular structures found in early/subtle LPP. Peripilar casts as well as compound hairs can be used as a clue to the diagnosis and to guide to the right site for a biopsy [12]. For the pathologist, it is important to utilize horizontal sections on pathology, as the findings of perifollicular fibrosis, inflammation and the loss of sebaceous glands may be focal and limited to only a few follicles (fig. 3b). Table 4 summarizes the main features of the different disorders characterized by hair thinning and a red, scaly scalp. It is our experience that scalp biopsies are crucial for the diagnosis in ambiguous cases.
Table 4.
Differential diagnosis of the scalp disorders characterizing the red, scaly and thinning scalp by dermatoscopy and pathology
| Diagnosis | Dermatoscopy | Pathology |
|---|---|---|
| AGA | Hair variability >20% | Terminal-to-vellus ratio <2:1 |
| One hair per follicular ostium | ||
| AGA with subtle LPP | Hair thinning >20% | Terminal-to-vellus ratio <2:1 |
| Groups of hairs (2–4) emerging from the same ostium in the area of thinning | Compound follicular structures with perifollicular fibrosis and lichenoid inflammation | |
| Perifollicular and interfollicular scale and erythema | Focal absence of sebaceous glands | |
| LPP | Absence of follicular ostia | Follicular dropout |
| Peripilar scaling | Compound follicular structures with perifollicular fibrosis and lichenoid inflammation | |
| Erythema | Absent sebaceous glands | |
| Seborrheic dermatitis | Perifollicular and interfollicular scale and erythema | Infundibular parakeratosis |
| Spongiosis | ||
| Dilated sebaceous canals with larger sebaceous glands | ||
Fibrosing alopecia in a pattern distribution was described as an unusual form of LPP showing concomitant miniaturization, mostly in women (15 females and 4 males) [13]. In the original description of Zinkernagel and Trueb [13], 19 cases are very similar to our cases, but 15/19 of their patients showed the loss of follicular ostia (8/26 in our series) and only 10/14 had follicular miniaturization on pathology (26/26 in our series). Women prevailed in their case series (15/19), whereas men prevailed in our series (17/26). A possible explanation for the female predominance in their cases may be the fact that men are usually not concerned about their common baldness and mild scaling. Therefore, the diagnosis may be missed and underreported [14].
Independent from the given name fibrosing alopecia in a pattern distribution or subtle LPP associated with AGA, the clinician should be aware of this condition and proceed with scalp biopsies for the diagnosis in patients with hair thinning and concomitant redness and scaling. This is particularly true for patients who do not improve with standard treatment and for patients requesting hair transplantation.
References
- 1.Jang WS, Son IP, Yeo IK, Park KY, Li K, Kim BJ, Seo SJ, Kim MN, Hong CK. The annual changes of clinical manifestation of androgenetic alopecia clinic in Korean males and females: a outpatient-based study. Ann Dermatol. 2013;25:181–188. doi: 10.5021/ad.2013.25.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.d'Ovidio R, Sgarra C, Conserva A, Angelotti UF, Erriquez R, Foti C. Alterated integrin expression in lichen planopilaris. Head Face Med. 2007;3:11. doi: 10.1186/1746-160X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevasco NC, Bergfeld WF, Remzi BK, de Knott HR. A case series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57:47–53. doi: 10.1016/j.jaad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Chiang YZ, Tosti A, Chaudhry IH, Lyne L, Farjo B, Farjo N, Cadore de Farias D, Griffiths CE, Paus R, Harries MJ. Lichen planopilaris following hair transplantation and face-lift surgery. Br J Dermatol. 2012;166:666–370. doi: 10.1111/j.1365-2133.2011.10692.x. [DOI] [PubMed] [Google Scholar]
- 5.Crisostomo MR, Crisostomo MC, Crisostomo MG, Gondim VJ, Crisostomo MR, Benevides AN. Hair loss due to lichen planopilaris after hair transplantation: a report of two cases and a literature review. An Bras Dermatol. 2011;86:359–362. doi: 10.1590/s0365-05962011000200024. [DOI] [PubMed] [Google Scholar]
- 6.Donovan J. Lichen planopilaris after hair transplantation: report of 17 cases. Dermatol Surg, 2012;38:1998–2004. doi: 10.1111/dsu.12014. [DOI] [PubMed] [Google Scholar]
- 7.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1:123–130. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horenstein MG, Bacheler CJ. Follicular density and ratios in scarring and nonscarring alopecia. Am J Dermatopathol. 2013;35:818–826. doi: 10.1097/DAD.0b013e3182827fc7. [DOI] [PubMed] [Google Scholar]
- 9.Yazdabadi A, Magee J, Harrison S, Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br J Dermatol. 2008;159:1300–1302. doi: 10.1111/j.1365-2133.2008.08820.x. [DOI] [PubMed] [Google Scholar]
- 10.Pincus LB, Price VH, McCalmont TH. The amount counts: distinguishing neutrophil-mediated and lymphocyte-mediated cicatricial alopecia by compound follicles. J Cutan Pathol. 2011;38:1–4. doi: 10.1111/j.1600-0560.2010.01645_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, Olszewska M, Rudnicka L. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–758. [PubMed] [Google Scholar]
- 12.Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013;27:1299–1303. doi: 10.1111/j.1468-3083.2012.04530.x. [DOI] [PubMed] [Google Scholar]
- 13.Zinkernagel MS, Trueb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205–211. doi: 10.1001/archderm.136.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Chiu HY, Lin SJ. Fibrosing alopecia in a pattern distribution. J Eur Acad Dermatol Venereol. 2010;24:1113–1114. doi: 10.1111/j.1468-3083.2010.03580.x. [DOI] [PubMed] [Google Scholar]